Salt Stress Induced Plant Physio-biochemical and Molecular Responses: A Review
Автор: Latif Ahmad Peer, Mohd. Yaqub Bhat, Abdul Hamid Wani
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.17, 2021 года.
Бесплатный доступ
Salt stress-induced limitation in crop growth and produce is a critical problem worldwide. The prerequisite to developing salt-tolerant plants of commercial importance is understanding the plant responses to salinity exposure at physiological, biochemical, and molecular levels, integrating various approaches to understanding underlying salt tolerance mechanisms, and utilizing naturally occurring genetic resources available for salt tolerance. In this review, plant responses and associated salt tolerance, at physiological and biochemical levels through ion homeostasis, osmolyte accumulation, hormonal regulation, antioxidant responses, and mitogen-activated protein kinase cascade signaling and molecular responses through transcription factors, different gene expressions, non-coding RNA production, and epigenetic modifications are presented.
Epigenetic modifications, ion homeostasis, non-coding RNA, salinity tolerance, transcriptional factors
Короткий адрес: https://sciup.org/143173877
IDR: 143173877
Текст научной статьи Salt Stress Induced Plant Physio-biochemical and Molecular Responses: A Review
A broad spectrum of abiotic stresses like acidity alkalinity, salinity, and microbial infections are harmful to the plants. Salinity stress is among the most crucial environmental stresses to the productivity of crops worldwide ( lowers 2004; Munns and Tester 2008). Soil salinity shows a high concentration of soluble salts in the soil moisture of the rhizosphere. According to the United Nations Environment Programme, nearly 50% of the world's cultivated land is saline ( lowers and Yeo 1995). Salt stress in plants can occur in two ways; first, high soil salt concentration makes it difficult for roots to absorb water, and second, the high content of salt within the plant may prove fatal as it is toxic (Munns and Tester 2008). Saline stress in plants may hamper the various processes such as flowering, fruit quality, seed germination, survival percentage, which leads to decreased crop productivity (Sairam and Tyagi 2004). Severe consequences of salinity on plant growth may be because of ion toxicity in cell and osmotic stress (Hussain et al., 2007). Lipids are major constituents in the cell membrane, which help to maintain cell tolerance to saline stress. Increased saline levels disturb the arrangement of lipids and proteins in the cell membrane, which leads to defective lipid metabolism (Guo et al., 2019). The most challenging circumstance for salt stress is the degradation of chlorophyll content, which terminates photosynthesis level in plants, and it also hampers the stomatal conductance, respiration rate (Doganlar et al., 2010).
The initial reaction shown by salt-stressed plants is water potential decrease, which hampers water use efficiency, resulting in significant toxic damage and reduced yield (James et al., 2011). Water relations and osmotic adjustment regulate salt-stressed plants' growth responses (Munns et al., 1983). Salinity stress inhibits water content in many crop yielding plants such as pea tomato, Mentha, balm, showing a deleterious reduction in crop yield (Ozturk and Unlukara 2004). Metabolic adjustments in response to salinity stress are dynamic and multifaceted, e.g., under salt stress, the most dramatic change occurs in the ice plant (Guan et al., 2020). Salt stress can bring out a shift from C 3 to CAM pathway in this succulent plant by the induction of some enzymatic machinery, e.g., Phosphoenolpyruvate
(Romano et al., 2020) carboxylase, within a few hours, and the transition occurs within 6 to 8 days. The striking feature of CAM plants is scotoactive stomata, by which they can use water efficiently by showing minimum transpiration rate (Guan et al., 2020).
Some metabolic changes are common to most plants, whereas others are specific, e.g., organic solutes of low molecular weight show salt-stress induced accumulation. These solutes comprise amino acids (proline or glutamate), betaines (glycine betaine and alanine betaine), and polyols. During nitrogen deficiency, plants usually accumulate sulfonium compounds, like dimethyl-sulfonium propionate, which are physiologically similar to nitrogen-containing betaines. These organic solutes act antagonistically to inorganic solutes like Na and Cl ions, even at high concentrations, and are not detrimental to the enzymes and cell organelles. That is why these organic compounds preferentially function as compatible osmolytes (Rhodes and Hanson 1993; Ashraf and Harris 2004). Studies revealed that higher plants could tolerate high salinity by exclusion or accumulation of salt. Salt excluders have the property to remove salts from the whole plant or particular organs. In such situations membrane permeability supports the intake of K+ over Na+. Thus, the crops which exclude salts are distinguished by the low concentration of Na+ and Cl+ ions (Chen et al., 2018). Salt accumulators possess the ability to uptake high salt content through the following two mechanisms. In the first mechanism, plants can withstand high concentrations of intercellular salts by tolerant cell membranes, a common feature of salt-stressed plants, e.g., Halophytes. In this way, the ratio of Na+ to K+ in tissues is apparent. The second mechanism involves eliminating excess salt that enters the plant from which roots can uptake ions to prevent their detrimental effects (Yensen 2006).
Salt tolerance through physiological and biochemical mechanisms
Salt stress negatively affects plant growth through various constraints like osmotic stress, wherein water uptake by plants is affected, triggering significant events like the arrest of the shoot and root (Munns et al., 2000; ricke et al., 2004). Therefore, the osmotic adjustment becomes the requirement for plants in such conditions. Though reduced, the root growth rate resumes within an hour by regaining cell turgor by inorganic ions uptake and changing cell wall composition like Na+ binding affecting Na+ passage and other ion bindings (Shabala and Lew 2002; Byrt et al., 2018). The assimilation of CO2 is also affected by osmotic stress through the rapid closing of stomata, resulting from reduced xylem pressure. The roots sense and send the hydraulic signals at fast speed while shoots transduce these signals immediately to alter its metabolism, and ion channels in guard cells decode the altered xylem pressure to alter stomatal conductance ( uruichi et al., 2008; Christmann et al., 2013; Shabala et al., 2016).
Salt stress-induced ion toxicity, mostly related to increased Na+ and Cl- accumulation, also restricts plant growth. Na+ toxicity lethal effect on plants though unknown can occur through enzyme inhibition; for example, many enzymes functioning in primary metabolism, regulated by K+, get inhibited through the replacement of K+ by Na+ (Wu et al., 2018). Salt sensitive plants exhibit increasing physiological dysfunctions with increasing shoot Cl-levels; thus, salt tolerance demands the exclusion of Cl- from shoots (Geilfus 2018; Teakle and Tyerman 2010). Halophytes can withstand Cl- concentration more than 1M without any adverse effect, and Cl- affects the plant by causing less availability of macronutrients like S and N, as Cl-share the anion transporters with SO 4 2- and NO 3 -(Bazihizina et al., 2019). Salt stress, mostly Na+ specific, cause PCD (programmed cell death) in plant roots, and events like cytochrome c release and DNA fragmentation occur rapidly (Jiang et al., 2008). Mutants of Arabidopsis lacking GORK, a K+ channel, show reduced or no such PCD events, suggesting entry of Na+ into cytosol causes membrane depolarisation, K+ efflux, activation of PCD executing endonucleases, and caspase-like proteases (Demidchik et al., 2010; Demidchik 2014).
Ion Homeostasis
NaCl is the primary soil salinity stress determinant, so the mechanisms underlying Na+ transport and its sequestration have remained the researcher's primary focus. The accumulation of a high concentration of Na+ in the root cell cytoplasm is prevented by excluding Na+ from uptake and vacuolar compartmentalization of Na+ (Theerawitaya et al., 2020) . Na+ uptake by roots is carried by various ion transporters in which two essential types include non-selectively operating cation channels, HKT2 (high-affinity K+ transporters), and CNGCs (cyclic nucleotide-gated) or GLRs (glutamate receptor-like) (Demidchik 2018; Mian et al., 2011). Other suggested transporters involved in Na+ uptake include HAK5, AKT1 LCT1 (low-affinity cation transporter1), and PIP2-1 (aquaporins) (Schachtman et al., 1997; Mian et al., 2011; Isayenkov and Maathuis 2019; Kronzucker and Britto 2011; Byrt et al., 2017). The counterbalance of Na+ uptake occurs through Na+ expulsion through SOS1 Na+/H+ exchanger and vesicles, and about 95% Na+ returns to the rhizosphere (Shi et al., 2002b; Shabala et al., 2020). Na+ vacuolar sequestration is done by Na+/H+ tonoplast antiporters of the NHX family, which show elevated transcript levels and activity in glycophytes upon salt exposure and constitutive activity in halophytes (Bassil and Blumwald 2014; Shabala and Mackay 2011). Two types of leak channels in tonoplasts SV and V (fast), need to be tightly regulated to block the leakage of Na+ back into the cytosol (Shabala et al., 2020). urther, Na+/H+ antiporter's higher affinity for K+ than Na+ suggests the operation of other mechanisms like vacuolar trafficking in delivering Na+ to the vacuole (Bassil et al., 2019; Baral et al., 2015). The loading of Na+ into xylem occurs both actively and passively, and the importance of mode of uptake depends on the time after salt exposure (Ishikawa et al., 2018b). NSCC mediate the passive Na+ xylem loading while SOS1 Na+/ H+ antiporter and HKT2 (K+/Na+ symporter) carry the active loading (Guo et al., 2010; El Mahi et al., 2019; Jabnoune et al., 2009). SOS1 Na+/H+ antiporter occurs abundantly in xylem parenchyma and HKT2 in stellar tissues of the root. Saline conditions favour passive K+ outward movement from parenchyma cells into the xylem due to depolarization, which creates a driving force for Na+ xylem loading (Ishikawa et al., 2018b). The CCC (cation-chloride cotransporters), which mediate K+ Cl-, and Na+ symport, create a driving force by transporting Cl- into the xylem passively, for active Na+ xylem loading (Ishikawa et al., 2018a). HKT1 (Class I)
transporters remove Na+ from xylem sap, and these transporters are more selective for Na+ than K+ (Munns et al., 2012). Also, Na+ is recirculated back to the roots from the shoots through the phloem involving mainly HKT1, and this Na+ gets stored in stellar parenchyma cells to prevent phototoxicity and damage to shoot meristematic tissues and growing leaves (Kobayashi et al., 2017; Shabala 2017).
Osmolytes and Osmoprotection
Osmolytes, compatible solutes or osmoprotectants are polar, soluble, uncharged, and chemically varied organic compounds, not interfering with cellular processes even if accumulated at high concentrations. These include glycine betaine, proline , polyols, sugar hydroxyproline, β-alanine, polyamines, and LEA (late embryogenesis abundant) proteins (Tahir et al., 2012; Wang and Nii 2000; Kerepesi and Galiba 2000; Saxena et al., 2013). Different plant species show variations for amounts of organic osmolytes synthesized and accumulated, like proline accumulated in varied plant groups, whereas β-alanine is accumulated only in some members of plumbaginaceae (Saxena et al., 2013; Hanson et al., 1994). The external osmolarity determines the osmolyte accumulation, and thus through water influx, osmolytes function in the protection of structures and maintenance of cell osmolarity (Hasegawa et al., 2000).
The quaternary ammonium compounds that physiologically act as potent compatible plant osmolytes under salt stress are glycine betaine, (β)-alanine betaine, proline betaine, choline-O-sulphate, hydroxyproline betaine, and pipecolate betaine (Ashraf and Harris 2004). GB shows ubiquitous occurrence in organisms like cyanobacteria, bacteria, fungi, algae, animals, and several families of plants like families Chenopodiaceae and Gramineae over an extensive range of abiotic stress conditions (Turkan and Demiral 2009; Lokhande and Penna 2012). This osmolyte is mostly found in chloroplasts and plays a significant role in stromal adjustment and thylakoid membrane protection, and hence and photosynthetic activity is maintained (Jagendorf and Takabe 2001). PS-II (photosystem II) complex is protected by GB in saline conditions (Annunziata et al., 2019). Against osmotic stress, GB prevents the membrane and enzyme (like Rubisco) destabilization induced by heat (Giri 2011). In angiosperms, synthesis of GB within the cell occurs from choline via ethanolamine, by two sequential oxidation reactions catalyzed by enzymes, namely CMO (choline monooxygenase) and BADH (betaine aldehyde dehydrogenase) respectively (Luo et al., 2012a; Missihoun et al., 2015). In some plants, another biosynthetic pathway involving two N-methyl transferase enzymes shows GB synthesis from glycine (Ahmad et al., 2012). Studies revealed that GB improves salt tolerance in maize, tobacco, potato, rice, barley, tomato belonging to different agronomical crops. The exploitation of these plants in biotechnology for GB synthesis has provided tolerance against various abiotic stresses (Sairam and Tyagi 2004; Turkan and Demiral 2009). GB's unique structure enables it to interact with macromolecules like enzymes through hydrophilic and hydrophobic domains. It functions in stabilization of proteins, osmotic adjustment, prevention of damage to photosynthetic apparatus, and lowering ROS (Reactive Oxygen Species) levels (Ashraf and oolad 2007; Saxena et al., 2013; Cha-Um and Kirdmanee 2010). Pre-treatment of rice seedlings with GB can prevent damages like grana disintegration, thylakoid swelling, mitochondrial swelling, and foliar spray of GB, leading to increased photosynthetic rate, pigment stabilization, and enhanced growth (Rahman et al., 2015; Ahmad et al., 2012).
In contrast to other amino acids like methionine, arginine, and cysteine, which show a decreased concentration, proline shows an increase in concentration under salt stress (El-Shintinawy and El-Shourbagy 2001). The accumulation of proline (Liu et al., 2006) provides a measure of improvement of salinity stress, and its intracellular accumulation serves as a nitrogen reserve for after recovery utilization (Saxena et al., 2013; Ben Ahmed et al., 2010). The Pro concentration is metabolically regulated, and synthesis of this imino acid occurs in plastids and cytoplasm, while it is broken down to L-glutamate (Kadioglu et al., 2012) in mitochondria. In plants, Pro has two precursors viz; glutamate (Kadioglu et al., 2012) and ornithine (Orn). Pro synthesis occurs from Glu via glutamic – ϒ- semialdehyde and pyrroline-5-carboxylate (P5C). The conversion of Glu to P5C is catalyzed by P5C synthase (P5CS) succeeded by P5C reductase (P5CR), which reduces P5C to Pro (Ashraf and oolad 2007). Probiosynthesis also takes place from another precursor Orn which is transaminated to P5C with the involvement of an enzyme mitochondrial Orn –ϒ-aminotransferase (Ong et al., 2016; Verbruggen and Hermans 2008). Pro biosynthesis is negatively regulated by a bHLH T MYC2, by limiting the expression of P5CS (Verma et al., 2020). Pro can quench 1O2, act as a free radical scavenger, buffer cellular redox potential, and mitigate cytoplasmic acidosis under stress (Babiychuk et al., 1995; Matysik et al., 2002; Lee et al., 2008). In Olea europaea, proline supplements improve salt tolerance by enhancing enzymatic antioxidants, photosynthesis osmotic balance, and plant growth, while in tobacco, it enhances salt tolerance through the acceleration of antioxidant defence pathway-specific enzymatic ac under stress (Babiychuk et al., 1995; Matysik et al., 2002; Lee et al., 2008). Inlular structures like proteins and membranes are stabilized by Pro through cluster formation with water, thereby preventing their denaturation, and membranes are protected through the maintenance of cellular ion homeostasis and osmotic balance (Ashraf and Harris 2004; Lee et al., 2008; Gleeson et al., 2005). Silicon supplementation increases the Pro content 3 to 6 days and decreases it 9 to 12 days after salt exposure and negative correlation between Pro and cytokinin exist after 3 days of stress, suggesting communication between cytokinin and Pro metabolism. Silicon induced Pro increase that can regulate cytokinin to confer salt tolerance (Zhu et al., 2020c)
Sugar and Sugar Alcohols:
The correlation between salt tolerance and fluctuations in soluble carbohydrate concentrations shows that the accumulation of carbohydrates like sugars (fructose, sucrose, glucose) and starch occurs due to salt stress (Parida and Das 2005). At higher salinity (400-500 mM NaCl), plants like Cakile maritime and Aster tripolium accumulate more elevated amounts of total soluble carbohydrates and proline (Megdiche et al., 2008; Geissler et al., 2010). Sugar alcohol and sugars function in storing carbon, scavenging radicals, osmoregulation, and osmoprotection (Ahmad and Sharma 2008; Lee et al., 2008; Adams et al., 2005). urthermore, there is deliberation about the sugar and sugar alcohols as molecular chaperones (Liu et al., 2006; Hasegawa et al., 2000). In Setaria sphacelata, starch and sugar show reduction in amounts under water stress for the shortterm while soluble sugars increase and starch decrease in long-term stress, suggesting a metabolic shift towards sucrose because starch turnover is more affected than sucrose (da Silva and Arrabaca 2004).
Trehalose, a non-reducing sugar, helps angiosperms protect from abiotic stress and alleviates salt-induced oxidative stress by preventing ROS accumulation (Mostofa et al., 2015). Salt tolerance increases in transgenic rice overexpressing OsTRE1, a trehalase gene (Islam et al., 2019). While functioning as osmoprotectant and osmolyte, it prevents membranes and proteins from undergoing denaturation (Ashraf and Harris 2004). Transgenic plants exhibit a reduction in photo-oxidative damage and increased photosynthetic rate with increased trehalose levels under salt stress. It protects biological molecules from injuries induced by desiccation through its potential for water absorption and in maize seedling growth improved via regulation of the glyoxalase system and antioxidant by trehalose through a reduction in ROS, methylglyoxal, malondialdehyde, and Na+/K+ (Rohman et al., 2019; Penna 2003). Sugar alcohols (Polyols) can be acyclic like mannitol or cyclic polyols like pinitol, having multiple functional OH-groups, and act as osmolytes, ROS scavengers, and chaperones (Ashraf and oolad 2007). These can work as osmolytes in osmotic adjustment and osmoprotectants through two indistinguishable means and osmotic adjustment occurs through the facilitation of cytoplasmic water retention and apoplast or vacuolar Na+ sequestration (Parida and Das 2005). Accumulation of polyols in grapes under water deficit conditions provides a means for refining water use efficiency and grapevine practices (Conde et al., 2015).
Polyamines
Polyamines or organic amines, including Spd (spermidine), Put (putrescine), and Spm (spermine)
increase salt tolerance when applied exogenously, or their levels increased in transgenic plants (Bueno and Cordovilla 2019; Rathinapriya et al., 2020; Ji et al., 2019). Different stresses induce polyamines that regulate the functioning of specific ion channels to control ionic influxes and play a protective role, as evidenced by the outcome of exogenous application (Zhao et al., 2007; Pottosin and Shabala 2014). Transgenic overexpression and loss of function mutants support polyamine's protective role (Ji et al., 2019; Zarza et al., 2017). The endogenous level of plant polyamines increase after salt exposure, and polyamine catabolism regulates the endogenous level and the oxidative catabolism catalyzing enzymes of polyamines, amine oxidases, are essential in imparting salt tolerance (Takahashi and Kakehi 2010). Polyamines maintain membrane integrity, regulate gene expression governing osmolytes' metabolism, reduce ROS production and control Na+ and Cl- accumulation in various plant organs (Ji et al., 2019; Rathinapriya et al., 2020; Seo et al., 2019). Spermidine and spermine, when exogenously applied to like in soybean seedlings, enhance photosynthesis and ROS metabolism, thereby improve growth and enhance salt tolerance (Wang and Yin 2014; Baniasadi et al., 2018). SAMDC (S-adenosylmethionine decarboxylase) upregulation and ODC (ornithine decarboxylase) and ADC (arginine decarboxylase) downregulation led to decreased Put and increased Spm and Spd accumulation, alleviating the salt-induced inhibition of growth (Takahashi et al., 2017). Spd and Spm affect many metabolic pathways, and in grape seedlings, polyamines with ABA alleviate salt stress (Paul and Roychoudhury 2017; Sun et al., 2018). ADC2 deletion increases salt sensitivity, whereas ADC expression leads to enhanced osmotic adjustment and better plant growth (Naka et al., 2010; Espasandin et al., 2018).
LEA Proteins
Late embryogenesis abundant proteins occur in plants and animals that prevent desiccation, protein aggregation, or osmotic stresses ( uruki et al., 2012; Hundertmark et al., 2012; Hand et al., 2011). Named after their finding in maturing seeds, constitutive expression, or salinity stress-induced expression of LEA proteins are reported (Cumming 1999; Liu et al., 2013). LEA proteins protect various plant molecular or cellular structures from damaging effects of H2O2 through hydration, buffering, ion sequestration, direct protection of membranes or other proteins, or unfolded protein renaturation and phosphorylation is supposedly to have an enormous impact on the functioning of LEA proteins under stress conditions (Zhang et al., 2000; Goyal et al., 2005; Liu et al., 2017). IpLEA, gene product from Ipomoea pes-caprae, belonging to LEA group 2 functions in drought and salt tolerance through the facilitation of water homeostasis or ROS scavenging (Zheng et al., 2019). LEA group 1 proteins (XsLEA1-8) in Arabidopsis thaliana, while avoiding damage to subcellular structures, enhance the drought and salt tolerance (Artur et al., 2019). LsEm1 (LEA group1), from lettuce, when overexpressed in rice, alters the expression of other genes like OsCDPK25, OsCDPK15, OsCDPK13, OsCDPK9, and rab21 positively leading to increased salt and drought tolerance (Xiang et al., 2018). CaDHN5 (Luo et al., 2019) overexpression upregulated several salt-responsive genes, increasing osmotic and salt tolerance (Luo et al., 2019).
HORMONAL REGULATION OF SALINITY STRESS
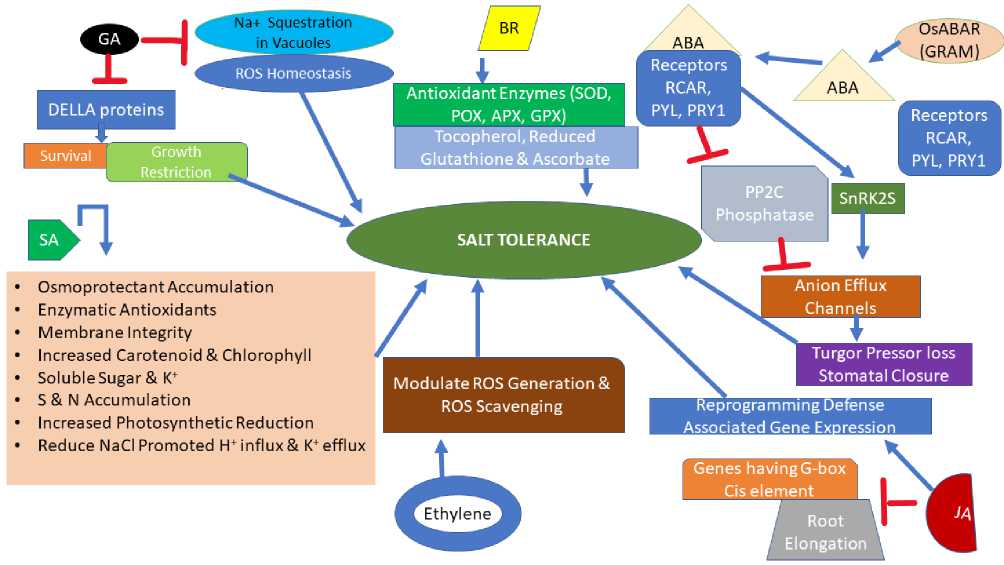
Many phytohormones like ethylene, GA, ABA jasmonic acid (JA), and salicylic acid coordinate and integrate, enabling plants to respond and adapt to salt stress while ABA among these acts in different abiotic stresses. Water deficit and osmotic stress induced by salt stress result in rapid ABA production in shoots and roots (Shi et al., 2002b). ABA binding to receptors like RCAR (REGULATORY COMPONENTS O ABA RECEPTORS), PYL (PRY1-LIKE), and PRY1 (PYRABACTIN RESISTANCE1) leads to inhibition of PP2C phosphatases and promotion of SnRK2s activity that can activate many anion efflux channels resulting in turgor pressure loss and stomatal closure (Duarte et al., 2019; Zhang et al., 2017). Previously ABA was thought to be produced in roots exposed to osmotic stress and regulate stomatal closure; however, evidence like upregulation of gene NCED3 encoding enzyme in leaf parenchyma upon osmotic stress, catalyzing the first step of ABA biosynthesis, suggest ABA delivery to shoot from roots is not necessary (Endo et al., 2008; Buckley
2019). Besides, synthesizing ABA in roots would require precursor transport from leaves (Zhang et al., 2018). The role of ABA produced in roots under salt stress is still unclear even though ABA produced in roots has a higher concentration than leaves (Shi et al., 2002a). ABA in osmotically stressed roots stimulates NADPH oxidase that induces H2O2 production and accumulation, triggering stomatal closure and thus provides a clue of its role in salt stress response (Nath et al., 2019). OsABAR1 encoding GRAM (glucosyltransferases-like GTPase activators and Myotubularin) protein positively regulates the ABA pathway and enhances tolerance to salt and drought through ABA (Zheng et al., 2020).
Several JA biosynthesis genes upregulate by salt stress, suggesting the role of JA signaling in salt stress-induced plant responses (Kazan 2015; Geng et al., 2013). Salt stress inhibits root elongation through JA signaling. Physical interaction of RSS3 (RICE SALT SENSITIVE3) protein with class C bHLH (basic-helixloop-helix) T s (transcription factors) and JAZ (JASMONATE ZIM_DOMAIN) proteins induce cell elongation whereas RSS3 mutants show enhanced expression of JA-responsive genes, suggesting JA functions in root growth inhibition under salt stress (Toda et al., 2013). GaJAZ1 affects salt tolerance in cotton through downregulating genes containing G-box ciselement and reprogramming of defence associated gene expression (Zhao et al., 2020). OsCYP94C2b gene, encoding an enzyme catalyzing the conversion of jasmonoyl isoleucine (active form) to the inactive one, when overexpressed, increases the chances of survival in rice, suggesting JA regulates salt tolerance negatively. Though, TaORP1, the gene for JA biosynthesis, overexpression, or JA exogenous application, result in enhanced salt tolerance, suggesting positive regulation of salt tolerance by JA (Kang et al., 2005; Dong et al., 2013). GA (Gibberellic acid) plays a critical role in plant growth regulation under stress conditions, and endogenous GAs reduce while DELLA proteins increase in seedlings on exposure to salt stress (Colebrook et al., 2014; Magome et al., 2008). DELLA proteins mediate salt-induced growth restriction and help plants survive (Achard et al., 2006).
GA deficiency in vivo enhances salt tolerance through Na+ sequestration in vacuoles and ROS homeostasis (Zhang et al., 2020f). Ethylene is vital in imparting salt tolerance as evidenced by increased salt tolerance by applying ACC (1-aminocyclopropane-1-carboxylic acid) the precursor of ethylene, and salt hypersensitivity due to mutations in genes involved in ethylene signaling (Cao et al., 2007; Peng et al., 2014). Ethylene positively regulates salt tolerance by modulating ROS scavenging and generation pathways (Peng et al., 2014).
SA enhances the osmoprotectant (glycine betaine, polyamines, proline) accumulation and enzymatic antioxidant activities under salt stress, suggesting its positive role in conferring salt tolerance (Misra and Misra 2012). SA induces salt tolerance by the restoration of membrane integrity and increased carotenoid and chlorophyll content, resulting in enhanced accumulation of soluble sugar and K+ in the root (El-Tayeb 2005). Increased antioxidant metabolism and enhanced sulfur and nitrogen assimilation promoted by SA lead to an increased photosynthetic reduction (Nazar et al., 2011). SA treatment enables plants to adapt under saline conditions by reducing NaCl promoted H+ influx and K+ efflux (Jayakannan et al., 2013). BR (Brassinosteroid) increase antioxidant enzyme (GPX, APX, POX, and SOD) activity and lead to the accumulation of tocopherol, reduced glutathione, and ascorbate (non-enzymatic antioxidants), thereby alleviating adverse salinity effects (El-Mashad and Mohamed 2012).
Antioxidant responses of salinity tolerance
ROS, mostly hydroxyl radical (OH-), hydrogen peroxide (H2O2), singlet oxygen (1O2), and singlet anion (O2.-), show an increased production under different environmental stresses, including the salt stress and mitochondria, chloroplasts, peroxisomes, and apoplast are their main sites of generation (Miller et al., 2010). NADPH oxidases AtRboh and AtRbohD, polyamine oxidase, diamine oxidase, and peroxidase mediate the apoplastic ROS production, and salt stress leads to the upregulation of AtRboh and AtRbohD genes, whereas plant mutant atrboh /artboarD exhibit salt hypersensitivity (Ma et al., 2012). Salt induced ROS production by AtRboh /AtRbohD leads to cytosolic K+ influx resulting in decreased Na+/K+ ratios. AtRboh restricts xylem Na+ distribution, thereby hampers Na+ transport from root to shoot, whereas AtRbohD helps in the propagation of environmental stimuli induced longdistance signal (Miller et al., 2009; Jiang et al., 2012). Salt induced antioxidative response against H2O2 production mediated by AtRboh /D helps decrease cellular oxidative damage (Ben Rejeb et al., 2015). The chloroplast is the primary singlet oxygen and hydrogen peroxide producer compared to other organelles (Davletova et al., 2005). During photosynthetic electron transport, the photoreduction of O2 to O2- takes place and is termed as Mehler Reaction. In the plastoquinone pool, the formation of superoxides is due to the reduction of molecular oxygen. In PS-I during ETC, this reduction is executed by plastosemiquinone, ferredoxin, or by sulfur redox centres (Dat et al., 2000). The conversion of superoxides to H2O2 occurs either naturally or by enzyme SOD action, and the hydroxyl radicals are produced from hydrogen peroxide (Pospisil et al., 2019). In plant cells, peroxisomes are the leading producers of intracellular hydrogen peroxide, and H2O2 production induced by high oxygen and low CO2 levels under salt stress enhance photorespiration (Wingler et al., 2000; Del Rio and Lopez-Huertas 2016). The sites for O2- production in peroxisomes are peroxisomal matrix and peroxisomal membrane. In the peroxisomal matrix the generation of O2- radicals occurs through xanthine and hypoxanthine oxidation to uric acid by enzyme xanthine oxidase (Gutteridge and Halliwell 2000). Salinity induces ROS production (O2--) in mitochondria through ubiquinone over-reduction leading to the release of electrons from the electron transport chain to oxygen (Miller et al., 2010).
Plants use low ROS levels as signals to regulate growth and development in response to different stresses, whereas higher ROS concentration under stress conditions like salt stress has harmful effects. ROS disrupts cell integrity and leads to membrane lipid peroxidation, protein denaturation, pigment breakdown damage to DNA and enzymes, and carbohydrate oxidation (Groß et al., 2013). ROS activates various ion channels like Ca+ channels sensitive to H2O2 in guard cells and root epidermal cells, Na+ cation channels, K+ efflux channels, Ca2+ pumps sensitive to OH-, disturbing ionic homeostasis (Demidchik 2018; Zepeda-Jazo et al., 2011). Plant ROS scavenging machinery detoxify the oxidative stress triggered by high salinity induced ROS accumulation, and ROS homeostasis maintenance plays a critical role in salt tolerance (Bose et al., 2014). ROS scavenging induced salt tolerance includes both the activity of enzymatic antioxidants like SOD (superoxide dismutase), (Groß et al., 2013) glutathione reductase CAT (catalases), and APX (ascorbate peroxidase) and non-enzymatic antioxidant (glutathione, ascorbate tocopherols) accumulation (Hanin et al., 2016). Enhanced glutathione levels in chloroplasts peroxisomes, and mitochondria increase, whereas reduced ascorbate levels, as in vtc2-1mutant, decrease salt tolerance (Bose et al., 2014; Koffler et al., 2015). Salt stress impairs the anthocyanin production, as seen in air1 (anthocyanin-impaired-response1) mutant evidencing the role of anthocyanin accumulation in salt tolerance (Van Oosten et al., 2013). Glutathione acts as a ROS scavenger through its reaction with H2O2 superoxide, and hydroxyl radicals, and while acting in the ascorbate-glutathione cycle, it helps in the regeneration of ascorbate ( oyer et al., 1997). In Allium cepa, exogenous application of glutathione restores the cell viability and plasma-membrane permeability under salt stress, and ascorbate plus glutathione application resulted in increased plant height and branch number and increased content of phenols, carbohydrates mineral ions, and xanthophylls (Aly-Salama and Al-Mutawa 2010) (Rawia et al., 2011). Ascorbate, an important antioxidant, mitigates the harmful impacts of salt stress and helps in plant recovery after salt stress exposure (Agarwal and Shaheen 2007; Munir and Aftab 2011). Plants can synthesize a lipophilic antioxidant known as alpha-tocopherol or Vit. E. In combination with other antioxidants, alpha-tocopherol scavenges free radicals (Munne-Bosch and Alegre 2003; Massacci et al., 2008). It plays a crucial role in protecting the structure and function of PS-II as it chemically combines with oxygen in the chloroplast (Lopez-Huertas et al., 2000). Alpha-tocopherol assists in membrane stabilization and mitigates tolerance of plants during oxidative stress (Munne-Bosch and Alegre 2003). Overexpression of OsVTE (encoding tocopherol cyclase in rice) IbTC (encoding tocopherol cyclase in sweet potato) enhanced the salt tolerance, and such a plant showed less accumulation of H2O2 (Ouyang et al., 2011; Kim et al., 2019). Superoxide anion is converted to H2O2 by SOD and H2O2 to water by APX in chloroplasts, and APX gene overexpression leads to enhancement in salt tolerance (Asada 2006; Badawi et al., 2004). Under salinity stress, the activity of SOD increases in Catharanthus roseus and Morus alba (Jaleel et al., 2008; Ahmad et al., 2010). In mitochondria, Mn-SOD (manganese SOD) and alternative oxidase (AOX) increase salt tolerance through ROS detoxification (Giraud et al., 2008). In rice, two isoforms of chaperone protein, NCA1a, and NCA1b interact mutually exclusive with CAT to control its activity and confer salt tolerance (Liu et al., 2019a).
MAPK signalling cascade and salt stress
Over the period, plants have developed various mechanisms to counter and overcome the stress conditions for their survival, and among these mechanisms, signaling pathways, production, and shifting of signal molecules are crucial. Signal molecules are the by-products of biochemical reactions received by plant receptors, usually found in the cellular membrane resulting in different gene expression, including stress genes, and help the plants tolerate and survive under stress conditions (Hamel et al., 2006; Colcombet and Hirt 2008). Various signaling pathways get triggered during stress conditions, including MAPK (mitogen-activated protein kinase) signaling cascade. MAPK components are a group of enzymes that enable plants to respond to various stimuli by different stresses and activate plant responses like the activation of antioxidant enzymes. MAPK components get triggered by the activation loop's phosphorylation or dephosphorylation by upstream kinases, and phosphatases can suppress such activation (Lee et al., 2009). Arabidopsis genome exhibits the ability to activate 5 MKP molecules, which include IBR5, PHS1, DSPTP1, AtMKP1, and AtMKP2. Among these molecules, AtMKP2 and DSPTP1 can dephosphorylate Arabidopsis MAPK molecules (MPK3,4, and 6) in-vitro, and MAPK component activation, and the expression of stress genes may increase plant response to stress (Lee and Ellis 2007).
The main MAPK molecules which get activated during stress are MPK6, MPK4, and MPK3, and under salt stress, M2K2 activates MPK4 (Colcombet and Hirt 2008). The upregulation of M2K2 and downregulation of MPK4 and MPK6 through MAPK cascade is related to Me2K (Teige et al., 2004). In Arabidopsis , AtMPK6 AtMPK4, and AtMPK3 are regulated by salt stress whereas salt stress results in MAPK's (ZmMPK5, ZmMAPK3, and ZmSIMK1) expression in corn ( Zea mays ) (Droillard et al., 2002; Droillard et al., 2004; Ding et al., 2009; Wang et al., 2010). Accumulation of ZmMPK3 RNA occurs in plants by treating it with ethylene, salicylic acid, hydrogen peroxide, ABA, or under salinity stress (Wang et al., 2010). In Arabidopsis ZmSIMK expression enhances plant salt tolerance (Kong et al., 2011). StMAPK3 regulates salt and osmotic tolerance through affecting the activities of enzymes like CAT, SOD, peroxidases, and concentration of Pro, H 2 O 2, and malondialdehyde (Zhu et al., 2020b). In Arabidopsis , overexpression of the AtM2K3 gene increases plant tolerance to salinity and its sensitivity to ABA, implicating the signalling role of AtM2K3 in ABA activation and plant tolerance under salinity (Hwa and Yang 2007). In salt-tolerant peppermint, MAPK signaling regulates essential oil biosynthesis under salt conditions (Li et al., 2016). Wheat MAPK phosphatase, TMKP1 when overexpressed, increases germination rates, enhanced antioxidant activities like SOD, CAT, and peroxidases, leading to salt tolerance enhancement (Zaidi et al., 2016). In Populus trichocarpa overexpression of MAPK kinase (PtMAPKK4) results in improved germination, growth, and tolerance.
Salt responses at the molecular level: gene expression and transcriptional regulation
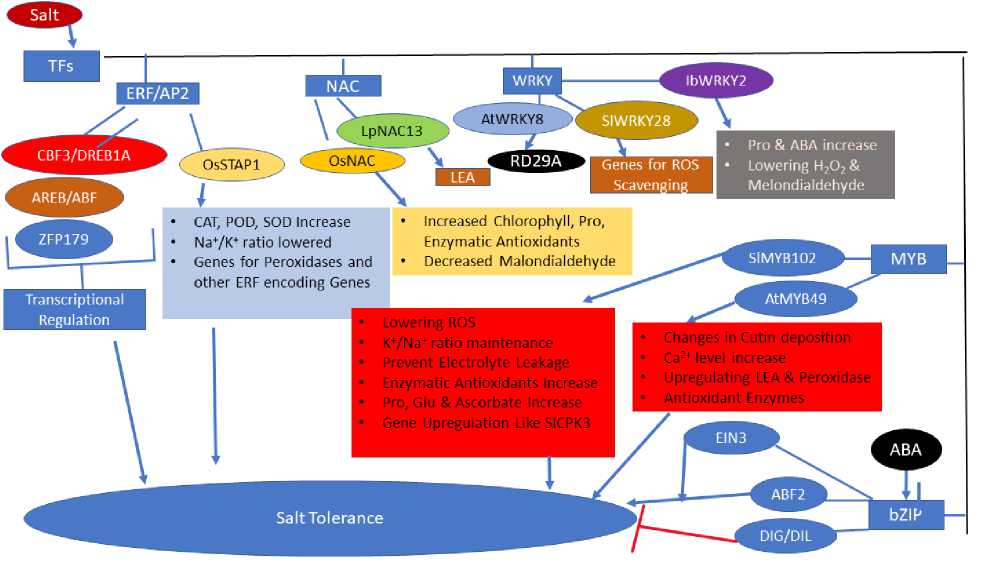
Gene expression in plants is regulated through varied mechanisms resulting in specific gene expression under specific conditions. Salt stress-induced differential expression of thousands of genes is determined by salt exposure duration or strength (Zeller et al., 2009). Transcriptome analyses of plants after salt stress alone or combined with other stresses propose widespread crosstalk between signaling pathways of salt stress and other stresses (Rasmussen et al., 2013). T s (transcription factors) constitute important regulators controlling gene expression, and T s from prominent T families like ER /AP2, NAC, bZIP, WRKY, MYB act in salt response. SlMYB102, R2R3-type MYB T overexpression confers salt tolerance through lowering ROS generation, K+/Na+ ratio maintenance, decreasing electrolyte leakage, enhancing the activity of SOD, CAT, APX, and peroxidase, increase in Pro, glutathione, and ascorbate concentrations, and upregulating several stress-associated genes like SICPK3, SICPK1, SIHAK5, SINHX4, SINHX3, SISOS2, and SISOS1 (Zhang et al., 2020e). AtMYB49 (R2R3-type MYB T ) increases salt tolerance through changes in cutin deposition, elevating Ca2+ level and through upregulating genes for LEA and peroxidases, improving antioxidant capacity (Zhang et al., 2020c). Another MYB T , VcMYB4a in Vaccinium corymbosum, is suggested an essential abiotic stress repressor as its overexpression leads to enhanced salt sensitivity (Zhang et al., 2020a).
Upregulation and downregulation of bZIP genes occur in salt-sensitive and salt-tolerant wheat cultivars, respectively, when exposed to an extended period of salinity, whereas in rice and wheat, NAC overexpression leads to salt tolerance (Johnson et al., 2002; Nakashima et al., 2007). LpNAC13 acting oppositely regulates drought tolerance negatively and salt tolerance positively, and its overexpression results in increased chlorophyll and proline content, increased enzymatic antioxidants and decreased malondialdehyde content under salt stress, whereas opposite results are found under drought conditions (Wang et al., 2020b). CB (C-repeat binding factor) from the AP2/ER family plays a positive role in the salt response, as evidenced by an increase in salt tolerance by overexpressing CB 3/DREB1A (dehydration responsive element binding protein 1A) and salt hypersensitivity in mutants of CB genes (Zhao and Zhu 2016; Kasuga et al., 1999). DREB2, DREB1/CB , and AREB/AB T s regulate abiotic stress responses transcriptionally, and T s, Z P179, and OsNAC5, are suggested to regulate the accumulation of LEA proteins, sugar, and proline under salinity stress, thereby conferring salt tolerance (Mizoi et al., 2012; ujita et al., 2013; Song et al., 2011). NnDREB2C overexpression in salt-stressed Arabidopsis increased germination, chlorophyll content, survival rates, lowered conductivity and resulted in increased tolerance to salt and drought through upregulation of PIP (Plasma membrane intrinsic proteins) genes (Ziyuan et al., 2020). OsSTAP1 encoding AP2/ER T overexpression enhances salt tolerance through increasing activities of CAT, POD, and SOD, lowering Na+/K+ ratios and upregulating stress associated genes like peroxidase and other ER encoding genes, suggesting its positive regulation of salt tolerance (Wang et al., 2020c). AtWRKY8 in Arabidopsis shows increased expression and binds directly to the target gene RD29A promoter under salt stress (Hu et al., 2013). IbWRKY2 enhances salt and drought tolerance in sweet potato through content increase for Pro and ABA, activity increase for SOD, and lowering H2O2 and malondialdehyde content (Zhu et al., 2020a). SlWRKY28 in Salix linearistipularis is found to improve tolerance to alkaline salt stress by regulating genes involved in the pathway of ROS scavenging (Wang et al., 2020a).
Regulation of gene expression by ABA mostly occurs through the bZIP T subfamily, AREB/AB , like AB 2 overexpression, enhances plant tolerance to multiple stresses (Choi et al., 2000; Kim et al., 2004). High salt and ABA hypersensitivity are exhibited by plants overexpressing DIG (Dynamic Influencer of Gene expression)/DIL (DIG-like), ABA-responsive T s (Song et al., 2016). MYC2, T involved in jasmonate signaling positively regulates salt tolerance, and EIN3 T involved in ethylene signaling enhances salt tolerance via DELLA proteins (Zhao et al., 2014; Peng et al., 2014). EIN3 act through ESE1 (Ethylene and Salt Inducible1) and ER 1 (Ethylene Response actor1) and activate salt responsive gene expression (Cheng et al., 2013; Zhang et al., 2011a). SER 1 (SALT-RESPONSIVE ER 1) directly binds to MAPK6, MAPK5, Z P179 (ZINC
INGER PROTEIN179), and DREB2A promoters and confers salt tolerance (Schmidt et al., 2013). Some T s can be controlled by different kinases that function in salinity tolerance like OsRMC encoding a receptor kinase that negatively regulates salt tolerance, is downregulated through binding of its promoter by two T s, OsEREBP1 and OsEREBP2 (Serra et al., 2013). Also, OsEREBP1 expression is not affected by ABA, salt, or severe cold but is regulated by moderate cold and drought slightly while ABA, cold, salinity enhance OsEREBP2 expression and drought, evidencing the role of OsEREBP2 in salt tolerance (Serra et al., 2013). OSBZ8, a bZIP T , in rice activated by phosphorylation through the SN -1 group of serine/threonine kinase, is expressed highly in salt-tolerant than the salt insensitive cultivars (Gupta et al., 2012). CaSBP12 (SBP-box T ) negatively regulates the salt tolerance through modulation of ROS signaling (Zhang et al., 2020b).
Plant processes, mainly under various stress conditions, are influenced by alternative splicing. Alternative splicing regulating proteins like Ser/Arg rich proteins can splice alternatively under salt and other abiotic stresses (Staiger and Brown 2013). In Arabidopsis , alternative splicing involves the symmetrical demethylation of Arg side chains by Type II Arg methyltransferase, PRMT5, and prmt5 mutants are salt sensitive (Zhang et al., 2011b). PRMT5 alters H4R3sme2 (H4 arginine3 symmetric demethylation) and LSm4 methylation status and impacts the salt response and plant growth (Zhang et al., 2011b). Salt, ABA, and mannitol upregulate the At-SKIP (Ski-interacting protein) expression, and altered tolerance of At-SKIP antisense lines or At-SKIP overexpressing lines in response to different stresses suggests the role of alternative splicing in such phenotypic expressions (Staiger and Brown 2013; Lim et al., 2010). In Arabidopsis , nuclear SUMO proteases, OTS1 (Conti et al., 2008) and OTS2 redundantly regulate salt stress responses, and the UBC32 (Ubiquitin conjugase) component of ERAD (endoplasmic reticulum-associated protein degradation) affect salt tolerance through BR (Cui et al., 2012; Conti et al., 2008). The existence of a mutual regulation mechanism between various signals, proteins, and genes is suggested to control various processes specific to abiotic stress adaptability of plants such as in-vitro salt tolerance increases when β-carotene hydroxylase downregulation leads the increased level of total carotenoid and β-carotene (Kim et al., 2012).
Regulation of salt tolerance in plants is also influenced through epigenetic modifications like acetylation, methylation, ubiquitination, and phosphorylation. Many HATs (histone acetyltransferases) that catalyze histone acetylation and HDACs (histone deacetylases) that catalyze histone deacetylation function in salinity stress response such as HDA6 interaction with HD2C results in repression of ABI1 and ABI2 (ABA-responsive genes), thereby decrease salt tolerance (Luo et al., 2012b). Salt tolerance is positively regulated by HDA5,14, 15,18 (class II enzymes), and negatively regulated by HDA6 9, 19 (class I enzymes) belonging to the RPD3 family of histone deacetylases (Ueda et al., 2017). HUB2 (E3 ligase from Arabidopsis) enhances salt and drought tolerance through increasing the monoubiquitination of H2B histone (Zhou et al., 2017; Chen et al., 2019). GmMYB84 encoding a T conferring salt tolerance in soybean depends on DNA methylation, and upstream 690nt to 950nt of its initiation codon, DNA methylation level decreases upon salt exposure leading to its higher expression (Zhang et al., 2020d).
Non-coding RNAs like miRNAs (microRNAs), siRNAs (small interfering RNAs), and lncRNAs (long non-coding RNAs) also mediate salt tolerance. miR-393 and miR-169 are induced, whereas miR-398 is inhibited by salt stress (Sunkar et al., 2012), and miR319 induces salt tolerance through the downregulation of essential genes involved in the methionine cycle and upregulating genes for ethylene synthesis (Liu et al., 2019b). A 24-nt nat-siRNA induces salt tolerance by downregulating the expression of enzyme P5CDH (delta1-pyrroline-5-carboxylate dehydrogenase), thereby inhibiting proline degradation (Borsani et al., 2005). DRIR (Drought Induced lncRNA) regulates salt tolerance by modifying the expression of multiple genes (Qin et al., 2017) and lncRNA973 in cotton increases salt tolerance through the regulation of T s, salt-stress responsive genes, and ROS scavenging genes (Zhang et al., 2019).
Stress priming or utilizing previous stress memory to enhance stress response by plants involves epigenetic modifications, inherited through mitosis or meiosis, like histone methylation and DNA methylation (Eichten et al., 2014). Salt and drought tolerance increase through seed priming using hyperosmotic reagents or NaCl (Sani et al., 2013; Cayuela et al., 1996). With mild seed priming using salt, the H3K27me3 (histone H3 lysine 27 trimethylation) islands get shortened and fractioned, which results in an alteration in the transcriptional responsiveness of many genes upon second stress exposure (Sani et al., 2013).
Na+ toxicity
Enzyme Inhibition
Membrane depolarization
[ K+ efflux through GORK" :
__like proteins______________
Salt Stress

• Replacement of K+ by Na+ resulting Na+ toxicity

CAIter metabolism
-
• Cl" leads less availability of macronutrients (S,N), by sharing anion transporters
Alter Ion channels in Guard cells
Plant Response and Tolerance
Excluding Na+ from uptake
Na+ Vacuolar Compartmentmentalization by Na+/H+ tonoplast antiporter
Hydraulic signals from roots
Homeostasis
la+ expulsion through SOS: Na+/H+exchanger
SalLSensing by Roots
Osmotic" Adjustment
-
• Uptake of Inorganic Ions
-
• Cell wall composition alteration
-
• Stomatai Closure
-
• Exclusion of Cl-or accumulation as in Halophytes
Na+ Uptake through channels: HKT2, CNGCs, HAK5,AKT1, LCT1, PIP2-1
J.
Na+ accumulation in Cytoplasm
-
Figure 1: igurerepresenting Plant response and tolerance to NaCl
Glycine Betaine Proline
Sugar and Sugar alcohol
Protein Stabilization Osmotic Adjustment Lowering ROS Content
Stromal Adjustment and Thylakoid membrane Protection
Source of Nitrogen source after stress recovery
Quench Singlet Oxygen
Buffer Cellular Redox Potentia
Mitigate Cytoplasmic Acidosis
Increase Enzymatic Antioxidant Activity
Enhance Photosynthesis
Osmotic Adjustment
Protein and Membrane Stabilization
Storing Carbon
Scavenging Radicals
Osmoregulation and Osmoprotection
Molecular Chaperones
Trehalose: Protect membrane and Prevent protein denaturation
Mannitol and Pinnitol: Cytoplasmic Water retention and Apoplastic/Vacuolar Na* sequestration; ROS scavenger;
Chaperones
Polyamines
LEA Proteins
Spermine, Putrescine, Spermine
-
• Regulate ion influxes through specific Ion Channels
-
• Membrane Integrity
-
• Regulate Osmolyte Metabolism Through Gene Expression regulation
-
• Reduce ROS Production
-
• Control Na* and Cl" Accumulation
-
• Enhance Phtosynthesis
-
• Protection from H2O2 Damage Through Hydration and Buffering Ion Sequestration
-
• Membrane and Protein Protection
-
• Unfolded Protein Renaturation and Phosphorylation
-
• Gene Expression Alteration of Genes like OsCDPK25, OsCDPKIS, OsCDPKIS, OsCDPK9, and rab21
Figure 2: Role of osmolytes under salinity stress
ABA
ABA
Ethylene
Na+ Squestration in Vacuoles
OsABAR (GRAM)
ROS Homeostasis
SALT TOLERANCE
• Osmoprotectant Accumulation
• Enzymatic Antioxidants
• Membrane Integrity
• Increased Carotenoid & Chlorophyll
• SolubleSugar & IC
• S & N Accumulation
• Increased Photosynthetic Reduction
• Reduce NaCI Promoted H* influx & K+ efflux
Receptors RCAR, PYL, PRY1
Antioxidant Enzymes (SOD, POX, APX, GPX) Tocopherol, Reduced
Gl utath lone & Ascorbate
Receptors RCAR, PYL, PRY1
Survival Growth
I Restriction
Reprogramming Defense Associated Gene Expression
Genes havingG-box Cis element
Root Elongation
Modulate ROS Generation & ROS Scavenging
PP2C Phosphatase
Turgor Pressor loss Stomatai Closure
Anion Efflux Channels
DELLA proteins
SnRK2S
Figure 3: Role of phytohormones in imparting salt tolerance [GA (Gibberellic Acid), BR (Brassinosteroid), ABA (Abscisic Acid), SA (Salicylic Acid), JA (Jasmonic Acid), GRAM (Glucosyltransferases-like GTPase Activators and Myotubularin), RCAR (Regulatory Components of ABA Receptors), PYL (PRY1-Like), and PRY1 (Pyrabactin
Resistance1)
AtWRKYS
LpNAC13
OsNAC
OsSTAPl
lbWRKY2
NAC
C3F3/DREB1A
RD29A
LEA
Scavenging
AREB/ABF
ZFP179
SIMYB102
EIN3
ABA
ABF2
bZIP
DIG/DIL
ncreased Chlorophyll, Pro, Enzymatic Antioxidants Decreased Malondialdehyde
CAT, POD, SOD Increase NaVK* ratio lowered Genes for Peroxidases and other ERF encoding Genes
• Lowering ROS
• K*/Na+ratio maintenance
• Prevent Electrolyte Leakage
• Enzymatic Antioxidants Increase
• Pro, Glu & Ascorbate ncrease
• Gene Upregulation Like SICPK3
• Changes in Cutin deposition
• Ca2t level ncrease
• Upregulating LEA& Peroxidase
• Antioxidant Enzymes
TFs
WRKY
ERF/AP2
SIWRKY28
Genes for ROS
AtMYB49
Transcriptiona Regulation
Salt Tolerance
• Pro & ABA increase
• Lowering H2O2& Melondialdehyde
I MYB I
Figure 4: Role of Transcription actors (T s) in salt tolerance.
Many studies have described that salinity stress induces within genome DNA methylation and epigenetic variation, either natural or induced by mutations in DNA methylation mechanisms result from gene expression alteration mediating salt tolerance (Karan et al., 2012;
Wang et al., 2014; Wang et al., 2015; Huang et al., 2013). A correlation between histone methylation alterations and inactivation or activation of genes induced by salt (Sun et al., 2019); however, the role of epigenetic changes induced by salt stress in salt tolerance is yet to be arrived at fully.
CONCLUSIONS AND FUTURE PERSPECTIVE
Plant salt tolerance includes a cascade of responses at physiological, biochemical, and molecular levels. Comprehensive research viz; physiological, biochemical and molecular studies have explained various salinity mechanisms regulating ion intake, exchange and balance, osmotic regulation, hormonal metabolism antioxidant metabolism, and stress signaling. Besides, rapid expression of NtNHX1 increases plants salt tolerance by enhancing vacuolar Na+ compartmentalization that declines the toxic accumulation of the ion in the cytoplasm that promotes growth in a saline environment. The determination of net Na+ flux across the plasma membrane regulates the expression of SOS 1 (Na+ efflux) and HKT1 (Na+ influx). Environmental stress, particularly osmotic and ionic stresses, are liable for the decrement in yield, especially in arid and semi-arid regions. Production of ROS in different cell organelles chloroplast, mitochondria, and peroxisomes is due to prolonged environmental stresses. The normal functioning of the cell is disturbed by ROS as it attacks biomolecules like DNA, lipids, proteins, and carbohydrates. Under extreme stress conditions, ROS finally leads to cell death. or overcoming oxidative stress, plants have enzymatic and non – enzymatic antioxidants. Many workers have addressed various benefits of SOD, CAT, APX, GR, MDHAR, AsA in overcoming oxidative damage to the cell. There is an accumulation of osmolytes and osmoprotectants, such as proline and glycine betaine, to overcome salt stress's detrimental effects. These compounds help in osmotic adjustment and protecting subcellular structures. Salt tolerance of plants can be enhanced by increasing CO 2 concentration as it alleviates oxidative stress, which activates the oxidative system and increases the accumulation of compatible substances.
LEA proteins play a significant role in plant stress tolerance, but the elaborated mechanisms of plant stress protection remain undetermined. Under abiotic stress distinct signaling pathways are regulated, including the MAPK cascade. MAPK molecules are a group of proteins that can negotiate various plant functions, like cell cycle, plant growth and development, plant response to stress. The integration of different MAPK pathways can be very beneficial for transgenic plant production which is more resistant to salinity stress. Maintaining plant behaviour, particularly Na+ cellular concentration under salt stress, is an important key issue to make plant salt tolerant. MAPK signaling can significantly affect such pathways by regulating proton pumps' activity, Na+ localization into vacuoles, and regulating the cell cycle. There is a crosstalk between different signaling pathways during the stress and the interactions with phytohormones. At the molecular level-specific T s, non-coding RNAs and epigenetic modifications play an essential role in countering salt stress and imparting salt tolerance.
The limitation of crop production due to salt stress and ensuring food security can be overcome by developing salt-tolerant crop plants by utilizing novel technologies like gene editing and resourceful genetic transformation. The prerequisite for these novel technologies is that candidate genes associated with salinity tolerance must be recognized and exploited. Though multiple studies are carried out, the are many areas of understanding salt-induced responses and plant adaptability which need more focus, including how a plant senses the salt stress at the cellular and whole plant level, what sort of changes occur at cellular levels like cell wall modifications, cell organelles response and signal integration, mechanisms of phytohormone involvement. The control of specific gene expression during salinity stress needs special attention to discover and understand the role of key players like T s, noncoding RNAs, and epigenetic modifications.
ACKNOWLEDGMENTS
The authors are thankful to Head, Department of Botany, University of Kashmir for providing necessary facilities for the completion of this manuscript.
Список литературы Salt Stress Induced Plant Physio-biochemical and Molecular Responses: A Review
- Achard P., Cheng H., De Grauwe L., Decat J., Schoutteten H., Moritz T., Van Der Straeten D., Peng J., and Harberd N. P. (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science, 311, 91-94.
- Adams Mark A., Richter Andreas, Hill Alan K., and Colmer Timothy D. (2005) Salt tolerance in Eucalyptus spp.: identity and response of putative osmolytes. Plant Cell Environ, 28, 772-787.
- Agarwal Sheela, and Shaheen Robina (2007) Stimulation of antioxidant system and lipid peroxidation by abiotic stresses in leaves of Momordica charantia. Braz. J. Plant Physiol, 19, 149-161.
- Ahmad P, Jaleel CA, and Sharma S. (2010) Antioxidant defense system, lipid peroxidation, rolinemetabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russ. J. Plant Physiol, 57, 509-517.
- Ahmad P., and Sharma Satyawati. (2008) Salt stress and phytobiochemical responses of plants. Plant Soil Environ, 54, 89-99.
- Ahmad Raza, Lim Chan Ju, and Kwon Suk-Yoon. (2012) Glycine betaine: a versatile compound with great potential for gene pyramiding to improve crop plant performance against environmental stresses. Plant Biotechnol. Rep., 7, 49-57.
- Aly-Salama Karima Hamed , and Al-Mutawa MM. (2010) Glutathione-triggered mitigation in salt-induced alterations in plasmalemma of onion epidermal cells. Int J Agric Biol, 11, 639-642.
- Annunziata M. G., Ciarmiello L. F., Woodrow P., Dell'Aversana E., and Carillo P. (2019) Spatial and Temporal Profile of Glycine Betaine Accumulation in Plants Under Abiotic Stresses. Front Plant Sci, 10, 230.
- Artur M. A. S., Rienstra J., Dennis T. J., Farrant J. M., Ligterink W., and Hilhorst H. (2019) Structural Plasticity of Intrinsically Disordered LEA Proteins from Xerophyta schlechteri Provides Protection In Vitro and In Vivo. Front Plant Sci, 10, 1272.
- Asada Kozi. (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol., 141, 391-396.
- Ashraf M., and Foolad M. R. (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot., 59, 206-216.
- Ashraf M., and Harris P. J. C. (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci., 166, 3-16.
- Babiychuk E., Kushnir S., Belles-Boix E., Van Montagu M., and Inze D. (1995) Arabidopsis thaliana NADPH oxidoreductase homologs confer tolerance of yeasts toward the thiol-oxidizing drug diamide. J. Biol. Chem., 270, 26224-26231.
- Badawi G. H., Kawano N., Yamauchi Y., Shimada E., Sasaki R., Kubo A., and Tanaka K. (2004) Overexpression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol. Plant., 121, 231-238.
- Baniasadi Fatemeh, Saffari Vahid Reza, and Maghsoudi Moud Ali Akbar. (2018) Physiological and growth responses of Calendula officinalis L. plants to the interaction effects of polyamines and salt stress. Sci. Hortic., 234, 312-317.
- Baral A., Shruthi K. S., and Mathew M. K. (2015) Vesicular trafficking and salinity responses in plants. IUBMB Life, 67, 677-686.
- Bassil E., and Blumwald E. (2014) The ins and outs of intracellular ion homeostasis: NHX-type cation/H(+) transporters. Curr. Opin. Plant Biol., 22, 1-6.
- Bassil E., Zhang S., Gong H., Tajima H., and Blumwald E. (2019) Cation Specificity of Vacuolar NHXType Cation/H(+) Antiporters. Plant Physiol., 179, 616-629.
- Bazihizina N., Colmer T. D., Cuin T. A., Mancuso S., and Shabala S. (2019) Friend or Foe? Chloride Patterning in Halophytes. Trends Plant Sci., 24, 142-151.
- Ben Ahmed C., Ben Rouina B., Sensoy S., Boukhriss M., and Ben Abdullah F. (2010) Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J. Agric. Food Chem., 58, 4216-4222.
- Ben Rejeb K., Lefebvre-De Vos D., Le Disquet I., Leprince A. S., Bordenave M., Maldiney R., Jdey A., Abdelly C., and Savoure A. (2015) Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arabidopsis thaliana. New Phytol., 208, 1138-1148.
- Borsani O., Zhu J., Verslues P. E., Sunkar R., and Zhu J. K. (2005) Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell, 123, 1279-1291.
- Bose J., Rodrigo-Moreno A., and Shabala S. (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot., 65, 1241-1257.
- Buckley T. N. (2019) How do stomata respond to water status? New Phytol., 224, 21-36.
- Bueno M., and Cordovilla M. P. (2019) Polyamines in Halophytes. Front Plant Sci, 10, 439.
- Byrt C. S., Munns R., Burton R. A., Gilliham M., and Wege S. (2018) Root cell wall solutions for crop plants in saline soils. Plant Sci., 269, 47-55.
- Byrt C. S., Zhao M., Kourghi M., Bose J., Henderson S. W., Qiu J., Gilliham M., Schultz C., Schwarz M., Ramesh S. A., Yool A., and Tyerman S. (2017) Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca(2+) and pH. Plant Cell Environ, 40, 802-815.
- Cao W. H., Liu J., He X. J., Mu R. L., Zhou H. L., Chen S. Y., and Zhang J. S. (2007) Modulation of responses. Plant Physiol., 143, 707-719.
- Cayuela E, Perez‐Alfocea F, Caro M, and Bolarin MC (1996) Priming of seeds with NaCl induces physiological changes in tomato plants grown under salt stress. Physiol Plant., 96, 231-236.
- Cha-Um Suriyan, and Kirdmanee Chalermpol. (2010) Effect of glycinebetaine on proline, water use, and photosynthetic efficiencies, and growth of rice seedlings under salt stress. TURK J AGRIC FOR., 34, 517-527.
- Chen H., Feng H., Zhang X., Zhang C., Wang T., and Dong J. (2019) An Arabidopsis E3 ligase HUB2 increases histone H2B monoubiquitination and enhances drought tolerance in transgenic cotton. Plant Biotechnol. J., 17, 556-568.
- Chen M., Yang Z., Liu J., Zhu T., Wei X., Fan H., and Wang B. (2018) Adaptation Mechanism of Salt Excluders under Saline Conditions and Its Applications. Int J Mol Sci, 19, 3668.
- Cheng M. C., Liao P. M., Kuo W. W., and Lin T. P. (2013) The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol., 162, 1566-1582.
- Choi H., Hong J., Ha J., Kang J., and Kim S. Y. (2000) ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem., 275, 1723-1730.
- Christmann A., Grill E., and Huang J. (2013) Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol., 16, 293-300.
- Colcombet J., and Hirt H. (2008) Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem. J., 413, 217-226.
- Colebrook E. H., Thomas S. G., Phillips A. L., and Hedden P. (2014) The role of gibberellin signaling in plant responses to abiotic stress. J. Exp. Biol., 217, 67-75.
- Conde A., Regalado A., Rodrigues D., Costa J. M., Blumwald E., Chaves M. M., and Geros H. (2015) Polyols in grape berry: transport and metabolic adjustments as a physiological strategy for waterdeficit stress tolerance in grapevine. J. Exp. Bot., 66, 889-906.
- Conti L., Price G., O'Donnell E., Schwessinger B., Dominy P., and Sadanandom A. (2008) Small ubiquitin-like modifier proteases OVERLY TOLERANT TO SALT1 and -2 regulate salt stress responses in Arabidopsis. Plant Cell, 20, 2894-2908.
- Cui F., Liu L., Zhao Q., Zhang Z., Li Q., Lin B., Wu Y., Tang S., and Xie Q. (2012) Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance. Plant Cell, 24, 233-244.
- Cumming A.C. (1999) LEA proteins. In Shewry, Peter R (ed.), Seed Proteins (Kluwar Dordrecht). da Silva J. M., and Arrabaca M. C. (2004) Contributions of soluble carbohydrates to the osmotic adjustment in the C4 grass Setaria sphacelata: a comparison between rapidly and slowly imposed water stress. J. Plant Physiol., 161, 551-555.
- Dat J., Vandenabeele S., Vranova E., Van Montagu M., Inze D., and Van Breusegem F. (2000) Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci., 57, 779-795.
- Davletova Sholpan, Rizhsky Ludmila, Liang Hongjian, Shengqiang Zhong, Oliver David J, Coutu Jesse, Shulaev Vladimir, Schlauch Karen, and Mittler Ron %J The Plant Cell. (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell, 17, 268-281.
- Del Rio L. A., and Lopez-Huertas E. (2016) ROS Generation in Peroxisomes and its Role in Cell Signaling. Plant Cell Physiol., 57, 1364-1376.
- Demidchik V. (2014) Mechanisms and physiological roles of K+ efflux from root cells. J. Plant Physiol., 171, 696-707.
- Demidchik V. (2018) ROS-Activated Ion Channels in Plants: Biophysical Characteristics, Physiological Functions and Molecular Nature. Int J Mol Sci, 19, 1263.
- Demidchik V., Cuin T. A., Svistunenko D., Smith S. J., Miller A. J., Shabala S., Sokolik A., and Yurin V. (2010) Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci., 123, 1468-1479.
- Ding Hai‐Dong, Zhang Xiao‐Hua, Xu Shu‐Cheng, Sun Li‐Li, Jiang Ming‐Yi, Zhang A‐Ying, and Jin Yin‐Gen. (2009) Induction of protection against paraquat‐induced oxidative damage by abscisic acid in maize leaves is mediated through mitogenactivated protein kinase. J. Integr. Plant Biol., 51, 961-972.
- Doganlar Zeynep, Demir Koksal, Başak Hakan, and Gul Iftikhar. (2010) Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. Afr. J. Agric. Res., 15, 2056-2065.
- Dong W., Wang M., Xu F., Quan T., Peng K., Xiao L., and Xia G. (2013) Wheat oxophytodienoate reductase gene TaOPR1 confers salinity tolerance via enhancement of abscisic acid signaling and reactive oxygen species scavenging. Plant Physiol., 161, 1217-1228.
- Droillard M., Boudsocq M., Barbier-Brygoo H., and Lauriere C. (2002) Different protein kinase families are activated by osmotic stresses in Arabidopsis thaliana cell suspensions. Involvement of the MAP kinases AtMPK3 and AtMPK6. FEBS Lett., 527, 43-50.
- Droillard M. J., Boudsocq M., Barbier-Brygoo H., and Lauriere C. (2004) Involvement of MPK4 in osmotic stress response pathways in cell suspensions and plantlets of Arabidopsis thaliana: activation by hypoosmolarity and negative role in hyperosmolarity tolerance. FEBS Lett., 574, 42-48.
- Duarte K. E., de Souza W. R., Santiago T. R., Sampaio B. L., Ribeiro A. P., Cotta M. G., da Cunha Badb, Marraccini P. R. R., Kobayashi A. K., and Molinari H. B. C. (2019) Identification and characterization of core abscisic acid (ABA) signaling components and their gene expression profile in response to abiotic stresses in Setaria viridis. Sci Rep, 9, 4028.
- Eichten S. R., Schmitz R. J., and Springer N. M. (2014) Epigenetics: Beyond Chromatin Modifications and Complex Genetic Regulation. Plant Physiol., 165, 933-947.
- El-Mashad A. A., and Mohamed H. I. (2012) Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma, 249, 625-635.
- El-Shintinawy F., and El-Shourbagy M. N. (2001) Alleviation of Changes in Protein Metabolism in NaCl-Stressed Wheat Seedlings by Thiamine. Biol. Plant., 44, 541-545.
- El-Tayeb MA (2005) Response of barley grains to the interactive e. ect of salinity and salicylic acid. Plant Growth Regul., 45, 215-224.
- El Mahi H., Perez-Hormaeche J., De Luca A., Villalta I., Espartero J., Gamez-Arjona F., Fernandez J. L., Bundo M., Mendoza I., Mieulet D., Lalanne E., Lee S. Y., Yun D. J., Guiderdoni E., Aguilar M., Leidi E. O., Pardo J. M., and Quintero F. J. (2019) A Critical Role of Sodium Flux via the Plasma Membrane Na(+)/H(+) Exchanger SOS1 in the Salt Tolerance of Rice. Plant Physiol., 180, 1046-1065.
- Endo A., Sawada Y., Takahashi H., Okamoto M., Ikegami K., Koiwai H., Seo M., Toyomasu T., Mitsuhashi W., Shinozaki K., Nakazono M., Kamiya Y., Koshiba T., and Nambara E. (2008) Drought induction of Arabidopsis 9-cisepoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol., 147, 1984-1993.
- Espasandin Fabiana D, Calzadilla Pablo I, Maiale Santiago J, Ruiz Oscar A, and Sansberro Pedro A %J Journal of plant growth regulation. (2018) Overexpression of the arginine decarboxylase gene improves tolerance to salt stress in Lotus tenuis plants. J. Plant Growth Regul., 37, 156-165.
- Flowers T. J. (2004) Improving crop salt tolerance. J Exp Bot, 55, 307-319.
- Flowers T. J., and Yeo A. R. (1995) Breeding for Salinity Resistance in Crop Plants: Where Next? Funct. Plant Biol., 22, 875-884.
- Foyer C. H, Lopez‐Delgado H., Dat J. F, and Scott I. M. (1997) Hydrogen peroxide‐and lutathioneassociated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant., 100, 241-254.
- Fricke W., Akhiyarova G., Veselov D., and Kudoyarova G. (2004) Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J. Exp. Bot., 55, 1115-1123.
- Fujita Y., Yoshida T., and Yamaguchi-Shinozaki K. (2013) Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant., 147, 15-27.
- Furuichi T., Tatsumi H., and Sokabe M. (2008) Mechano-sensitive channels regulate the stomatal aperture in Vicia faba. Biochem. Biophys. Res. Commun., 366, 758-762.
- Furuki T., Shimizu T., Chakrabortee S., Yamakawa K., Hatanaka R., Takahashi T., Kikawada T., Okuda T., Mihara H., Tunnacliffe A., and Sakurai M. (2012) Effects of Group 3 LEA protein model peptides on desiccation-induced protein aggregation. Biochim. Biophys. Acta, 1824, 891-897.
- Geilfus C. M. (2018) Review on the significance of chlorine for crop yield and quality. Plant Sci., 270, 114-122.
- Geissler N., Hussin S., and Koyro H. W. (2010) Elevated atmospheric CO2 concentration enhances salinity tolerance in Aster tripolium L. Planta, 231, 583-594.
- Geng Y., Wu R., Wee C. W., Xie F., Wei X., Chan P. M., Tham C., Duan L., and Dinneny J. R. (2013) A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell, 25, 2132-2154.
- Giraud E., Ho L. H., Clifton R., Carroll A., Estavillo G., Tan Y. F., Howell K. A., Ivanova A., Pogson B. J., Millar A. H., and Whelan J. (2008) The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol., 147, 595-610.
- Giri J. (2011) Glycinebetaine and abiotic stress tolerance in plants. Plant Signal Behav, 6, 1746-1751.
- Gleeson Deirdre, Lelu-Walter Marie-Anne, and Parkinson Michael. (2005) Overproduction of proline in transgenic hybrid larch (Larix x leptoeuropaea (Dengler)) cultures renders them tolerant to cold, salt and frost. Mol. Breed., 15, 21-29.
- Goyal Kshamata, Walton Laura J, and Tunnacliffe Alan (2005) LEA proteins prevent protein aggregation due to water stress. Biochem. J., 388, 151-157.
- Groß F., Durner J., and Gaupels F. (2013) Nitric oxide, antioxidants and prooxidants in plant defence responses. Front Plant Sci, 4, 419.
- Guan Q., Tan B., Kelley T. M., Tian J., and Chen S. (2020) Physiological Changes in Mesembryanthemum crystallinum During the C3 to CAM Transition Induced by Salt Stress. Front Plant Sci, 11, 283.
- Guo K. M., Babourina O., Christopher D. A., Borsic T., and Rengel Z. (2010) The cyclic nucleotide-gated channel AtCNGC10 transports Ca2+ and Mg2+ in Arabidopsis. Physiol. Plant., 139, 303-312.
- Guo Q., Liu L., and Barkla B. J. (2019) Membrane Lipid Remodeling in Response to Salinity. Int J Mol Sci, 20, 4264.
- Gupta Bhaskar, Gupta Kamala, and Sengupta Dibyendu Narayan. (2012) Spermidine-mediated in vitro phosphorylation of transcriptional regulator OSBZ8 by SNF1-type serine/threonine protein kinase SAPK4 homolog in indica rice. Acta Physiol. Plant., 34, 1321-1336.
- Gutteridge J. M., and Halliwell B. (2000) Free radicals and antioxidants in the year 2000. A historical look to the future. Ann. N. Y. Acad. Sci., 899, 136- 147.
- Hamel L. P., Nicole M. C., Sritubtim S., Morency M. J., Ellis M., Ehlting J., Beaudoin N., Barbazuk B., Klessig D., Lee J., Martin G., Mundy J., Ohashi Y., Scheel D., Sheen J., Xing T., Zhang S., Seguin A., and Ellis B. E. (2006) Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci., 11, 192-198.
- Hand Steven C, Menze Michael A, Toner Mehmet, Boswell Leaf, and Moore Daniel. (2011) LEA proteins during water stress: not just for plants anymore. Annu. Rev. Physiol, 73, 115-134.
- Hanin M., Ebel C., Ngom M., Laplaze L., and Masmoudi K. (2016) New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front Plant Sci, 7, 1787.
- Hanson A. D., Rathinasabapathi B., Rivoal J., Burnet M., Dillon M. O., and Gage D. A. (1994) Osmoprotective compounds in the Plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance. Proc Natl Acad Sci U S A, 91, 306-310.
- Hasegawa P. M., Bressan R. A., Zhu J. K., and Bohnert H. J. (2000) Plant Cellular and Molecular Responses to High Salinity. Annu. Rev. Plant Physiol., 51, 463-499.
- Hoque M. A., Banu M. N., Okuma E., Amako K., Nakamura Y., Shimoishi Y., and Murata Y. (2007) Exogenous proline and glycinebetaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J. Plant Physiol., 164, 1457-1468.
- Hu Y., Chen L., Wang H., Zhang L., Wang F., and Yu D. (2013) Arabidopsis transcription factor WRKY8 functions antagonistically with its interacting partner VQ9 to modulate salinity stress tolerance. Plant J., 74, 730-745.
- Huang C. F., Miki D., Tang K., Zhou H. R., Zheng Z., Chen W., Ma Z. Y., Yang L., Zhang H., Liu R., He X. J., and Zhu J. K. (2013) A Pre-mRNA-splicing factor is required for RNA-directed DNA methylation in Arabidopsis. PLoS Genet., 9, e1003779.
- Hundertmark M., Popova A. V., Rausch S., Seckler R., and Hincha D. K. (2012) Influence of drying on the secondary structure of intrinsically disordered and globular proteins. Biochem. Biophys. Res. Commun., 417, 122-128.
- Hussain Town, Chandrasekhar Thummala, Hazara Mahamed, Sultan Zafar, Saleh Brhan, and Ghanta Rama. (2007) Recent advances in salt stress biology - a review. Mol. Biol. Rev., 3, 8-13.
- Hwa Chi-Min, and Yang Xian-Ci. (2007) The AtMKK3 pathway mediates ABA and salt signaling in Arabidopsis. Acta Physiol. Plant., 30, 277-286.
- Isayenkov S. V., and Maathuis F. J. M. (2019) Plant Salinity Stress: Many Unanswered Questions Remain. Front Plant Sci, 10, 80.
- Ishikawa Tetsuya, Cuin Tracey Ann, Bazihizina Nadia, and Shabala Sergey. (2018a) Chapter Nine - Xylem Ion Loading and Its Implications for Plant Abiotic Stress Tolerance. In Maurel, Christophe (ed.), Adv. Bot. Res. (Academic Press).
- Salt Stress Induced Plant Physio-biochemical and Molecular Responses... Ishikawa Tetsuya, Cuin Tracey Ann, Bazihizina Nadia, and Shabala Sergey. (2018b) Xylem Ion Loading and Its Implications for Plant Abiotic Stress Tolerance. In Maurel, Christophe (ed.), Membrane Transport in Plants (Academic Press).
- Islam M. O., Kato H., Shima S., Tezuka D., Matsui H., and Imai R. (2019) Functional identification of a rice trehalase gene involved in salt stress tolerance. Gene, 685, 42-49.
- Jabnoune M., Espeout S., Mieulet D., Fizames C., Verdeil J. L., Conejero G., Rodriguez-Navarro A., Sentenac H., Guiderdoni E., Abdelly C., and Very A. A. (2009) Diversity in expression patterns and functional properties in the rice HKT transporter family. Plant Physiol., 150, 1955-1971.
- Jagendorf A. T., and Takabe T. (2001) Inducers of glycinebetaine synthesis in barley. Plant Physiol., 127, 1827-1835.
- Jaleel Cheruth Abdul, Gopi Ragupathi, Kishorekumar Ashok, Manivannan Paramasivam, Sankar Beemarao, and Panneerselvam Rajaram. (2008) Interactive effects of triadimefon and salt stress on antioxidative status and ajmalicine accumulation in Catharanthus roseus. Acta Physiol. Plant., 30, 287.
- James R. A., Blake C., Byrt C. S., and Munns R. (2011) Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot., 62, 2939-2947.
- Jayakannan M., Bose J., Babourina O., Rengel Z., and Shabala S. (2013) Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot., 64, 2255-2268.
- Ji M., Wang K., Wang L., Chen S., Li H., Ma C., and Wang Y. (2019) Overexpression of a SAdenosylmethionine Decarboxylase from Sugar Beet M14 Increased Araidopsis Salt Tolerance. Int J Mol Sci, 20(8). 1990.
- Jiang A. L., Cheng Y., Li J., and Zhang W. (2008) A zincdependent nuclear endonuclease is responsible for DNA laddering during salt-induced programmed cell death in root tip cells of rice. J. Plant Physiol., 165, 1134-1141.
- Jiang C., Belfield E. J., Mithani A., Visscher A., Ragoussis J., Mott R., Smith J. A., and Harberd N. P. (2012) ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. Embo J, 31, 4359-4370.
- Johnson R. R., Wagner R. L., Verhey S. D., and Walker-Simmons M. K. (2002) The abscisic acidresponsive kinase PKABA1 interacts with a seedspecific abscisic acid response element-binding factor, TaABF, and phosphorylates TaABF peptide sequences. Plant Physiol., 130, 837-846.
- Kadioglu Asim, Terzi Rabiye, Saruhan Neslihan, and Saglam Aykut. (2012) Current advances in the investigation of leaf rolling caused by biotic and abiotic stress factors. Plant Science, 182, 42-48.
- Kang D‐J, Seo Y‐J, Lee J‐D, Ishii R, Kim KU, Shin DH, Park SK, Jang SW, Lee I‐J , and Science Crop. (2005) Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salttolerant and salt‐sensitive rice cultivars. J. Agron, 191, 273-282.
- Karan R., DeLeon T., Biradar H., and Subudhi P. K. (2012) Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PloS One, 7, e40203.
- Kasuga M., Liu Q., Miura S., Yamaguchi-Shinozaki K., and Shinozaki K. (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol., 17, 287-291.
- Kazan K. (2015) Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci., 20, 219-229.
- Kerepesi Ildikó, and Galiba Gábor. (2000) Osmotic and Salt Stress-Induced Alteration in Soluble Carbohydrate Content in Wheat Seedlings. Crop Sci., 40, 482-487.
- Kim S. E., Lee C. J., Ji C. Y., Kim H. S., Park S. U., Lim Y. H., Park W. S., Ahn M. J., Bian X., Xie Y., Guo X., and Kwak S. S. (2019) Transgenic sweetpotato plants overexpressing tocopherol cyclase display enhanced α-tocopherol content and abiotic stress tolerance. Plant Physiol. Biochem., 144, 436-444.
- Kim S. H., Ahn Y. O., Ahn M. J., Lee H. S., and Kwak S. S. (2012) Down-regulation of beta-carotene hydroxylase increases beta-carotene and total carotenoids enhancing salt stress tolerance in transgenic cultured cells of sweetpotato. Phytochemistry, 74, 69-78.
- Kim S., Kang J. Y., Cho D. I., Park J. H., and Kim S. Y. (2004) ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J., 40, 75-87.
- Kobayashi N. I., Yamaji N., Yamamoto H., Okubo K., Ueno H., Costa A., Tanoi K., Matsumura H., Fujii-Kashino M., Horiuchi T., Nayef M. A., Shabala S., An G., Ma J. F., and Horie T. (2017) OsHKT1;5 mediates Na(+) exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J., 91, 657-670.
- Koffler Barbara Eva, Luschin-Ebengreuth Nora, and Zechmann Bernd. (2015) Compartment specific changes of the antioxidative status in Arabidopsis thaliana during salt stress. J. Plant Biol., 58, 8-16.
- Kong X., Pan J., Zhang M., Xing X., Zhou Y., Liu Y., Li D., and Li D. (2011) ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant Cell Environ, 34, 1291-1303.
- Kronzucker H. J., and Britto D. T. (2011) Sodium transport in plants: a critical review. New Phytol., 189, 54-81.
- Lee Geungjoo, Carrow Robert N., Duncan Ronny R., Eiteman Mark A., and Rieger Mark W. (2008) Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ. Exp. Bot., 63, 19-27.
- Lee J. S., and Ellis B. E. (2007) Arabidopsis MAPK phosphatase 2 (MKP2) positively regulates oxidative stress tolerance and inactivates the MPK3 and MPK6 MAPKs. J. Biol. Chem., 282, 25020-25029.
- Lee J. S., Wang S., Sritubtim S., Chen J. G., and Ellis B. E. (2009) Arabidopsis mitogen-activated protein kinase MPK12 interacts with the MAPK phosphatase IBR5 and regulates auxin signaling. Plant J., 57, 975-985.
- Li Z., Wang W., Li G., Guo K., Harvey P., Chen Q., Zhao Z., Wei Y., Li J., and Yang H. (2016) MAPKmediated regulation of growth and essential oil composition in a salt-tolerant peppermint (Mentha piperita L.) under NaCl stress. Protoplasma, 253, 1541-1556.
- Lim G. H., Zhang X., Chung M. S., Lee D. J., Woo Y. M., Cheong H. S., and Kim C. S. (2010) A putative novel transcription factor, AtSKIP, is involved in abscisic acid signalling and confers salt and osmotic tolerance in Arabidopsis. New Phytol., 185, 103-113.
- Liu J., Cui L., Xie Z., Zhang Z., Liu E., and Peng X. (2019a) Two NCA1 isoforms interact with catalase in a mutually exclusive manner to redundantly regulate its activity in rice. BMC Plant Biol., 19, 105.
- Liu Xiaojing, Duan Deyu, Li Weiqiang, Tadano T., and Khan M. Ajmal. (2006) A Comparative Study On Responses Of Growth And Solute Composition In Halophytes Suaeda Salsa And Limonium Bicolor To Salinity. In Ecophysiology of High Salinity Tolerant Plants, edited by Khan, M. Ajmal and Darrell J. Weber, 135-143. Dordrecht, Springer Netherlands.
- Liu Y., Li D., Yan J., Wang K., Luo H., and Zhang W. (2019b) MiR319 mediated salt tolerance by ethylene. Plant Biotechnol. J., 17, 2370-2383.
- Liu Y., Yang M., Cheng H., Sun N., Liu S., Li S., Wang Y., Zheng Y., and Uversky V. N. (2017) The effect of phosphorylation on the salt-tolerance-related functions of the soybean protein PM18, a member of the group-3 LEA protein family. Biochim Biophys Acta Proteins Proteom, 1865, 1291-1303.
- Liu Yang, Wang Li, Xing Xin, Sun Liping, Pan Jiaowen, Salt Stress Induced Plant Physio-biochemical and Molecular Responses... Kong Xiangpei, Zhang Maoying, and Li Dequan (2013) ZmLEA3, a multifunctional group 3 LEA protein from maize (Zea mays L.), is involved in biotic and abiotic stresses. Plant Cell Physiol., 54, 944-959.
- Lokhande V H, and Penna S. (2012) Prospects of halophytes in understanding and managing abiotic stress tolerance. In Ahmad P, and Prasad M. (ed.), Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change (Springer, New York).
- Lopez-Huertas E., Charlton W. L., Johnson B., Graham I. A., and Baker A. (2000) Stress induces peroxisome biogenesis genes. EMBO J., 19, 6770-6777.
- Luo D., Hou X., Zhang Y., Meng Y., Zhang H., Liu S., Wang X., and Chen R. (2019) CaDHN5, a Dehydrin Gene from Pepper, Plays an Important Role in Salt and Osmotic Stress Responses. Int J Mol Sci, 20, 1989.
- Luo D., Niu X., Yu J., Yan J., Gou X., Lu B. R., and Liu Y. (2012a) Rice choline monooxygenase (OsCMO) protein functions in enhancing glycine betaine biosynthesis in transgenic tobacco but does not accumulate in rice (Oryza sativa L. ssp. japonica). Plant Cell Rep., 31, 1625-1635.
- Luo M., Wang Y. Y., Liu X., Yang S., Lu Q., Cui Y., and Wu K. (2012b) HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J. Exp. Bot., 63, 3297-3306.
- Ma L., Zhang H., Sun L., Jiao Y., Zhang G., Miao C., and Hao F. (2012) NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na(+)/K(+)homeostasis in Arabidopsis under salt stress. J. Exp. Bot., 63, 305-317.
- Magome H., Yamaguchi S., Hanada A., Kamiya Y., and Oda K. (2008) The DDF1 transcriptional activator upregulates expression of a gibberellindeactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J., 56, 613-626.
- Massacci A., Nabiev S. M., Pietrosanti L., Nematov S. K., Chernikova T. N., Thor K., and Leipner J. (2008) Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol Biochem, 46, 189-195.
- Matysik Jörg, Alia Alia, Bhalu B., and Mohanty Prasanna. (2002) Molecular mechanism of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci., 82, 525-532.
- Megdiche W., Hessini K., Gharbi F., Jaleel C. A., Ksouri R., and Abdelly C. (2008) Photosynthesis and photosystem 2 efficiency of two salt-adapted halophytic seashore Cakile maritima ecotypes. Photosynthetica, 46, 410-419.
- Mian A., Oomen R. J., Isayenkov S., Sentenac H., Maathuis F. J., and Very A. A. (2011) Overexpression of an Na+-and K+-permeable HKT transporter in barley improves salt tolerance. Plant J., 68, 468-479.
- Miller G., Schlauch K., Tam R., Cortes D., Torres M. A., Shulaev V., Dangl J. L., and Mittler R. (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal, 2, ra45.
- Miller G., Suzuki N., Ciftci-Yilmaz S., and Mittler R. (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ, 33, 453-467.
- Misra Neelam, and Misra Rahul (2012) Salicylic acid changes plant growth parameters and proline metabolism in Rauwolfia serpentina leaves grown under salinity stress. AEJAES, 12, 1601-1609.
- Missihoun T. D., Willee E., Guegan J. P., Berardocco S., Shafiq M. R., Bouchereau A., and Bartels D. (2015) Overexpression of ALDH10A8 and ALDH10A9 Genes Provides Insight into Their Role in Glycine Betaine Synthesis and Affects Primary Metabolism in Arabidopsis thaliana. Plant Cell Physiol., 56, 1798-1807.
- Mizoi J., Shinozaki K., and Yamaguchi-Shinozaki K. (2012) AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta, 1819, 86-96.
- Mostofa M. G., Hossain M. A., and Fujita M. (2015) Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma, 252, 461-475.
- Munir Neelma, and Aftab Faheem (2011) Enhancement of salt tolerance in sugarcane by ascorbic acid pretreatment. Afr. J. Biotechnol, 10, 18362-18370.
- Munne-Bosch S., and Alegre L. (2003) Drought-induced changes in the redox state of alpha-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol., 131, 1816-1825.
- Munns R., Greenway H., and Kirst G. O. (1983) Halotolerant Eukaryotes. In Lange, O. L., P. S. Nobel, C. B. Osmond and H. Ziegler (eds.), Physiological Plant Ecology III (Springer Berlin Heidelberg, Berlin, Heidelberg).
- Munns R., James R. A., Xu B., Athman A., Conn S. J., Jordans C., Byrt C. S., Hare R. A., Tyerman S. D., Tester M., Plett D., and Gilliham M. (2012) Wheat grain yield on saline soils is improved by an ancestral Na(+) transporter gene. Nat. Biotechnol., 30, 360-364.
- Munns R., Passioura J. B., Guo J., Chazen O., and Cramer G. R. (2000) Water relations and leaf expansion: importance of time scale. J. Exp. Bot., 51, 1495-1504.
- Munns R., and Tester M. (2008) Mechanisms of salinity tolerance. Annu. Rev. Plant Biol., 59, 651-681.
- Naka Y., Watanabe K., Sagor G. H., Niitsu M., Pillai M. A., Kusano T., and Takahashi Y. (2010) Quantitative analysis of plant polyamines including thermospermine during growth and salinity stress. Plant Physiol. Biochem., 48, 527-533.
- Nakashima K., Tran L. S., Van Nguyen D., Fujita M., Maruyama K., Todaka D., Ito Y., Hayashi N., Shinozaki K., and Yamaguchi-Shinozaki K. (2007) Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J., 51, 617-630.
- Nath Manoj, Bhatt Deepesh, Jain Ajay, Saxena Saurabh C., Saifi Shabnam K., Yadav Sandep, Negi Manisha, Prasad Ram, and Tuteja Narendra. (2019) Salt stress triggers augmented levels of Na+, Ca2+ and ROS and alter stress-responsive gene expression in roots of CBL9 and CIPK23 knockout mutants of Arabidopsis thaliana. Environ. Exp. Bot., 161, 265-276.
- Nazar R., Iqbal N., Syeed S., and Khan N. A. (2011) Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol., 168, 807-815.
- Ong R. G., Higbee A., Bottoms S., Dickinson Q., Xie D., Smith S. A., Serate J., Pohlmann E., Jones A. D., Coon J. J., Sato T. K., Sanford G. R., Eilert D., Oates L. G., Piotrowski J. S., Bates D. M., Cavalier D., and Zhang Y. (2016) Inhibition of microbial biofuel production in drought-stressed switchgrass hydrolysate. Biotechnol Biofuels, 9, 237.
- Ouyang S., He S., Liu P., Zhang W., Zhang J., and Chen S. (2011) The role of tocopherol cyclase in salt stress tolerance of rice (Oryza sativa). Sci China Life Sci, 54, 181-188.
- Ozturk A, and Unlukara A. (2004) Effects of salt stress and water deficit on plant growth and essentialoil content of lemon balm (Melissa officinalis L.). Pak. J. Bot., 36, 787-792.
- Parida A. K., and Das A. B. (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol. Environ. Saf., 60, 324-349.
- Paul Saikat, and Roychoudhury Aryadeep. (2017) Seed priming with spermine and spermidine regulates the expression of diverse groups of abiotic stressresponsive genes during salinity stress in the seedlings of indica rice varieties. Plant Gene, 11, 124-132.
- Peng J., Li Z., Wen X., Li W., Shi H., Yang L., Zhu H., and Guo H. (2014) Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring Salt Stress Induced Plant Physio-biochemical and Molecular Responses... ROS accumulation in Arabidopsis. PLoS Genet., 10, e1004664.
- Penna S. (2003) Building stress tolerance through overproducing trehalose in transgenic plants. Trends Plant Sci., 8, 355-357.
- Pospisil P., Prasad A., and Rac M. (2019) Mechanism of the Formation of Electronically Excited Species by Oxidative Metabolic Processes: Role of Reactive Oxygen Species. Biomolecules, 9, 258.
- Pottosin I., and Shabala S. (2014) Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front Plant Sci, 5, 154.
- Qin T., Zhao H., Cui P., Albesher N., and Xiong L. (2017) A Nucleus-Localized Long Non-Coding RNA Enhances Drought and Salt Stress Tolerance. Plant Physiol., 175, 1321-1336.


