Selection of high yield m6 wheat mutant lines obtained from stem rust resistant parents
Автор: Arabi Mohammed Imad Eddin, Jawhar Mohammed, Al-Shehadah Eyad
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.19, 2023 года.
Бесплатный доступ
Wheat is a major cereal crop grown worldwide. Mutation breeding with the objective to improving grain yield was performed for selection of mutant lines. Dry dormant seeds with approx. 11 % moisture of three Australian genotypes (Mendos, Coorong and Egret) were treated with 100, 160 and 200 Gy Co-60 gamma radiation and mutants were selected between the M1 and M5 generations for yield production under field experimental conditions. Decrease in survival rate (%) of plants was observed. Five seeds from each spike per plant of M1 plants were collected, bulked dose-wise and grown separately as M2. Fifteen promising mutant lines retested in a M5-trial for yield in comparison with the parent cultivars. Significant differences ( P 2) and C-17-31 (976 g/m2) as compared with their mothers and the local control genotype Bouhouth10 (857 g/m2). The obtained results suggest that LD50 (160 Gy) gamma radiation treatment can be useful from breeding point of view for selecting higher yielding wheat plants, and the results showed that the Australian parents had Sr26 resistance gene against stem rust disease caused by Puccinia graminis f. sp . tritici , therefore, their utilization is suggested in wheat breeding programs to achieve resistant cultivars.
Wheat, grain yield, cobalt-60 radiation-induced mutagenesis, genetic variability, mutation, stem rust
Короткий адрес: https://sciup.org/143179375
IDR: 143179375
Текст научной статьи Selection of high yield m6 wheat mutant lines obtained from stem rust resistant parents
Bread wheat ( Triticum aestivum .) is the main cereal crop of the world and is the stable food of millions of people worldwide. Its demand is increasing due to the growing global population together with the nutritional changes in countries with rapidly economic growth (Connor and Minguez 2012; Enghiad et al., 2017). In Syria, wheat production vary from season to season based on climatic conditions and cultivated cultivar. Therefore, development of high yielding wheat cultivars with acceptable quality has always been a main goal of breeding programs around the world (Laidig et al., 2017).
Improvement of wheat depends on the level of genetic variation and the desirable characters (Kahrizi et al., 2010). Mutagenesis is an important tool in crop improvement and is free of the regulatory restrictions imposed on genetically modified organisms (Ahloowalia et al., 2004; Stacy et al., 2021). Induced genetic diversity is a proven strategy in the improvement of all major food crops, and the use of mutagenesis to create novel variation is particularly valuable in those crops with restricted genetic variability (Begna 2021). The use of physical mutagen, like gamma radiation for inducing variation, is well established.
It is well known that gamma rays are shortwavelength electromagnetic irradiations with a high penetration depth that are produced when certain elements undergo radioactive disintegration. It has important effects on plant growth and development by changes of morphological, physiological, biochemical, genetic, and cytological in cells and tissues (Kiani et al., 2022). These induced mutation help to develop many agronomical important traits such as grain yield and improvement tolerance or resistance to abiotic and biotic stresses use in major crops (Zakir 201 ).
Improving grain yield is a main challenge in wheat production for meeting nutritional demands. However, due to the limited genetic diversity among existing wheat genotypes, the development of superior varieties is hampered greatly (Dwivedi et al., 2017). Most of the bread wheat cultivars were developed from local landraces as Syria is the centre of diversity of these species (Al Darvish et al., 2022). In addition, wheat stem rust caused by Puccinia graminis f. sp. Tritici, is a devastating fungal disease of wheat in Syria and worldwide. Thus, the current work aimed at screening wheat superior mutant lines with a high potential grain yield under Syrian field conditions which are typical of Mediterranean environments. In addition, the presence of stem rust resistance Sr26 gene was checked in the used parents .
MATERIALS AND METHODS
Plant materials and irradiation treatment
Dried seeds (approx. 11% moisture content) of the three bread wheat genotypes from Australia (Mendos, Coorong and Egret) were irradiated by doses of 100, 160 and 200 Gy from a Co60 source at the Atomic Energy Commission of Syria. Grains were planted immediately after irradiation in order to obtain M 1 plants. Data on seed germination and surviving plants were recorded considering whole plots of M1 population. The local check genotype Bouhouth10 was introduced in the experiments.
Field experiments
Field experiments were performed under natural disease infection in Syria, at a site of 970 m altitude (550-mm rainfall average). The M 1 generation was grown according to the standard agricultural practice in a bulk. Soil fertilizers; 50 kg/ha of nitrogen in the form of Urea (46%) were drilled in equal portions before sowing and after tillerring, and 27 kg/ha superphosphate (33% P 2 O 5 ) was drilled before sowing. . The untreated seeds (0 Gy) from all the wheat genotypes were also planted after every five rows as control for comparison with the M1 population.
Plant selection from M1 to M5 generations
Single spikes were harvested from each plant in order to develop the M2 generation. The plants of M3 and M4 generations were planted in randomized blocks in three replications. Selection of the best lines from M1 to M5 was carried out based on individual plants. The best lines were tested with their parent variety in order to select advanced mutants. Grains of the best mutants were individually selected in each generation. After harvesting the M5 plants, the high-yielding potential mutant lines were selected.
1000-grain weight and yield estimation
The three central rows of each plot of each mutant line were harvested for yield measurements. After threshing 1000 seeds of each genotype were weighed using an electrical balance and mean values were used for data analysis.
DNA extraction from wheat parents
Genomic DNA was isolated from young leaves the three Australian parents (Mendos, Coorong and Egret) using the CTAB extraction method described by Doyle and Doyle (19 7). DNA concentration was determined by the use of BioSpecNanoDNA spectrophotometer. Quantified DNA samples were diluted to 25 ng/μL.
Sr26 Analysis
The reaction consisted of 1x PCR buffer, 0.2 mM of each dNTP, 40 nM forward Sr26#43-F primer (5'- AAT CGT CCA CAT TGG CTT CT -3), 300 nM reverse primer, 300 nM Sr26#43-R primer (5'- CGC AAC AAA ATC ATG CAC TA -3') (Mago et al., 2005), 0.1 L Taq polymerase (MBI Fermentas, York, UK), and 40 to 0 ng genomic DNA in a final volume of 12 L. The PCR cycling conditions were 95C for 2 min, 35 cycles of 94 C for 60 sec, 60C for 60 sec, and 72C for 60 sec with a final extension at 72C for 10 min. Amplifications were performeda Gene Amp 9700 Thermocycler (Applied Biosystems, USA).
Statistical analysis
Data was subjected to analysis of variance using the STAT-ITCF statistical programme (2nd Version). Means were compared using Newman-Keuls test at 5% probability level (Anonymous 19 ). The LD 50 was calculated using the survival percentage of irradiated vs nonirradiated seedlings, in which a larger genetic variability is expected to be found in advanced wheat mutant lines.
RESULTS AND DISCUSSION
The results on survival rate of plants at maturity derived from irradiated seeds are graphically illustrated (Fig. 1). Survival of plants decreased to a considerable level as compared to non-irradiated seeds (Fig. 1). In case of 160 Gy, it was 50% while it was 95% in the non- irradiated population. Different effects of gamma irradiation on survival (%) of wheat have been documented (Borzouei et al., 2010; Ahumada-Flores et al., 2021).
Significant differences ( P <0.05) in 1000-grain weight and grain yield were detected among mutants, with values being consistently higher in promising mutants lines than in the parents. Fifteen mutants were selected and evaluated according to the results of the grain yield conducted up to M5. Table 1 shows the statistical procedure of grain yield of wheat mutants and parents. Higher mean grain yield were found in the two promising mutants C-17-3 (1061 g/m2) and C-17-31 (976 g/m2) as compared with their mothers and the local control genotype Bouhouth10 ( 57 g/m2). (Table 1; Fig 2).
On the other hand, statistical analysis showed highly significant difference for 1000-grain weight among wheat lines shown in Table 1. The maximum 1000 grains weight was found in mutant line C-17-3 (34.33g), while the minimum 1000 grains weight was recorded in line E-26-3 (29.11g). The result indicates that mutant line C-17-3 performed better in soil and climatic condition of Syria.
The increase in wheat grain yield may be attributed to the mutagen induced enhanced mitotic division, alteration in physiological, biochemical and metabolic pathways and interaction of mutagens with yield governing genes (Louali et al., 2015; Ahmed et al., 201 ; Hong et al., 2022). Al-Salhi et al. (2004) and Hameed et al. (200 ) reported that seeds irradiation with gamma rays effects protein synthesis, leaf gas exchange, Leaf water potential, hormonal balance and enzyme activity which might be the reasonable explanations of the different effects of gamma radiations on the grain yield in the current investigation. However, the present results suggest that gamma rays in general LD 50 (160 Gy) can be useful from breeding point of view for selecting higher yielding wheat plants in early generations.
On the other hand, Gamma rays are efficient in broadening genetic variation and increasing grain yield of wheat cultivars, helping plant breeders to perform an efficient selection in the mutated generations (Al-Naggar et al., 2007; Bano et al., 2017). Frey (1969) reported that mutagen derived variability for quantitative characters in agricultural crops is heritable and response to selection is suitable. We assumed that the causes of the higher yields of our 15 selected mutants must have been phenotypical traits rather than physiological traits under the growing reasons. Firstly, the differences in traits between higher yield mutants and their originals were consistent over the years. Secondly, we used phenotypic traits for our selection of the mutants. In general, gamma irradiation might have different effects on traits through the production of free radicals including plant yield (Kiani et al., 2022).
A dominant marker Sr26, was identified in the used Australian parents (Mendos, Coorong and Egret) (Fig. 3), therefore, their utilization is suggested in wheat breeding programs to achieve resistant cultivars. Moreover, Our results are in agreement with previous works (Jamil and Khan 2002; Bano et al., 2017) reported that higher yield were observed from wheat mutants. Mutant populations have also been used widely to improve yield of many cereal crops, including rice (Viana et al., 2019), durum wheat (Louali et al., 2015) and the cowpea (Raina et al., 2022).
Table 1. Grain yield and 1000-grain weight of wheat mutants as compared with the local genotype.
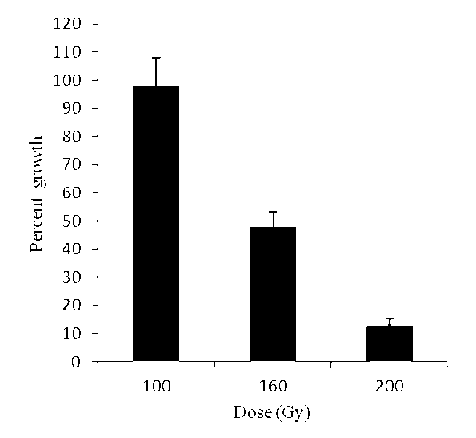
Figure 1. Effects of different doses of gamma irradiation on plant survival of wheat genotypes
|
No. |
Lines |
grain yield (g/m2) |
1000-grain weight (g) |
|
1 |
C-17-3 |
1061 |
34.33 |
|
2 |
C-17-31 |
976 |
33.12 |
|
3 |
C-17-10 |
4 |
32. 5 |
|
4 |
C-17-33 |
67 |
32.5 |
|
5 |
C-17-20 |
722 |
31.15 |
|
6 |
C-17-11 |
71 |
31.1 |
|
7 |
E-26-4 |
6 9 |
29.55 |
|
C-17-6 |
6 5 |
29. 5 |
|
|
9 |
C-17-4 |
676 |
29.3 |
|
10 |
E-26-14 |
662 |
29.55 |
|
11 |
C-17-22 |
610 |
29.44 |
|
12 |
M-27- |
579 |
31.2 |
|
13 |
E-26-3 |
520 |
29.35 |
|
14 |
M-27-5 |
443 |
31.2 |
|
15 |
M-27-6 |
324 |
31. 5 |
|
16 |
Bouhouth10 |
739 |
33. |
|
17 |
Mendos (M-27) |
34 |
33.125 |
|
1 |
Coorong (C-17) |
62 |
29.11 |
|
19 |
Egret (E-26) |
394 |
29. |
|
LSD |
101 |
2.1 |

Figure 2. Some wheat mutants obtained from the three Australian genotypes used in this study
Ml 2 3
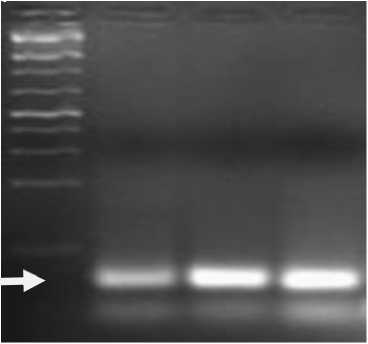
Figure 3 . Agarose gel electrophoresis showing the amplification product of marker Sr26 in Australian parents; Mendos (1), Coorong (2) and Egret (3). M, molecular weight marker (HinfI; MBI Fermentas, York, UK)
CONCLUSIONS
This study aimed to develop M5 mutant lines of spring wheat by using gamma irradiation treatment and identify genetic variability in grain yield. We have successfully selected higher yield 15 mutants retested in a M5-trial for yield in comparison with the parent genotypes. Significant differences (P<0.05) in grain yield and 1000-grain weight were detected among mutants, with values being consistently higher promising mutants lines than in the parents. The two promising mutants C-17-3 and C-17-31 showed the best performance by producing highest 1000-grain weight and hence highest grain yield. However, data suggest that 160 Gy of gamma radiation can be useful from breeding point of view for selecting higher yielding wheat plants in early generations, and the Australian parents having Sr26 resistance gene against stem rust disease can be suggested in wheat breeding programs.
ACKNOWLEDGMENT
The authors would like to gratefully acknowledge the Director General of AECS and the Head of Molecular biology and Biotechnology Department for their much appreciated help during the period of this research. We express gratitude also to Prof. Robert Park (University of
Sydney, Australia) for supplying us with wheat genotypes. Thanks are also extended to Dr. A. Al-Daoude for reading the manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Selection of high yield m6 wheat mutant lines obtained from stem rust resistant parents
- Ahmed MM, Abdalla IG, Salih AM, Hassan AB. (2018). Effect of gamma radiation on storability and functional properties of sorghum grains (Sorghum bicolor L.). Food Sci. Nutr., 7: 1933-1939.
- Ahloowalia BS, Maluszynski M, Nichterlein K. (2004). Global impact of mutation-derived varieties. Euphytica. 135: 187-204.
- Ahumada-Flores S, G ́omez Pando LR, Parra Cota FI,Cruz Torres ED, Sarsu F, los Santos Villalobos SD. (2021). Gamma irradiation induces changes of phenotypic and agronomic traits in wheat (Triticum turgidum ssp. durum). Appl. Radiat. Isot. 167: 109490
- Al-Darvish M, Al Kaddour A, Bourgol A, Alramadan Y, Hallak Y, Kell S. (2022). Survey and conservation of crop landraces in northwest Syria. Genet. Res. 5: 51-58.
- Al-Naggar AMM, Atta MM, Shaheen AM, Al-Azab KF. (2007). Gamma rays and EMS induced drought tolerant mutants in bread wheat. Egypt. J. Plant Breed., 11: 135-165.
- Anonymous (1988). STAT-ITCF, Programme, MICROSTA, realized by ECOSOFT, 2nd Version. Institut Technique des cereals et des Fourrages, Paris, 55pp.
- Al-Salhi M, Ghannam MM, Al-Ayed MS, El-Kameesy SU, Roshdy S. (2004). Effect of gamma irradiation on the biophysical and morphological properties of corn. Nahrung., 48: 95-98.
- Connor DJ, Minguez MI. (2012). Evolution not revolution of farming systems will best feed and green the world. Global Food Secur., 1: 106-113.
- Begna T. (2021). Role and economic importance of crop genetic diversity in food security. J. Agric. Sc. Food Technol., 7: 164-169.
- Bano S, Soomro ZA, Kaleri AA, Akram R, Nazeer S, Laghari AL, Chandio IA, R. Keerio R, Wahocho NA. (2017). Evaluation of M2 wheat (Triticum aestivum L.) mutants for yield and its contributing traits. J. Basic Appl. Sci., 13: 359-362.
- Borzouei A, Kafi M, Khazaei H, Naseriyan B, Majdabadi A. (2010). Effects of gamma radiation on germination and physiological aspects of wheat (Triticum aestivum L.) seedlings. Pak. J. Bot., 42: 2281-2290.
- Doyle JJ, Doyle JL. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull., 19: 11-15.
- Dwivedi SL, Scheben A, Edwards D, Spillane C, Ortiz R. (2017). Assessing and exploiting functional diversity in germplasm pools to enhance abiotic stress adaptation and yield in cereals and food legumes. Front. Plant Sci., 8: 1461.
- Enghiad A, Ufer D, Countryman A, Thilmany D. (2017). An overview of global wheat market fundamentals in an era of climate concerns. Int. J. Agron., 19: 1-15.
- Frey KJ. (1969). Release of mutagen-induced genetic variability in oats by outcrossing. Japan J. Genet. 44: 396-403.
- Jamil M, Khan U.Q. (2002). Study of genetic variation in yield and yield components of wheat cultivar Bukhtwar-92 as induced by gamma radiation. Asian J. Plant Sci., 1: 579-580.
- Hameed A, Mahmud TS, Atta BM, Haq MA, Sayed H. (2008). Gamma irradiation effects on seed germination and growth, protein content, peroxidase and protease activity, lipid peroxidation in desi and kabuli chickpea. Pak. J. Bot., 40: 1033-1041.
- Hong MJ, Kim DY, Jo YD, Choi H I, Ahn JW, Kwon SJ, Kim SH, Seo YW, Kim JB. (2022). Biological effect of gamma rays according to exposure time on germination and plant growth in wheat. Appl. Sci., 12: 3208.
- Laidig F, Piepho HP, Rentel D, Drobek T, Meyer U, Huesken A. (2017). Breeding progress, environmental variation and correlation of winter wheat yield and quality traits in German official variety trials and on-farm during 1983–2014. Theor. Appl. Genet., 130: 223-245.
- Louali Y, Belbekri N, Bouldjej R, Ykhlef N, Djekoun A. (2015). Effect of gamma irradiation on morphological, biochemical, physiological character and cytological studies, of durum wheat mutants. Int. J. Advanced Res., 3: 246-256.
- Kahrizi D, Maniee M, Mohammadi R et al. (2010). Estimation of genetic parameters related to morpho-agronomic traits of durum wheat (Triticum turgidum var. durum). Biharean Biol., 4: 93-97.
- Kiani D, Borzouei A, Ramezanpour S. et al. (2022). Application of gamma irradiation on morphological, biochemical, and molecular aspects of wheat (Triticum aestivum L.) under different seed moisture contents. Sci. Rep., 12: 11082.
- Mago R, Bariana HS, Dundas IS, Spielmeyer W, Lawrence GJ, Pryor AJ, Ellis JG. (2005). Development of PCR Markers for the Selection of Wheat Stem Rust Resistance Genes Sr24 and Sr26 in Diverse Wheat Germplasm. Theor. Appl. Genet., 111: 496-504.
- Raina A, Laskar RA, Wani MR, Jan BL, Ali S, Khan S. (2022). Gamma rays and sodium azide induced genetic variability in high-yielding and biofortified mutant lines in cowpea (Vigna unguiculata L.). Front. Plant Sci., 13: 911049.
- Stacy DS, Laurie JD, Bilichak A, Kumar S, Singh J. (2021). Genetic variation and unintended risk in the context of old and new breeding techniques. Critical Rev. Plant Sc., 40: 68-108
- Viana VE, Pegoraro C, Busanello C, Costa de Oliveira A. (2019). Mutagenesis in rice: The basis for breeding a new super plant. Front Plant Sci., 8: 1326.
- Zakir M. (2018). Mutation Breeding and its Application in Crop Improvement under current environmental situations for biotic and abiotic stresses. Int. J. Res. Stud. Agri. Sci., 4: 1-10


