Seasonal variation in hematological and histomorphological parameters in the garden lizard, calotes versicolor
Автор: Singh Tumul, Singh Ramesh, Tripathi Manish Kumar
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.18, 2022 года.
Бесплатный доступ
Seasonal variations in the physiological processes help animal to adapt to the environmental condition, however, informations on this subject in reptiles are scarce. In the present study, garden lizards ( Calotes versicolor ) were used to study the annual variation in blood immune responses and changes in the lymphoid organs. The lizards were weighed, anaesthetized under mild anesthesia and blood was collected in heparinized syringe. Blood was then processed for determining total leucocyte count, differential leucocyte count and nitroblue tetrazolium reduction test (Slide assay) for assessment of reactive oxygen production by leucocytes. For histomorphological studies, spleen, thymus and testes were excised rapidly, cleaned and weighed and their weight was transformed to thymosomatic, splenosomatic and gonadosomatic indices respectively. Total leucocyte count did not change significantly, however, differential leucocyte count varied during different seasons. Thymosomatic index was lesser in summer and rainy months. Splenosomatic index started increasing from May and remained high up to August (the hot and humid months of the year and reproductively active months). Gonadosomatic index was low from September to March. It started increasing from April and remained at highest level from May to August. Environmental factors play an important role in body physiology, oxidation, and intermediary metabolism and gonadal activity of all the vertebrates, including reptiles. The annual variation in physiology is pivotal to help animals cope with seasonal stressors.
Seasonal variation, reptiles, leucocytes, annual rhythm, lymphoid organs
Короткий адрес: https://sciup.org/143178806
IDR: 143178806
Текст научной статьи Seasonal variation in hematological and histomorphological parameters in the garden lizard, calotes versicolor
Adaptations in physiology and behavior are strategies that non tropical species use to adjust to seasonal changes in metabolic fuel and inclement conditions. The animals need to adapt themselves through various means like migration, hibernation and specially reproduction in order to cope up with these changes. Breeding, moulting, migration, territorial defense and other energetically expensive activities are synchronized to coincide with the availability of food or favorable local conditions; these typically occur during long days (Bronson, 1989; Wingfield and Kenagy, 1991; Wingfield and Farner, 1993). The photoperiod length is used by most of the organisms as a seasonal marker, because it is the most reproducible and predictable sign of the changing season (Goldman, 2001; Prendergast et al., 2002). Maintaining optimal immune function related to survival is energetically expensive; the cascade of mitotic activities among immune cells, the onset and maintenance of inflammation and fever, and the production of humoral immune factors, all require significant energy (Kelley, 1985). Mounting an immune response requires resources that could otherwise be allocated to other biological functions (Sheldon and Verhulst, 1996). Thus, activation of immune function is costly (Nelson et al. , 2002; Demas et al. , 2003a). Consequently, animals may maintain the highest level of immune function that is energetically possible, given the constrain of processes essential for survival, growth, reproduction, thermogenesis, foraging and other activities (Festa-Bianchet, 1989).
Seasonal variation in lymphoid organ mass has been reported in reptiles: Clemnys leprosa, Spalerosophis diadema, Testudo mauritonica, Scincus scincus, Psammophis schokari and Mauremys capsica (Aime, 1912; Hussein et al. , 1979a, b; El Ridi et al. , 1981) Effect of seasonal changes on the structure of lymphoid organs, cell viability, proportion of T and B cells, antibody titers, responses to mitogens and mixed leucocyte reactions have been reported in Egyptian Lizards (El Ridi et al. , 1981). Seasonal variations in Cell-Mediated and Humoral Immunity have been reported in the snake, Psammophis schokari (El Ridi et al. , 1981) and the lizard, Scincus scincus (Hussein et al. , 1979b).
There is a profound modulation of immune reactivity by neurotransmitters and hormones and, conversely, immune cells-derived soluble mediators, cytokines, have an effect on neuroendocrine function. In last two decade, the role of melatonin as a neuromodulator has been accepted (Guerrero and Reiter, 1992; Maestroni, 1993; Poon et al. , 1994b; Haldar and Rai, 2007). Thus, it is reasonable to postulate that seasonal changes in the duration of melatonin secretion may mediate seasonal alterations in immune function (Brainard et al. , 1988; Champney and McMurray, 1991; Champney et al. , 1997). Likewise it is reasonable to speculate that individuals within a species that vary in reproductive responsiveness to photoperiod may also vary in immune-responsiveness to melatonin. In addition to melatonin, gonadal steroids, prolactin and glucocorticoids also affect the immune functions (Bartness et al. , 1993).
Our goal is to emphasize a new dimension to the study of seasonality by shifting the focus from reproduction to immunity and to emphasize the immunomodulatory role of melatonin with respect to various seasonal factors. In the present study, male garden lizard ( Calotes versicolor ) were used, which shows inverse circannual variations in the activity of pineal and testes (Haldar and Thapliyal, 1977). It is a seasonally breeding lizard that faces annually changing environmental factors and synchronizes its reproductive cycle with changing environmental conditions. It is also evident that pineal circadian rhythm has significant influence over rhythm of blood constituents of the lizard, Calotes versicolor (Haldar and Thapliyal, 1977). Our aim was to study seasonal variation in total leucocyte count, lymphocyte count and Nitroblue Tetrazolium (NBT) reduction slide assay for assessment of reactive oxygen production by leucocytes. Annual variations in histomorphology of spleen and thymus were also studied.
MATERIALS AND METHODS
Animals
Calotes versicolor (Daudin), the garden lizard often called a bloodsucker is the most common lizard seen in parks and gardens. It is commonly found in low shrubs and bushes; it may also climb up tree trunks to avoid being detected. These are available throughout the year. The species is identified by the short crest above the neck, the presence of small spines above the tympanum and by the lack of a shoulder fold, colour ranges from brownish-buff to grayish. Sexes are separate, and in the breeding season the throat of the male becomes red and black. It is insectivorous and feed on crickets, maggots, flies, ants but may also feed on small lizards, earthworms, caterpillars, millipedes etc. It runs swiftly on ground. The lizards were caught locally in suburbs of Varanasi (latitude 25°18’N: longitude 83°01’E) during the second half of every month. Adult male garden lizards were selected for the study. They measured snout-vent length 10±2 cm and average body weight 30±2 g. They were immediately brought to laboratory, housed in vivarium (wire net cages of size 18 x12x 10 inch), and they were provided with food (crickets, maggots, flies) and water ad libitum.
Isolation of Blood
The lizards were weighed and anaesthetized under mild anesthesia. Blood was collected in heparinized syringe with a hypodermic 20 gauze needle through cardiac puncture. Blood was then processed for determining Total Leucocyte Count (TLC), Differential Leucocyte Count (DLC) and NBT reduction test (Slide assay) for assessment of reactive oxygen production by leucocytes. Care was taken to see that the whole operation was completed in minimum time.
Total Leucocyte Count
For TLC, 20 µl of blood was diluted twenty times with Turk’s fluid (0.2% gentian violet solution in 3% acetic acid). The diluted blood was applied on Hemocytometer Neubauer counting chamber (ROHEM, India), and cells from the four chambers of a square millimeter were counted under the microscope, and number of leucocytes/mm 3 was determined.
Differential Leucocyte Count
For DLC, a uniform blood film was smeared on clean glass slide. The blood smear was air dried and stained in a mixture of Giemsa and Leishman stain. After washing under tap water, the slides were dried, dehydrated, cleared in xylene and mounted in DPX. The purpose of a differential leucocyte count is to establish the relative percentage frequency of each cell type. Stained slide was observed under oil immersion objective from the upper edge of the smear to the extreme lower edge. Hundred leucocytes were identified and counted. Once the relative percentage frequency of each type of cells was obtained, their number is calculated in mm3 blood from total leucocyte count.
NBT reduction test (Slide assay)
NBT solution (0.1%) was prepared in Phosphate-Buffered saline (PBS; pH 7.2) and stirred at room temperature for 1 hour. The solution was stored at 4° C. For assay, 20µl of blood was mixed with 50µl of NBT solution. The mixture was applied on acid cleaned cover glass and incubated for 1 hour at room temperature in moist chamber. Following incubation, the cover glass was washed with PBS and mounted on slide with PBS. NBT reduction results formation of blue formazan deposit in leucocytes. Number of blue cells was scored in 20 optic fields at 4x10 X magnification randomly.
Histomorphological Study
For histomorphological studies, spleen, thymus and testes were excised rapidly, cleaned and weighed and their weight was transformed to the indices as below-
Thymus Weight (mg)
Thymosoinatic Index = — --------—"—~ xlOO
Body Weight (g)
Spleen Weight (mg)
Splenosomatic Index = ------------------- <100
Body Weight (g)
„ , ■ т i Testis Weight (mg)
Gonadosomatic Index = --------——-—— xlOO
Body Weight (g)
The excised tissue was fixed in Bouin’s fluid. Paraffin sections (5µ thick) were prepared, stained with Ehrlich’s acid hematoxylin and eosin, and mounted in DPX.
Statistical Analysis
The Data are expressed as Mean ± SEM. Statistical analysis of the data is done by One Way ANOVA. Post hoc analysis was done using Newman Keuls multiplerange test.
RESULTS
Thymosomatic index was lesser in summer and rainy months. It started increasing from month of October when temperature and photoperiod started decreasing. It remained high up to month of March (Fig. 1). Splenosomatic index varied between 8.51 to 48.40 mg/100 g body weight during the different months of the study. It started increasing from May and remained high up to August (the hot and humid months of the year and reproductively active months). Lowest spleen mass was recorded during autumn and winter months (Fig. 2). Gonadal mass was also analyzed in lizards to relate the reproductive status of the lizard with immune parameter. Gonadosomatic index was low from September to March. It started increasing from April and remained at highest level from May to August (Fig. 3). Total leucocytes count was found to be in the range of 8810 per mm3 to 16590 per mm3 in the garden lizard during the whole period of study from. It started increasing from July and remained more or less in higher side up to month of February next year. It again remained low during March to May next year and again increasing in June-July. However, when the variation was analyzed with ANOVA, it was found to be insignificant (Fig. 4). Eosinophil number was found to increase in month of June to August, then to decrease in September to October and again to increase during November to February. It again decreased during March to May (Fig. 5). Variation in basophil number between different months was found to be significant (Fig. 6). Neutrophil number in lizard blood was found to be low during summer and rainy season when days were long and hot It started increasing following October and remained almost higher during winter and spring months (Fig. 7). Lymphocyte number varied significantly (p>0.05) between different months of a year, but a definite pattern was not found in the variation (Fig. 8). Monocyte number varied between months insignificantly, but the variation did not show any definite pattern (Fig. 9). The number of NBT positive cells remained low during June-July, but increased abruptly during Aug-Sept; it again decreased significantly in Oct and continued to remain low in winter months up to Jan. Increased number of NBT positive cells was recorded in Feb and April, that decreased to low level in May (Fig. 10).
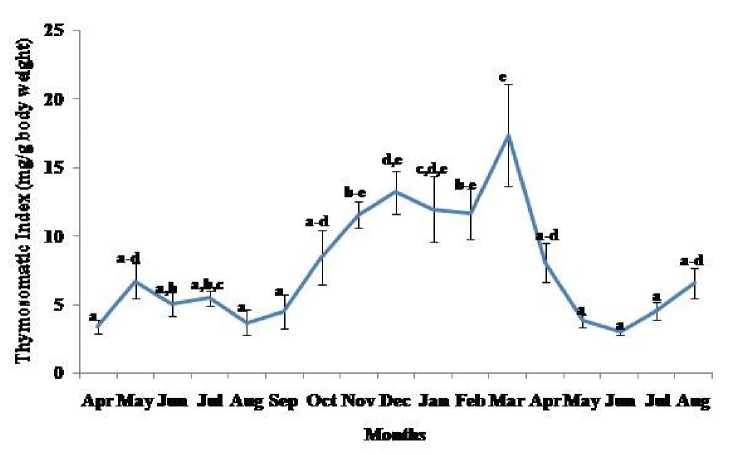
Figure 1. Annual variation in thymus mass (Mean ± SEM) in the garden lizard, Calotes versicolor . Data were analyzed by ANOVA. Post hoc analysis was performed using Neuman Keuls Multiple Range Test (P < 0.05).
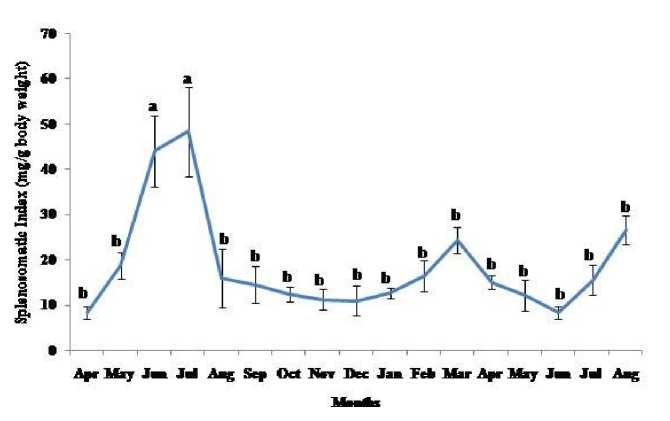
Figure 2. Annual variation in spleen mass (Mean ± SEM) in the garden lizard, Calotes versicolor . Data were analyzed by ANOVA. Post hoc analysis was performed using Neuman Keuls Multiple Range Test (P < 0.05).
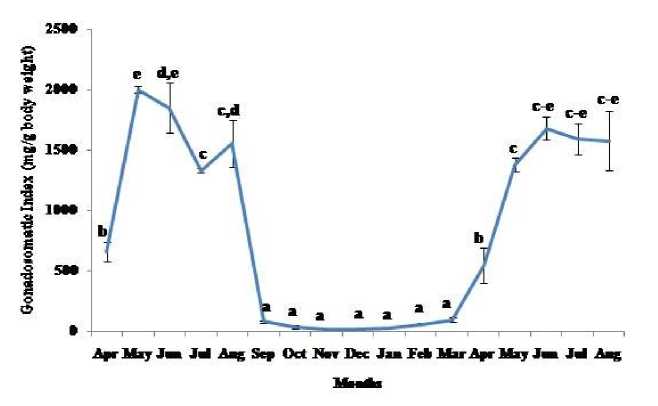
Figure 3. Annual variation in gonad mass (Mean ± SEM) in the garden lizard, Calotes versicolor . Data were analyzed by ANOVA. Post hoc analysis was performed using Neuman Keuls Multiple Range Test (P < 0.05).
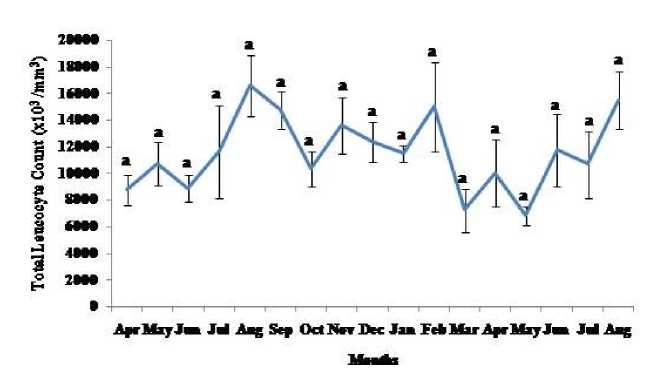
Figure 4. Seasonal variation in total leucocyte count (Mean ± SEM) in the garden lizard, Calotes versicolor . Data were analyzed by ANOVA. Post hoc analysis was performed using Neuman Keuls Multiple Range Test (P < 0.05).
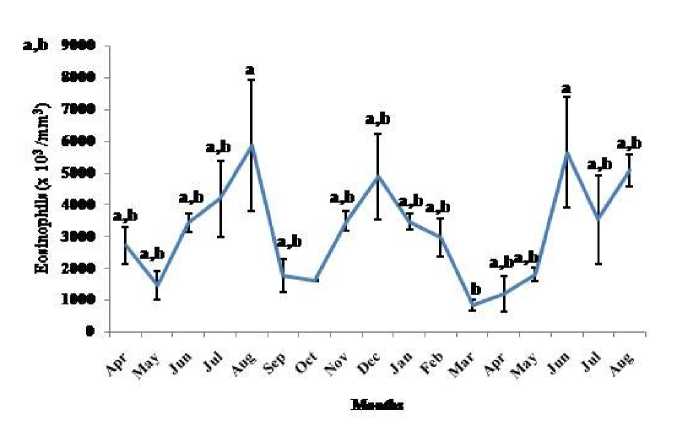
Figure 5. Seasonal variation in eosinophil number (Mean ± SEM) in the garden lizard, Calotes versicolor . Data were analyzed by ANOVA. Post hoc analysis was performed using Neuman Keuls Multiple Range Test (P < 0.05)
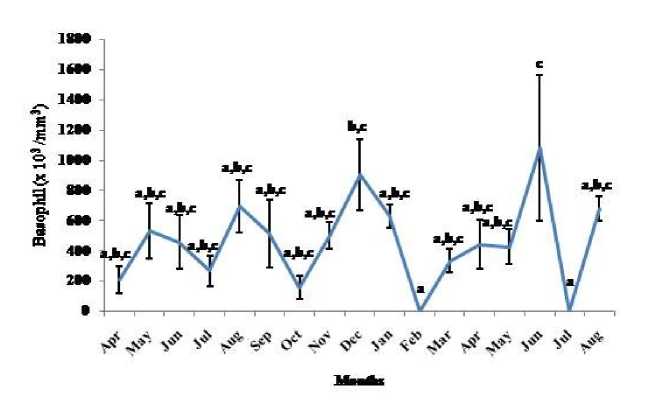
Figure 6. Seasonal variation in basophil number (Mean ± SEM) in the garden lizard, Calotes versicolor . Post hoc analysis was performed using Neuman Keuls Multiple Range Test (P < 0.05).
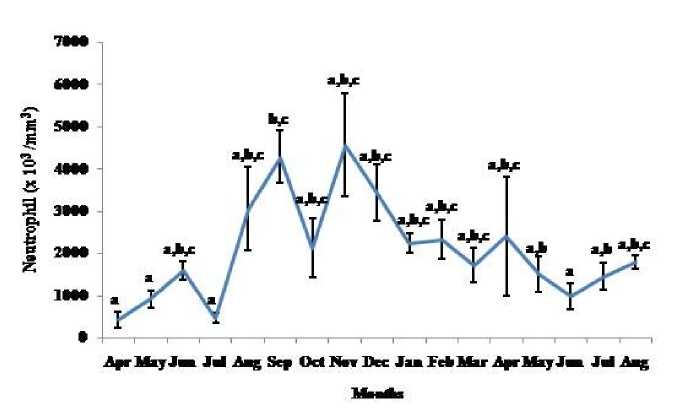
Figure 7 . Seasonal variation in neutrophil number (Mean ± SEM) in the garden lizard, Calotes versicolor . Post hoc analysis was performed using Neuman Keuls Multiple Range Test (P < 0.05).
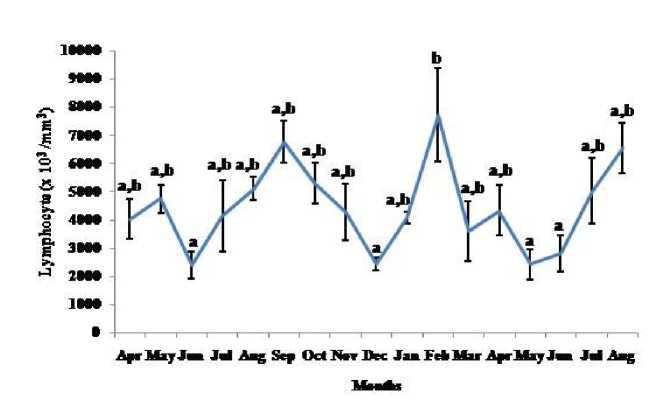
Figure 8. Seasonal variation in lymphocyte number (Mean ± SEM) in the garden lizard, Calotes versicolor . Post hoc analysis was performed using Neuman Keuls Multiple Range Test (P < 0.05).
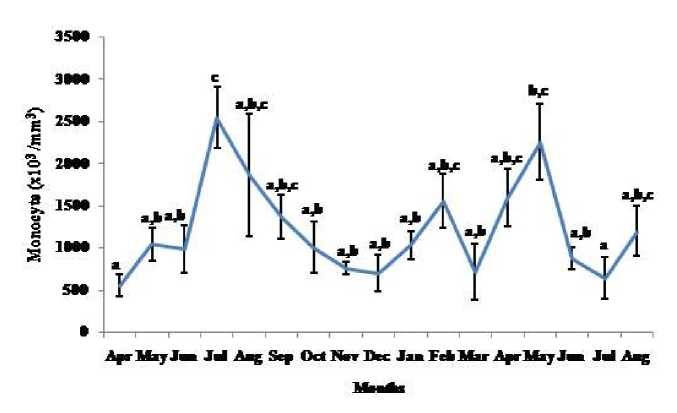
Figure 9. Seasonal variation in monocytes number (Mean ± SEM) in the garden lizard, Calotes versicolor . Post hoc analysis was performed using Neuman Keuls Multiple Range Test (P < 0.05).
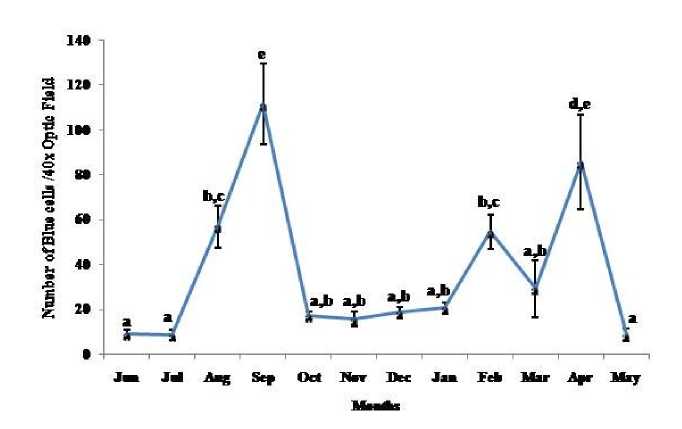
Figure 10. Annual variation in oxidative burst (NBT Reduction Test-Slide Assay) in the garden lizard, Calotes versicolor. Data were analyzed by ANOVA. Post hoc analysis was performed using Neuman Keuls Multiple Range Test (P < 0.05).
DISCUSSION
Two pairs of thymic lobes have consistently been found present in the lizard. The morphological features described in the present investigation are generally in agreement with the studies by El Ridi et al (1981) and Hussein et al (1978a, b). Spleen is a secondary lymphoid organ. It contains specialized vascular spaces, meshwork of reticular cells and rich supply of macrophages. The blood may move through spleen with little filtering or enter the splenic reticular tissue where macrophages are plentiful. The proportion of white pulp and germinal center increased in spleen of lizard during the months from June to August. Germinal centre is known as the centre of lymphocyte proliferation. It seems that the proliferated lymphocytes released in circulation are responsible for increased lymphocyte count during these months. Also, these months are hot and humid, and these environmental conditions are suitable for flair up of microbial and fungal pathogens. Lymphocyte mediated immune response against these pathogens may be the defense prerequisite and that may be responsible for increased lymphocyte count and increased proportion of germinal centre/white pulp in spleen. During the period June to August, the garden lizard is reproductively active. The metabolic activity will be correlated with enhanced RBC production as well as wear and tear of these corpuscles, and these factors may also be responsible for increased size of spleen, as the spleen is the discriminatory filter organ placed in path of blood circulation.
Seasonal cycles of regression and regeneration and development are reported to be the feature of reptilian thymus (Nelson and Demas, 1996; Nelson et al. , 2002) Seasonal changes in thymic mass are apparently first reported in turtles, Clemnys leprosa and Testudo mauritonica by Aime (1912), being reduced during winter, regenerated in spring and continued to enlarge throughout summer. In another report on turtle, Mauremys caspica ; thymus attained maximal development in spring and depleted in summer (Leceta and Zapata, 1985; Leceta et al. , 1989). Thymus of the lizard Scincus scincus becomes involuted and thymic cells undifferentiated as compared to summer (Hussein et al. , 1978a, b). Thymus begins to regenerate in spring.
In an Egyptian Colubrid snake, El Ridi et al. (1981) have reported thymic mass maximal in spring and autumn and involuted in summer and winter with most of the organ being composed of fibrous tissue. Interestingly in the present study on the garden lizard, C. versicolor , thymic mass was reported to be high during autumn and winter and lesser during spring and summer. Also, an inverse relationship between thymus and gonad seems apparent in this lizard, as revealed by statistical analysis. This observation shows analogy to the fact that testosterone suppresses the immune function (of thymus), and seasonal changes in thymus are due to changes in circulating steroid hormones (Nelson et al. , 2002). Testosterone treatment in turtles is reported to decrease in size of thymus and depletion of lymphocytes (Varas et al. , 1992). Seasonal changes in thymus tissue have been reported in other non-mammalian vertebrate groups as well. Thymus is found regressed in fish species that die following spawning. On other hand other fish species alive after spawning show seasonal change in thymus and the changes are independent of breeding. Well developed thymus seen in spring and summer starts degenerating in autumn and winter (Honma and Tamura, 1984; Alvarez et al. , 1988). In frog ( Rana temporaria ), maximal development of thymus occurs in summer with marked atrophy in winter such that cortex and medulla are not clearly distinct (Plytyez et al. , 1995). Zapata et al. (1992) have suggested that the environment does not trigger seasonal change in thymic mass in anurans; rather, these are regulated by some form of endogenous Circannual rhythm. In avian species also, thymus has been reported to be minimal when the gonads were undergoing vernal recrudescence (Nelson et al. , 2002).
There are very few reports on annual or seasonal variation of leucocytes, the primary immune cells. In other reptilian species, as reviewed by Duguy (1970), the basophil, lymphocyte and neutrophils are found to be minimum during months of hibernation and high during summer months; whereas eosinophil showed reverse pattern, being maximum during hibernation and minimum during summer. In recent observation on the living fossil, tuatara, Sphenodon, Burnham et al. (2006), have reported total leucocyte count as well as lymphocyte and heterophil to be higher in winter and spring months. So far non-human mammals are concerned, significant fluctuation in WBC count has also been reported, but fluctuations are independent of specific seasons in cotton rats, Sigmodon hispidus (Lochmiller et al., 1994). In the same species, reduction in peripheral white blood cell count in response to sheep blood cells injection has been reported in winter, as compared with that in summer. Recently Singh et al. (2020) have reported that photoperiodic modulation affects the responses of blood immune cells in a reptile Natrix piscator. Mann et al. (2000) have reported that circulating number of WBC and neutrophil as well as lymphocyte proliferation in response to mitogens are higher in winter than summer. Periodic change in the number of peripheral white blood cells and its types might result due to several factors like distribution of circulating and marginal cell components of tissues, influx from storage site, cell proliferation and release of de novo cells into circulation as well as cell destruction and removal.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Seasonal variation in hematological and histomorphological parameters in the garden lizard, calotes versicolor
- Aime P. (1912). Note syr le thymus chez les cheloniens. C.R. Soc. Seances Soc. Biol. Fil. 72:889-890.
- Alvarez E., Razquin B.E., Villena A.J., Fierro P.L. and Zapata, A.A. (1988). Alteration in the peripheral lymphoid organs and differential leukocyte count in saprolegnia infected brown trout, Salmo trata L: A morphometric study. Vet. Immunol. Immunopathol., 64, 267-278.
- Bartness T.J., Bradley M.H., Hastings E.L., Bittman, and Goldman B.D. (1993). The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J. Pineal Res.,15, 161-190.
- Brainard G.C., Watson-Whitmeyer R.L., Knobler M. and Lubin F.D. (1988). Neuroendocrine regulation of immune parameters: photoperiod control of spleen in the Syrian Hamsters. Ann. N. Y. Acad. Sci., 540, 704-706.
- Bronson F.H. and Heidman P.D. (1994).Seasonal regulation of reproduction in mammals. In Physiol. of Rep. Vol 2 2nd ed. E. Knobil and J.D.Neill, Eds. Raven Press NY. 541-584.
- Burnham D.K., Keall S.N., Nelson N.J. and Daugherty C.H. (2006). Effects of sampling date, gender, and tick burden on peripheral blood cells of captive and wild tuatara (Spendodon punctatus). New Zealand Journal of Zoology., 33, 241-248.
- Champney T.H. and Mc Murray D.N. (1991). Spleen morphology and lymphoproliferative activity in short photoperiod exposed hamsters. In: Fraschini F and Reiter R.J. (Eds.). Role of Melatonin and Pineal Peptides in Neuroimmunomodulation, Plenum Press, London 219-225.
- Champney T.H., Prado J., Youngblood T., Appel K. and McMurray D.N. (1997). Immune responsiveness of splenocytes after chronic daily melatonin administration in male Syrian hamsters. Immunol. Lett, 58, 95-100.
- Demas G.E., Bartness T.J., Nelson R.J. and Drazen D.L. (2003a). Photoperiod modulates the effects of norepinephrine on lymphocyte proliferation in Siberian hamsters. J. Am. J. Physiol. Regul. Integr. Comp. Physiol., 285, R873-R879.
- Duguy. R. (1970). Numbers of blood cells and their variation. In: Biology of the reptilia Vol.III (Carl Gans and T.S. Parson, editors), Academic Press, London. pp 93-109.
- EL-Ridi R., Badir N. and EL Rouby S. (1981). Effect of seasonal vaaariation on immune system the snake, Psammophis schokari. J. Exp. Zool., 216, 357.
- Festa-Bianchet M. (1989). Individual differences, parasites, and costs of reproduction for bighorn ewes (Ovis Canadensis). J. Anim. Ecol., 58, 785795.
- Goldman B.D. (2001). Mammalian photoperiodic system formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms, 16, 283-301.
- Guerrero J.M. and Reiter R.J. (1992). A brief survey of pineal gland immune system interrelationships. Endocrine Res., 18, 91-113.
- Haldar C. and Rai S. (2007). Melatonin and Immunomodulation in seasonally breeding Mammals. In Hormone Biotechnology, (Ed) Maitra S.K. Daya Publishing House. 187-211.
- Haldar C. and Thapliyal J.P. (1977). Effect of pinealectomy on the annual testicular cycle of Calotes versicolor. Gen. Comp. Endocrinol., 32, 395-399.
- Honma Y. and Tamura, E. (1984). Anatomical and behavioral differences among thre spine sticklebacks-The marine form, the landlocked form and their hybrids. Acta. Zool. doi.org/10.1111/j.1463-6395.1984.tb00812.x
- Hussein M.F., Badir N., EL-Ridi R. and Akef M. (1979a). Lymphoid tissues of snake Spalerosophis diadema in different seasons. Dev.Comp. Immunol., 3, 77.
- Hussein M.F., Badir N., EL-Ridi R. and EL Deeb S. (1979 b). Effect of seasonal variations on lymphoid tissues of the lizard Scincus scincus. J. Exp. Zool., 209, 91.
- Leceta J., Garrido E., Torrobo M. and Zapata A. (1989). Ultrastructural changes in the thymus of the turtle, Mauremys capsica in relation to seasonal cycle. Cell tissue Res., 256, 213-220.
- Leceta J. and Zapata A. (1985). Seasonal changes in the thymus and spleen of the turtle, Mauremys caspica. A morphometrical, light microscopical study. Dev.Comp. Immunol., 9, 653-659.
- Lochmiller R.L., Vesty M.R. and McMurray S. T. (1994). Temporal variation in humoral and cell mediated immune response in a Sigmodon hispidus population. Ecology., 75, 236-245.
- Maestroni G.J.M. (1993). The immunoendocrine role of melatonin. J. Pineal Res.14:1-10.
- Mann D.R., Akibami M.A., Gould K.G. and Ansari A.A. (2000). Seasonal variations in cytokine expression and cell-mediated immunity in male rhesus monkeys. Cell. Immunol., 200, 105-115.
- Nelson R.J. and Demas G.E. (1996). Seasonal changes in immune function. Q. Rev. Biol., 71, 511-548.
- Nelson R.J., Demas G.E., Klein S.L. and Kriegsfeld L.J. (2002). Seasonal Patterns of Stress and Immune Fucntion and Disease, Cambridge University Press, New York.
- Poon A.M.S., Liu Z.M., Pang C.S., Brown G.M. and Pang S.F. (1994b). Evidence for a direct action on the immune system. Biol. Signals., 3, 107-117.
- Prendergast B.J., Edward K.E.W., Yellon S.M. and Nelson R.J. (2002). Photorefractoriness of Immune Function in Male Siberian Hamsters (Phodopus sungorus). J. Neuroendocrinol., 14, 318-329.
- Sheldon B.C. and Verhulst S. (1996). Ecological immunity: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol., 11, 317-321.
- Singh A., Singh R. and Tripathi M.K. (2020). Photoperiodic Manipulation Modulates the Innate and Cell Mediated Immune Functions in the Fresh Water Snake, Natrix piscator. Sci. Rep., 10:14722, doi.org/10.1038/s41598-020-71777-2
- Varas A., Torroba M. and Zapata, A.G. (1992). Changes in the thymus and spleen of the turtle Mauremis caspica after testosterone injection: A morphometric study. Dev. Comp. Immunol., 16, 165-174.
- Wingfield J.C. and Farner D.S. (1993). Endocrinology of reproduction in wild species. Avian Biol. 9:163-327.
- Wingfield J.C. and Kenagy G.J. (1991). Natural regulation of reproductive cycles. In Vertebrate Endocrinology: Fundamentals and Biomedical Implications (ed M Schreibman and R.E. Jones) NY. Acad. Press.181-241.
- Zapata A.G., Varas A. and Torroba, M. (1992). Seasonal variations in the immune system of lower vertebrates. Immunol. Today., 13: 142-147.


