Shade and drought stress-induced changes in phenolic content of wild oat (Avena fatua L.) seeds
Автор: Gallagher Robert S., Granger Kristen L., Keser Lidewij H., Rossi Jairus, Pittmann Dennis, Rowland Sebastian, Burnham Mark, Fuerst E. patrick
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.6, 2010 года.
Бесплатный доступ
Plants develop under a wide range of maternal environments, depending on the time of emergence, prevailing competition from other plants, and presence or absence of other biotic or abiotic stress factors. Stress factors, such as light limitation and drought, during plant development typically reduces the reproductive allocation to seeds, resulting in fewer and often smaller seeds. Such stress factors may also influence seed quality traits associated with persistence in the soil, such as seed dormancy and chemical defense. For this research, we hypothesized that light limitation and drought during wild oat (Avena fatua L.) seed development would result in reduced allocation to seed phenolics and other aliphatic organic acids previously identified in the seeds of this species. Wild oat isolines (M73 and SH430) were grown in the greenhouse under cyclic drought conditions (2005 only) or two levels of shade (50 and 70%; 2005 and 2006) achieved with standard black shade cloth. The soluble and cellular bound chemical constituents were identified and quantified using gas chromatography - mass spectrometry. The shade and drought stress treatments often significantly affected the mass of the caryopsis and hull seed fractions, as well as the phenolic content of these seed fractions, depending upon isoline, seed fraction, phenolic fraction, and specific phenolics analyzed. Phenolic content of the hull was reduced by the stress environments by up to 48%, whereas there was some evidence of an increase in the soluble phenolic content of the caryopsis in response to the stress environments. Ferulic and p-coumaric acids were the most abundant phenolic acids in both soluble and bound fractions, and bound phenolics comprised generally 95% or more of total phenolics. There was no discernable evidence that the aliphatic organic content was affected by the stress environments. Our results indicate that plant stress during seed development can reduce both the physical and chemical defense in seeds, which may result in seeds that are less persistent in the soil seed bank and potentially less of a weed management concern.
Seed chemistry, secondary compounds, seed defense, seed longevity
Короткий адрес: https://sciup.org/14323505
IDR: 14323505
Текст научной статьи Shade and drought stress-induced changes in phenolic content of wild oat (Avena fatua L.) seeds
Abbreviations : Gas Chromatography - Mass Spectrometry (GC/MS); Selective Ion Monitoring (SIM) .
Wild oat (Avena fatua L.) is a dominant weed species in many of the dryland small grain growing regions around the world. The pernicious nature of this species is related to its longevity in the soil seed bank, prolonged emergence phenology and its competitive nature. Wild oat seeds persist in the soil weed seed bank for up to 9 years, depending upon environment, cropping system, and genetics (Chancellor, 1976; Holm et al., 1977). In eastern Washington, wild oat typically emerges over an extended period during the spring and early summer following accumulations of 200 to 700 growing degree (°C) days (Page et al., 2007a, 2007b). During this time, fall planted crops, such as winter wheat, are already well established and have a substantial competitive advantage over wild oat. In contrast, spring planted crops, such as spring wheat or pulse crops, are less competitive with wild oat. The degree of competition wild oat experiences, in conjunction with abiotic factors such as water and nutrient availability, will influence not only reproductive output (reviewed by Bazaaz et al., 2000), but also possibly the seed quality traits associated with seed persistence in the soil, such as primary dormancy, resistance to pathogens and oxidative stress tolerance (reviewed by Gallagher and Fuerst, 2006). Understanding the environmental conditions that result in sub-optimal seed quality in wild oat is essential for the development of threshold-based criteria for its management, which is the cornerstone of integrated pest management (IPM) systems.
Wild oat seeds that develop under drought and shade conditions have substantially lower levels of primary dormancy and a shorter seed bank persistence than seed that developed under nonstressed conditions (Peters, 1982; Sawhney and Naylor 1982). In addition to being less dormant, wild oat seeds from stressed environments (i.e. drought or shade) tended to have a lower mass and lighter pigmentation (visual observation) than seeds from the non-stressed environments (R.S. Gallagher, unpublished). Reductions in seed mass can negatively impact seedling vigor (Gallagher and Fuerst, 2006), whereas brownish pigmentation may be linked to the presence of secondary compounds, such as phenolics (Debeaujon et al., 2000). Although in some plant tissues, such as leaves, phenolic compound concentration can increase in response to stressors such as drought to protect the photosynthetic machinery from oxidative damage (Hura et al., 2008), our observations of lighter pigmentation, as well as a study on soybean (Caldwell et al., 2005), suggest that drought could decrease the amount of phenolics in seeds. We hypothesized that light limitation or drought stress during seed development will reduce the allocation of secondary chemical constituents associated with seed persistence and vigor. These constituents include phenolic compounds (acids and alcohols) and relatively (10 C or less) short-chained aliphatic organic acids (Gallagher et al., 2010). Phenolics may serve multiple roles in seeds, including the promotion of seed dormancy, deterring pathogen attack, and slowing oxidative degradation of cell constituents (reviewed by Shirely, 1998; Gallagher and Fuerst, 2006). The primary aliphatic organic acids found in wild oat seeds are associated with the tricarboxylic acid cycle (succinic, fumaric and malic acids) (Aldasoro and Nicolas, 1980) and may have direct antimicrobial activity (Leeming et al., 1986) or play a role in induction of phenolic-based defense from pathogens (Jung et al., 2009).
The objective of this study was to identify and quantify the phenolic and aliphatic organic acid constituents in wild oat seeds that developed under light limitation (i.e. shade) or drought stress, and compare the chemical composition and concentration of these compounds to those from plants grown under unstressed conditions.
MATERIALS AND METHODS
Plant Materials. The wild oat seeds for this research were from the isolines SH430 and M73 commonly used in seed ecology/physiology studies for their non-dormant and highly dormant characteristics, respectively (Adkins et al., 1986). The first grow-out for this experiment was conducted from July to November 2005 in a greenhouse at the plant growth facilities located at Washington State University (WSU), Pullman WA, and consisted of: 1) no stress; 2) drought stress; 3) 50% shade, and 4) 70% shade treatment regimes. Since we anticipated a large difference in the total number of seeds per plant produced among the stress treatments, a different number of pots (1 plant per pot) were used for each regime. There were 31 pots for the ‘no stress’; 168 pots for the ‘drought stress’; 50 pots for the ‘50% shade’; and 140 pots for ‘70% shade’. Pots were arranged in single stress treatments clusters, separated by polyester row cover, to prevent cross pollination among the treatments and isolines (Imam and Allard, 1965). Pots measuring 10 x 10 x 35 cm (Stuewe and Sons, Inc., Tangent, OR) were filled 5 cm from the top with Sun-Gro Sunshine® Mix #1 (SunGro® Horticulture, Vancouver, British Columbia, CA). The mix for each pot contained ~15 g of Osmocote® (19:6:12) slow release fertilizer which was supplemented with biweekly additions of ~100 ml Miracle-Gro® soluble fertilizer solution (1% w/v; 18:18:21 + micronutrients) per pot. Multiple seeds (3-4) were planted per pot and thinned to 1 per pot after 2 wks. Stress regimes were imposed when the plants reached the 5 tiller stage. Drought conditions were simulated by imposing a cyclical watering regime during which the pots were watered to container capacity based on gravimetric water content, allowed to dry to ~30% container capacity for 2 to 3 days and then re-watered to full capacity, for total of six cycles (Fig. 1). This protocol was based on preliminary experiments conducted by our group to ascertain the degree of drought stress that could be imposed without causing plant death. The gravimetric water content was determined by weighing 3 indicator pots for each isoline, but did not factor out the wild oat biomass accumulation in the calculation, which was determined to be small compared to the weight of the potting mix and water. The ‘no stress’ and shade treatment pots were maintained at a water content of 80% of container capacity or higher throughout the grow-out periods. The shade treatments were imposed by using black shade cloth (50% and 70%; Gempler, Madison WI) that was supported on a PVC pipe framework completely surrounding shade treatment clusters. This shade cloth had a negligible effect (<2%) on the red to far-red light ratio of the light reaching the plants (660/730 Sensor, Skye Instruments, Wales UK), but is reported by the manufacturer to reduce ultraviolet light levels. Aphid pests (species unidentified) were controlled as needed using registered insecticides applied at labeled rates. Greenhouse temperatures ranged from 25 to 35°C for the duration of the grow-out period, and natural lighting was supplemented with six 400 W high pressure sodium lamps per 1.2 x 2.4 m greenhouse bench, set to a 16 h photoperiod. Since seed production in the shade treatments was insufficient to provide seeds for all the intended seed ecology experiments, an additional grow-out for the shade and the ‘no stress’ treatments was repeated in 2006 (July – November) at the greenhouses associated with the Department of Crop and Soil Sciences at Penn State (University Park, PA) under similar growth conditions and methodologies as used in the WSU grow-out. Seed harvest began approximately 3 mos after planting, and was accomplished by gently shaking the panicle and catching seeds in a small wash basin on a daily basis. Weekly seed pools were kept separate and stored at -20°C until extracted for the chemical constituents. To evaluate the potential effect of maturation timing with in selected treatments, the weekly seed pools in the 2005 grow-out from the ‘no stress’ and ‘drought stress’ treatment clusters were pooled into four maturation groups based on total seed weight; attempting to achieve four groups of near equal mass, whereas the weekly seed harvests from each respective shade treatments were pooled. For the 2006 grow-out, the weekly seed harvests for each treatment were grouped into two pools: early or late maturing.
Chemical Extraction. The phenolic compounds and aliphatic organic acids constituents in the seeds were extracted and purified by methods outlined by Gallagher et al. (2010). Briefly, fifty seeds from each environment were separated into the caryopsis and hull (palea and lemma) fractions (collectively referred to as “seed fractions”) and lyophilized. The homogenized seed material was extracted by a procedure that resulted in separate soluble and chemically bound (“bound” hereafter) fractions for the hull and caryopsis seed material. The liberated chemical constituents in the bound fraction were the result of hydrolysis with NaOH, whereas the soluble fraction was comprised of the free chemical constituents and the hydrolyzed soluble esters.
Three replicates of 50 seeds each for each isoline, stress environment, and maturation group were extracted according to this procedure.
GCMS Analysis. The chemical constituents in the seeds were identified and quantified by gas chromatography (GC) and mass spectrometry (MS) according to methods nearly identical to those outlined in Gallagher et al., (2010). Briefly, optimal quantities to achieve an acceptable level of MS detection of the extracted seed and chemical fractions were brought to a total volume of 445 ul and derivatized with 45 ul of BSTFA + 1% TMCS (Supleco, Bellefonte, PA) just prior to the initiation of the GCMS analysis. These derivatized samples were analyzed in positive electronic ionization mode on a Thermo Scientific® Trace Ultra® GC with a PTV® inlet, DSQ® MS and a TriPlus® autosampler (Thermo Fisher Scientific, West Palm Beach, FL). The GC column was a 30 m by 0.25 mm ID Rxi®-5ms 30 (Restek, Bellefonte, PA). The injection volume was 1 µl sample + 1 µl internal standard (20 mg l-1 hexachlorobenzene in chloroform) injected simultaneously by the autosampler. Xcalibur® 1.4 (Thermo Fisher Scientific, West Palm Beach, FL) was used as the primary software interface with the GCMS and for data processing. Identification of the individual chemical constituents was based on matching the retention time of a dominant quantification ion and the appropriate ratio of the qualification ions as determined by previous analysis of the BSTFA derivatives of the chemical standards. Quantification of the chemical constituents was based on calibration curves established with the individual chemical standards. Standard curves were based on a ratio of the abundance of the quantification ion at the various calibration levels with the abundance of the quantification ion for the internal standard. Quality control samples were regularly included in the sequence and were comprised of know concentrations of the target compounds. We are reporting a more diverse suite of phenolic compounds than that outlined in our previous study (Gallagher et al., 2010), due to a refinement of the selective ion monitoring (SIM) methodologies as our research evolved. In standard full scan monitoring, base-line MS signal noise can obscure the presence of low concentration compounds which can be readily detected with SIM scans.
Data Analysis. The data were subjected to analysis of variance procedures (GLM) and least squared mean separation tests (95% confidence intervals) in SAS version 9.1 (SAS Institute, 2004). Preliminary analysis revealed no significant trends among the maturation groups within each stress environments. As such, maturation group data were pooled within each stress environment. Data for the 2005 and 2006 grow-outs were analyzed separately since these grow-outs occurred at two different locations, and consisted of different treatment configurations.. Treatments consisted of 1) no stress, 2) drought, 3) 50% shade and 4) 70% shade in the 2005 grow-out, and 1) no stress, 2) 50% shade and 3) 70% shade in the 2006 grow-out. Dependent variables included the mass of the seed fractions, the concentration of the individual chemical constituents within the seed and chemical fractions, and the total phenolic concentration within the seed and chemical fractions.
RESULTS
Seed Mass. The dry mass of the caryopsis and hull seed fractions was often significantly affected by one or more of the stress environments for both seed isolines and grow-out periods (Table 1). In the M73 isoline, shade stress reduced the mass per caryopsis by an average of 22 and 16% compared to the no stress treatment in the 2005 and 2006 grow-outs, respectively. In the SH430 isoline, shade stress did not affect caryopsis mass in 2005, but reduced caryopsis mass by an average of 21% compared to the no stress treatment in the 2006 grow–out. In the M73 isoline, shade stress reduced hull mass by 43 and 38% in the 2005 and 2006 grow-outs, respectively, compared to the no stress treatment. Similar results were observed with the SH430 isoline, with hull mass reduced by 23 and 33% compared to the no stress treatment in the 2005 and 2006 grow-outs, respectively. Drought stress reduced caryopsis mass by 13% and hull mass by 10% in the M73 isoline, but had no effect on mass of the seed fractions in the SH430 isoline (2005 only).
Seed Phenolics – 2005 Grow-Out . Analyses of variance indicated that the effect of stress environments on seed phenolic concentration (ng seed-1) depended on the wild oat isoline. For both isolines, however, the most prominent phenolic constituents in both the bound and soluble fractions were the cinnamic acid derivatives ferulic, p -coumaric, phenylproprionic, and syringic acids; the benzoic acid derivatives OH-benzoic and vanillic; and hydroquinone (an alcohol) ( Tables 2 & 3 ). In addition, low concentrations (<1 ng seed) of catechol, protocatechuic, and cinnamic, hydrocaffeic and sinapic acids were often detected (data not shown). There were no discernable trends for the effects of the stress environments on the concentration of the aliphatic acids in either isoline (data not shown).
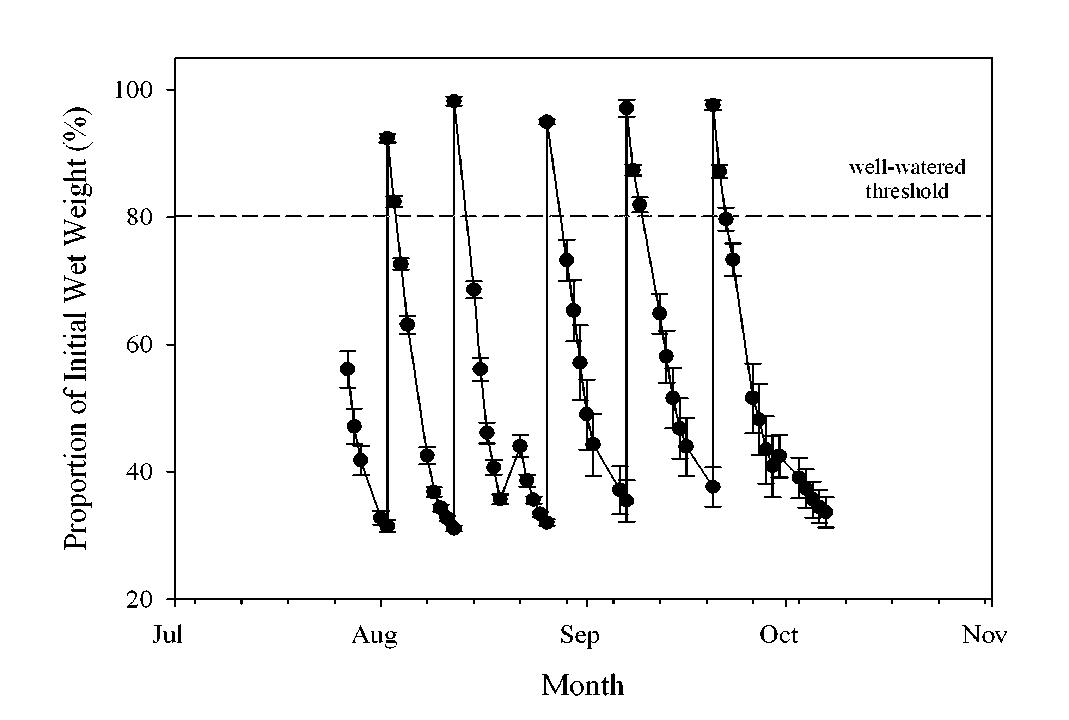
Fig 1. Gravimetric soil water content throughout the life cycle of the drought-grown wild oat plants.
Error bars represent one standard error of the mean.
Seed Maturation Environment
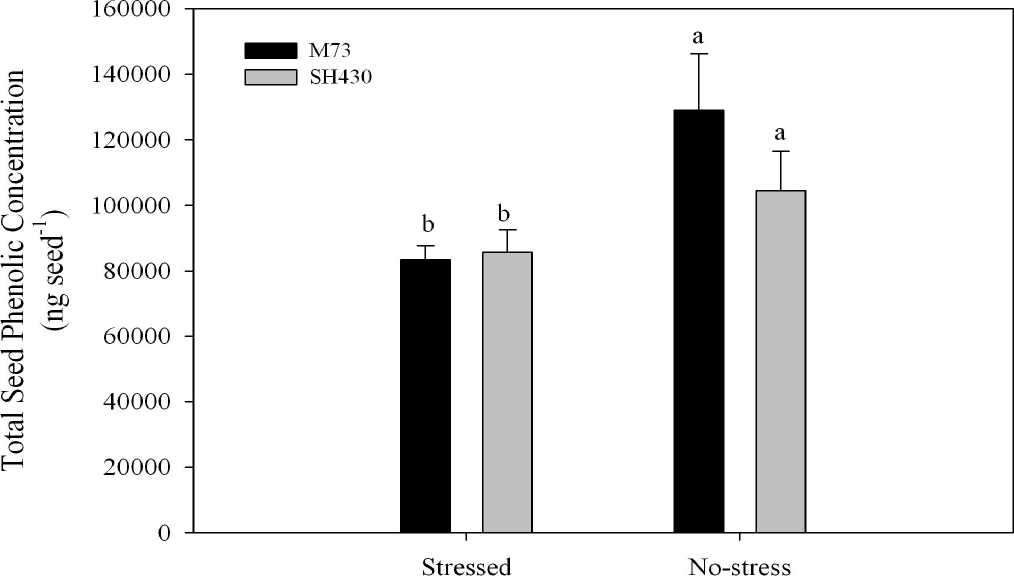
Fig. 2 . Total phenolic concentration (ng seed-1) on a whole seed basis (caryopsis plus hull) for the
2005 grow-out. Data for the drought, 50% shade and 75% shade environments were pooled to comprise the ‘Stressed’ values. Graph values accompanied by the same letters are not significantly different according to a least squared means test at a 95% confidence interval. Error bars represent one standard error of the mean.
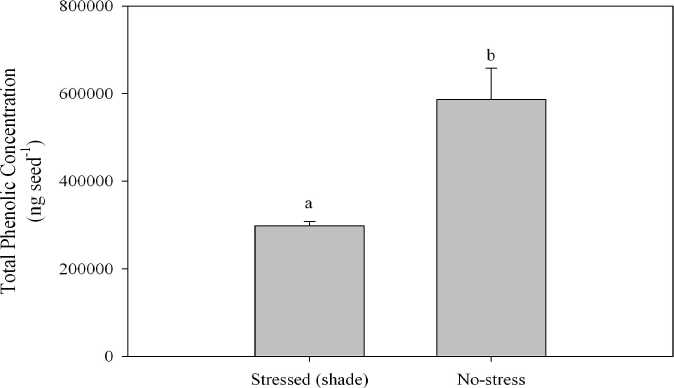
Environment
Fig. 3. Total phenolic concentration (ng seed-1) on a whole seed basis for the 2006 growout. Graph values accompanied by the same letters are not significantly different according to a least squared means test at a 95% confidence interval. Isolines and shade environments were pooled for analysis. Error bars represent one standard error of the mean.
Table 1. The effect of seed maturation environment on dry mass of the caryopsis and hull seed fractions in the two wild oat isolines for the 2005 and 2006 grow-out periods. Means for each isoline within a seed fraction and grow-out period followed by the same letter are not significantly different according to a least squared mean test at a 95% confidence interval.
|
Isoline |
Stress Environment |
Tissue Mass |
|||
|
2005 |
2006 |
||||
|
Caryopsis |
Hull |
Caryopsis |
Hull |
||
|
M73 |
No-stress |
mg seed-1 13.6 a |
6.8 a |
14 a |
8.1 a |
|
50% Shade |
10.5 b |
4.2 b |
11.0 b |
5.6 b |
|
|
70% Shade |
10. 7 b |
3.6 b |
12.5 b |
4.3 b |
|
|
Drought |
11.7 b |
6.1 b |
--- |
--- |
|
|
SH430 |
No-stress |
15.4 a |
7.1 a |
16.9 a |
8.4 a |
|
50% Shade |
16.4 a |
5.6 b |
12.3 b |
5.5 b |
|
|
70% Shade |
16.1 a |
5.2 b |
14.3 b |
5.7 b |
|
|
Drought |
16.1 a |
6.6 a |
--- |
--- |
|
Table 2 . 2005 grow-out of wild oat isoline M73: The effect of shade or drought stress during seed development on the concentrationsof total and specific phenolic constituents. . Means within a column for each seed and chemical fraction followed by the same letter are not significantly different according to least squared means test at the 95% confidence interval.
|
SeedChemical Fraction |
Stress Environment |
Total Phenolics† |
Cinnamic Acid Derivatives |
Benzoic Acid Derivatives |
Alcohols |
||||
|
Ferulic |
p- Coumaric |
Phenyl-proprionic |
Syringic |
OH Benzoic |
Vanillic |
Hydroquinone |
|||
|
Caryopsis-Bound |
No stress |
5457 a |
4273 a |
914 a |
ng seed-56 a |
1 98 a |
17 a |
84 a |
1 b |
|
50% Shade |
4689 a |
3667 a |
739 a |
50 a |
134 a |
20 a |
78 a |
1 b |
|
|
70% Shade |
4525 a |
3672 a |
629 a |
58 a |
49 a |
32 a |
75 a |
1 b |
|
|
Drought |
5256 a |
3972 a |
832 a |
37 a |
236 a |
24 a |
65 a |
11 a |
|
|
Caryopsis-Soluble |
No stress |
477 a |
299 a |
40 b |
9 a |
25 a |
33 b |
53 b |
<1 b |
|
50% Shade |
763 a |
309 a |
104 a |
37 a |
48 a |
78 a |
148 a |
36 a |
|
|
70% Shade |
806 a |
350 a |
128 a |
2 a |
47 a |
79 a |
173 a |
26 a |
|
|
Drought |
351 a |
179 a |
46 b |
3 a |
48 a |
26 b |
47 b |
<1 b |
|
|
Hull-Bound |
No stress |
112474 a |
97440 a |
13837 a |
136 a |
720 a |
35 a |
255 a |
9 a |
|
50% Shade |
71276 b |
57441 b |
12095 a |
175 a |
1290 a |
28 a |
215 a |
11 a |
|
|
70% Shade |
67945 b |
54880 b |
11619 a |
185 a |
814 a |
27 a |
384 a |
6 a |
|
|
Drought |
80686 b |
69860 b |
10331 a |
143 a |
34 a |
28 a |
240 a |
9 a |
|
|
Hull-Soluble |
No stress |
3340 a |
2467 a |
359 a |
97 a |
55 a |
33 a |
310 a |
1 c |
|
50% Shade |
2525 b |
1688 b |
250 a |
171 a |
89 a |
33 a |
251 a |
42 a |
|
|
70% Shade |
2058 b |
1322 b |
206 a |
166 a |
75 a |
30 a |
227 a |
31 b |
|
|
Drought |
1910 b |
1262 b |
191 a |
60 a |
122 a |
22 a |
237 a |
1 c |
|
† A sum of all phenolic compounds detected in the samples.
JOURNAL OF STRESS PHYSIOLOGY & BIOCHEMISTRY Vol. 6 No. 4 2010
Table 3 . 2005 grow-out of wild oat isoline SH430: The effect of shade or drought stress during seed development on the concentrationsof total and specificphenolic constituents. Means within a column for each seed and chemical fraction followed by the same letter are not significantly different according to least squared means test at the 95% confidence interval.
|
SeedChemical Fraction |
Stress Environment |
Total Phenolics† |
Cinnamic Acid Derivatives |
Benzoic Acid Derivatives |
Alcohols |
||||
|
Ferulic |
p- Coumaric |
Phenyl-proprionic |
Syringic |
OH Benzoic |
Vanillic |
Hydroquinone |
|||
|
Caryopsis-Bound |
50% Shade |
7966 a |
5953 a |
868 a |
ng seed- 61 a |
1 803 a |
24 a |
87 a |
2 a |
|
70% Shade |
8472 a |
7062 a |
961 a |
58 a |
43 a |
29 a |
109 a |
<1 a |
|
|
Drought |
6165 a |
4832 a |
848 a |
44 a |
185 a |
32 a |
103 a |
1 a |
|
|
No stress |
6626 a |
5176 a |
897 a |
48 a |
26 a |
30 a |
121 a |
2 a |
|
|
Caryopsis-Soluble |
50% Shade |
1446 a |
616 a |
113 a |
85 a |
245 a |
48 a |
168 a |
2 a |
|
70% Shade |
1069 a |
501 a |
116 a |
57 a |
81 b |
42 a |
134 a |
2 a |
|
|
Drought |
582 b |
301 a |
55 a |
22 a |
39 b |
33 a |
100 a |
1 a |
|
|
No stress |
826 b |
440 a |
56 a |
76 a |
66 b |
54 a |
129 a |
1 a |
|
|
Hull-Bound |
50% Shade |
75769 a |
60235 a |
14775 a |
208 a |
29 b |
41 a |
416 a |
9 a |
|
70% Shade |
62253 a |
47737 a |
13946 a |
137 a |
11 b |
35 a |
352 a |
7 a |
|
|
Drought |
77285 a |
63271 a |
12670 a |
225 a |
557 a |
45 a |
451 a |
9 a |
|
|
No stress |
90946 a |
76474 a |
12591 a |
267 a |
992 a |
50 a |
515 a |
9 a |
|
|
Hull-Soluble |
50% Shade |
2190 a |
957 a |
274 a |
209 a |
363 a |
45 a |
341 a |
<1 a |
|
70% Shade |
1300 a |
619 a |
217 a |
110 a |
57 a |
32 a |
263 a |
<1 a |
|
|
Drought |
2104 a |
1017 a |
282 a |
202 a |
109 a |
48 a |
438 a |
3 a |
|
|
No stress |
2176 a |
1093 a |
281 a |
326 a |
79 a |
46 a |
338 a |
4 a |
|
† A sum of all phenolic compounds detected in the samples.
JOURNAL OF STRESS PHYSIOLOGY & BIOCHEMISTRY Vol. 6 No. 4 2010
с\ с\
Table 4. 2006 grow out: The effect of shade during seed development on the concentrationsof total and specific phenolic constituents. Isolines pooled for the analysis. Means within a column for each seed and chemical fraction followed by the same letter are not significantly different according to least squared means test at the 95% confidence interval.
|
Cinnamic Acid Derivatives |
Benzoic Acid Derivatives |
Alcohols |
|||||||
|
Seed/ Chemical Fraction |
Stress Treatment |
Total Phenolics† |
Ferulic |
p -coumaric |
Syringic |
OH Benzoic |
Vanillic |
Gallic |
Hydroquinone |
|
Caryopsis Bound |
No stress |
33674 a |
24569 a |
2539 a |
ng seed-1 118 a |
1894 a |
202 a |
196 a |
3991 a |
|
50% shade |
28943 a |
17829 a |
1782 a |
177 a |
2739 a |
183 a |
265 a |
5871 a |
|
|
70% shade |
37450 a |
24881 a |
2484 a |
119 a |
1468 a |
227 a |
139 a |
7886 a |
|
|
Caryopsis Soluble |
No stress |
7267 a |
2159 a |
242 a |
176 a |
3251 a |
238 a |
33 a |
1148 a |
|
50% shade |
6444 a |
1502 b |
155 b |
132 a |
2877 a |
200 a |
24 a |
1507 a |
|
|
70% shade |
7615 a |
1696 b |
175 b |
205 a |
3176 a |
268 a |
28 a |
1972 a |
|
|
Hull Bound |
No stress |
583847 a |
491498 a |
42960 a |
170 a |
7969 a |
770 a |
2036 a |
22427 a |
|
50% shade |
272580 b |
208850 b |
28314 a |
106 a |
5976 a |
473 a |
1170 a |
27625 a |
|
|
70% shade |
332516 b |
255538 b |
36026 a |
134 a |
7659 a |
680 a |
2982 a |
29415 a |
|
|
Hull Soluble |
No stress |
19287 a |
12157 a |
1804 a |
212 a |
1937 a |
1042 a |
26 a |
1613 a |
|
50% shade |
6516 b |
2438 b |
482 b |
109 b |
1590 a |
574 b |
13 a |
1306 a |
|
|
70% shade |
8336 b |
2570 b |
561 b |
140 b |
2389 a |
668 b |
33 a |
1974 a |
|
† A sum of all phenolic compounds detected in the samples.
JOURNAL OF STRESS PHYSIOLOGY & BIOCHEMISTRY Vol. 6 No. 4 2010
M73 caryopsis phenolics. In the bound fraction of the M73 caryopsis, there was no evidence that the stress environments affected total phenolic concentration or the concentration of any of the phenolic constituents (Table 2). Total phenolic concentration averaged 4980 ng seed-1 for all treatments, with ferulic and p -coumaric acid constituting 78 and 15% of the total phenolics, respectively. All other compounds comprised 2% or less of the total phenolics. For the soluble fraction of the M73 caryopsis, the stress environments did not affect total phenolic concentration, which averaged 600 ng seed-1 for all the treatments, and was made up of 47% ferulic acid. Concentrations of p -coumaric, OH benzoic and vanillic acids, as well as hydroquinone, were significantly greater in the shade environments compared to the drought and no stress treatments. The pooled concentration of these four compounds was on average 204% higher in the shade environments compared to the no-stress treatment.
M73 Hull phenolics. In the bound fraction of the M73 hull, total phenolic concentration was reduced by 34% in the stressed environments compared to the no stress treatment, averaging 73302 ng seed-1 in the shade and drought environments compared to 112474 ng seed-1 in the no stress treatment (Table 2). Ferulic acid was again the most abundant phenolic constituent, comprising 86% of the total phenolics in the no-stress environment and an average of 82% of the total phenolics in the stressed environments. There was no evidence that the stress environments affected the concentrations of the other phenolic constituents, of which p-coumaric was the most abundant, constituting 12% of the total phenolic concentration. In the soluble fraction of the M73 hulls, the total phenolic concentration was reduced by 35% in the stressed environments compared to the no-stress treatment, averaging 2164 ng seed-1 in the stressed environments compared to 3340 ng seed-1 in the no-stress treatment. Ferulic acid made up on average 65% of the total phenolics in the stressed environments, and 74% of total phenolics in the no-stress treatment. There were no significant differences among the treatments for the other phenolic constituents, which were comprised primarily of p-coumaric (205 ng seed-1) and vanillic (256 ng seed-1) acids. The total phenolic concentration pooled among seed and phenolic fractions for the M73 isoline significantly lower in the stress environments compared to the no stress treatments, average ~81000 ng seed-1 for the stressed environment and ~129000 ng seed-1 for the no-stress treatment (Fig. 2).
SH430 caryopsis phenolics. In the bound fraction of the SH430 caryopsis, there were no significant differences among the treatments for total phenolic concentration or the concentrations of the constituent phenolic compounds. Total phenolics averaged 7307 ng seed-1 for all treatments, and were comprised of 78% ferulic acid and 12% p-coumaric acid. In the soluble fraction of the SH430 caryopsis, total phenolic concentration was on average 52% higher in the shade environments compared no stress treatment. There was a similar trend of higher concentrations of the many of the constituent compounds in the shade environments compared to the no stress treatment, but this was generally not significantly different. Ferulic acid constituted on average 43% of the total phenolic concentration in the shade environments and 53% of total phenolic concentration in the no stress environment. The other prominent phenolic constituents included p-coumaric (43 to 53% of total) and the benzoic acid derivatives (15 to 22% of total).
SH430 hull phenolics. In the bound fraction of the hull, the stress environments had no effect on total phenolic concentrations, averaging 76563 ng seed-1 for all treatments. Ferulic and p -coumaric acid made up 81 and 17% of the total phenolic concentrations, respectively, with the other compounds comprising less than 1% of the total phenolics. Syringic acid concentrations, however, were reduced by 98% in the shade environments compared to the no-stress environment. In the soluble fraction of the hull, the stress environments did not affect total phenolic concentration, averaging 1942 ng seed-1 for all the environments, and were comprised of 47% ferulic and 13% p -coumaric acids, and 19% benzoic acid derivatives. The total phenolic concentration pooled among seed and chemical fraction for the SH430 isoline were significantly lower in the stress environments compared to the no stress treatment, averaging ~85000 ng seed-1for the stressed environment and ~104000 ng seed-1 for the no-stress treatment ( Fig. 2 ).
Seed Phenolics – 2006 Grow-Out. For the wild oat seeds from the 2006 grow-out (Table 4), analyses of variance indicated that the effect of stress environments on seed phenolic concentration was relatively consistent between the two wild oat isolines, and consequently data were pooled across isolines. As in the 2005 grow-out, the most prominent phenolic constituents were the cinnamic acid derivatives ferulic, p-coumaric, and syringic acids; the benzoic acid derivatives OH-benzoic vanillic and gallic acids; and hydroquinone (an alcohol). In addition, low concentrations (<1 ng seed) of phenylproprionic, protocatechuic, cinnamic, hydrocaffeic and sinapic acids, as well as catechol were often detected (data not shown). There were no discernable trends for the effects of the stress environments on the concentration of the aliphatic acids in either isoline (data not shown).
Caryopsis phenolics. In the bound fraction of the caryopsis, the shade environments did not affect the concentration of total phenolics or the concentration of any of the constituent compounds ( Table 4 ). The average total phenolic concentration for the treatments was 33355 ng seed-1, and was made up of 67% ferulic acid, 7% p -coumaric acid, and 7% of the benzoic acid derivatives. In the soluble fraction of the caryopsis, total phenolic concentrations did not differ significantly among the treatments, but there were reductions for some of the constituent compounds. The average total phenolic concentration for the environments was 7108 ng seed-1, and was constituted of 30% ferulic acid and 50% of the benzoic acid derivatives within the no stress treatment. The concentrations of ferulic and p -coumaric acids were on average 26 and 31%, respectively, lower in the shade treatments relative to the no stress treatment.
Hull phenolics. In the bound fraction of the hull, the total phenolic concentrations were 48% lower in the shade environments compared to the no stress treatment, averaging 302548 ng seed-1 in the shade environments and ~540000 ng seed-1 in the no stress treatment. Ferulic acid constituted 84% of total phenolics in the no-stress environment and an average of 76% in the shade environments. In the no stress treatment environment, p-coumaric acid comprised 7% of total phenolics, whereas the benzoic acid derivatives in total were less than 2% of total phenolics. In the soluble fraction of the hull, total phenolic concentrations were 61% lower in the shade environments compared to the no stress treatments, averaging ~7400 ng seed-1 in the shade environments and ~19000 ng seed-1 in the no-stress treatments. Ferulic acid concentrations were reduced by an average of 79% in the shade environments, making up 79% of total phenolics in the no-stress environments, and 33% of total phenolics in the shade environments. Similarly, p-coumaric, syringic and vanillic acids were reduced in the shade environments by an average of 71, 41 and 40%, respectively, compared to the nostress environment. Total phenolics on a whole seed basis ranged from ~280000 to ~590000 ng seed-1, being reduced by an average of 49% in the shade environments compared to the no stress environment (Fig. 3).
DISCUSSION
The shade and drought stress treatments often significantly affected the mass of the caryopsis and hull seed fractions ( Table 1 ), as well as the phenolic content of these seed fractions, depending upon isoline, seed fraction, phenolic fraction, and specific phenolics analyzed ( Tables 2-4 ). In general, hull mass was reduced more than caryopsis mass by the stress environments. Soluble phenolic content was much lower than bound phenolic content, and phenolics were much more abundant in the hull than caryopsis. Ferulic and p -coumaric acids were the predominant phenolic acids in both soluble and bound fractions. Differences in phenolic concentrations among the stress environments in the SH430 seeds ( Table 3 ) were less pronounced than observed in the M73 seeds from the 2005 grow-out ( Table 2 ). However, the two isolines generally yielded similar analyses in the 2006
grow-out period and consequently were pooled for analysis ( Table 4 ).
Based on these results, our initial hypothesis that drought stress and light limitation during seed development can reduce the allocation to the chemical constituents in wild oat seeds was confirmed for total phenolics and some of the individual phenolic constituents, but not for the aliphatic organic acids evaluated in this study. Reduced allocation to seed phenolics appears to be related in part to the reduced allocation to the mass of seed fractions, particularly in the M73 seed from the 2005 grow-out (Table 1). There was, however, strong evidence in the seeds from the 2006 grow-out that reduced allocation to phenolics can be independent of the reduction to seed biomass caused by the stress environments (Table 4). Overall, the shade environments reduced the hull mass by an average 35% across isolines and grow-outs, whereas drought (only evaluated in the 2005 grow-out) reduced hull mass by 10% (Table 1). In the M73 isoline in 2005 and both isolines in 2006, shade reduced bound and soluble phenolic concentrations in the hull by 45%. Drought reduced the phenolic concentration (bound and soluble) of the hull by 28%. Decreases in total phenolic concentrations in the hull on a mass basis (ng g-1 of seed tissue) were evident in both isolines in 2006, averaging a total reduction of 27% in the shade environments compared to the no stress treatment (calculated from values in Tables 1 and 4). These data suggest that abiotic stress during seed development may translate to less robust physical and chemical barriers to microbial invasion (hull or seed coat) that could lead to higher levels of microbial seed decay (Halloin, 1983; Kremer et al., 1984; Kemer, 1993). The hull may also play both a physical and chemical role in the regulation of seed dormancy in wild oat by restricting water uptake and gas exchange and being the source of soluble germination inhibitors (Simpson, 1980). Although there was a correlation in the M73 seeds between seed dormancy and phenolics in the hull, reduced allocation to phenolics also occurred in the non-dormant SH430 isoline, suggesting these phenolics are not sole regulators of primary seed dormancy.
There was some evidence that concentration of soluble phenolics may increase in the caryopsis in response to shade. In 2005, this occurred on both a seed basis (Tables 2 and 3) and a mass basis (data not shown), but only on seed basis in 2006, indicating it was related primarily to the reductions in the caryopsis mass. It is unclear if such soluble phenolics remain in the caryopsis or diffuse into the spermosphere. A number of studies have sought to characterize the composition of seed exudates that passively diffuse from seeds into the surrounding soil. Although there is some variation between species, phenolics and aliphatic organic acids are common components of seed exudates (reviewed by Nelson, 2004). Malic and succinic acids were detected in exudates of Eurasian catchfly seeds (Bruun et al. 2001). These aliphatic acids plus phenolic acids p-hydroxybenzoic, protocatechuic, vanillic, and syringic were detected in pea, cotton, and barley seed exudates (Kovacs, 1971). Additionally, caffeic, p-coumaric, and ferulic acids were found in seed exudates from two Festuca species (Klejdus and Kuban, 2000). This release of compounds from seeds into the spermosphere can have ecological impacts through interactions with soil fauna (Nelson, 2004) or with other seeds and plants (Klejdus and Kuban, 2000), and through effects on micronutrient solubility (Jones, 1998).
We are uncertain whether phenolic concentrations on a per seed or per mass basis are the most ecologically relevant. Each seed is a physiologically distinct reproductive unit; therefore phenolic concentration per seed may be the most pertinent and thus these data have been reported on a seed basis. As previously discussed, soluble chemical constituents are likely to be mobile in the spermosphere; in which case absolute quantity per seed will likely regulate the ecological function. In contrast, however, bound chemical constituents may be evaluated more appropriately on a mass basis if seed-organism interactions are sensitive to localized tissue concentrations.
Total phenolic concentrations in the no stress treatments varied considerably between the two grow-outs. In the 2005 grow-out, total phenolic concentrations were similar those reported previously by our group (Gallagher et al., 2010), but nearly 5 fold higher than in the 2006 grow-out. In the M73 isoline, per plant biomass and seed yield was 76 and 43% lower, respectively in the 2006 versus 2005 grow-out (data not shown). Although the temperature, light and fertility conditions were similar between the two grow-outs, we did note that aphid infestations were visually more prevalent in the 2006 grow-out. These data may indicate aphid-induced increase in seed phenolics. Herbivore-induced defense mechanisms, including those associated phenolic biosynthesis, have been widely reported in vegetative plant tissue (Karban and Myers, 1998), but it is unclear if these mechanisms translate to seeds. Likewise, aphids have been specifically identified to induce phenolic-based (i.e.
salicylic acid) defense mechanisms in Arabidopsis tissue (Moran and Thompson, 2000).
As outlined in our previous report on seed phenolics in wild oat (Gallagher et al., 2010), ferulic acid was the most abundant phenolic compound, constituting an average of 82% of total seed phenolics on a whole seed basis. Previous studies have found that ferulic acid plays important roles, particularly as a component of cell walls—forming linkages that help maintain cell structure (Parr et al., 1976) and protect against pathogens (Ikegawa, et al., 1996). Other prevalent phenolic compounds included p -coumaric, OH-benzoic and vanillic, with OH-benzoic and vanillic being relatively prominent in the soluble fractions of the caryopsis and hull. Although p- coumaric acid has been identified as a possible substrate for polyphenol oxidase (Mayer, 2006), the precise ecological role of this and other compounds remains unclear.
In summary, the environmental conditions in which wild oat seeds mature can influence the phenolic concentrations in the seeds, primarily reflected in the palea and lemma secondary structures. In this experiment, light limitation and drought stress often resulted in reduced biomass allocation to these structures, as well as decreasing their phenolic concentrations (chiefly ferulic and p-coumaric acids). We hypothesize that reduced physical and chemical defense capabilities of the hull may contribute to a shorter primary dormancy period and greater susceptibility to soil pathogens. More research, however, is needed to elucidate the precise ecological and physiological roles of these compounds in seed persistence in the soil. Data from this and related studies (Granger et al., 2009) suggest that weeds developing under highly competitive conditions are less likely to produce seeds that will persist in the soil seed bank and be a source of future weed infestations. As such, weed seed quality traits, such as chemical composition and dormancy level, may be a factors to include when establishing criteria for management thresholds in IPM systems. Such ecologically-based management thresholds will help enable farmers to be less reliant on environmentally-sensitive inputs, such as herbicides and tillage, to control weeds.
ACKNOWLEDGEMENT
We greatly appreciate the GC/MS support provided by Thermo Fisher Scientific (Dr. M. Bonilla, Dr. G. Harkey, B. Drakontaidis and M. Hendry), the Restek Corp, and Dr. E. Conklin. Special thanks to W. McCoy, L. Koller, C. Tien, J. Vincenty, and C. Ye for their technical support on this aspect of the project. We thank Dr. Richard Alldredge (Washington State University) for his guidance on the statistical analysis. Funding for this research was provided in part by the USDA NRI Biology of Weedy and Invasive Species Program (Award No. 2005-35320-15375), The Pennsylvania State University, and Washington State University.
Список литературы Shade and drought stress-induced changes in phenolic content of wild oat (Avena fatua L.) seeds
- Adkins, S.W., Loewen, M., and Symons S. (1986) Variation within pure lines of wild oats (Avena fatua) in relation to degree of primary dormancy. Weed Sci., 34, 859-864.
- Aldasoro, J. and Nicolas, G. (1980) Fermentative products and dark CO2 fixation during germination of seeds of Cicer Arientinum. Phytochem., 19, 3-5.
- Bazaaz, F.A., Ackerly, D.A., and Reekie, E.G. (2000) Reproductive allocation in plants. In Seeds: The Ecology of Regeneration of Plant Communities 2nd, Fenner, M. Ed.; CABI Publishing Wallingford, UK, pp. 1-30.
- Bruun, H.H. Van Rossum, F., and Ström, L. (2001) Exudation of low molecular weight organic acids by germinating seeds of two edaphic ecotypes of Silene nutans L. Acta Oecologica, 22, 285-291.
- Caldwell C.R., Britz, S.J, and Mirecki, R.M. (2005) Effect of temperature, elevated carbon dioxide, and drought during seed development on the isoflavone content of dwarf soybean [Glycine max (L.) Merrill] grown in controlled environments. J. Sci. Food. Agric., 53,1125-1129.
- Chancellor, R.J. (1976) Seed behavior. In Wild Oats in World Agriculture; Jones, D. P., Ed.; Agricultural Research Council: London, UK, pp. 65-87.
- Debeaujon, I., Leon-Kloosterziel, K.M, and Koorneef, M. (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol., 122, 403-413.
- Gallagher, R.S. and Fuerst, E.P. (2006) The ecophysiology of seed longevity. In Seed Science and Technology; Basra, A. S., Ed.; Haworth Food Products Press: Binghamton, New York, pp. 521-557.
- Gallagher, R.S., Ananth, R., Bradley, B., Granger, K.L, Anderson, J.V., and Fuerst, E.P. (2010) Phenolic and short-chained aliphatic organic acid constituents of wild oat (Avena fatua L.). J. Agric. Food Chem, 58,218-225.
- Halloin, J.M. (1983) Deterioration resistance mechanisms in seeds. Phytopath. 73, 335-339.
- Holm L.G., Plucknett D.L., Pancho J.V, and Herberger J.P. (1977) Avena fatua L. and other members of the wild oat group. In The Worlds Worst Weeds: Distribution and Biology; Hawaii Univ. Press: Honolulu, HI, pp. 105-113.
- Hura, T., Hura, K., and Grzesiak, S. (2008) Contents of total phenolics and ferulic acid, and PAL activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J. Agron. Crop Sci., 194, 104-112.
- Ikegawa, T., Mayama, S., Nakayashiki, H., and Kato, H. (1996) Accumulation of diferulic acid during the hypersensitive response of oat leaves to Puccinia coronata f.sp. avenae and its role in the resistance of oat tissues to cell wall degrading enzymes. Physiological and Molecular Plant Pathology, 48, 245-255.
- Jones, D.L. (1998) Organic acids in the rhizosphere -a critical review. Plant and Soil,., 205, 25-44.
- Jung, H.W., and Tschaplinski, T.J., and Wang, L, Glazebrook, J., and Greenberg, J.T. (2009) Priming in systemic plant immunities. Science, 324, 89-91.
- Karban, R. and Myers, J.H. (1989) Induced plant responses to herbivory. Annu. Rev. Ecol. Syst., 20, 331-48.
- Klejdus, B. and Kuban, V. (2000) High performance liquid chromatographic determination of phenolic compounds in seed exudates of Festuca arundinacea and F. pretense. Phytochem. Anal., 11, 375-379.
- Kovacs, M.F. (1971) Identification of aliphatic and aromatic acids in root and seed exudates of peas, cotton, and barley. Plant and Soil., 34, 441-451.
- Kremer, R.J., Hughes, L.B., and Aldrich, R.J. (1984) Examination of microorganisms and deterioration resistance mechanisms associated with velvetleaf seed. Agron. J., 76, 745-749.
- Kremer, R.J. (1993) Management of weed seed banks with microorganisms. Ecol. Applications, 3, 42-52.
- Leeming, J.P.,Holland, K.T., and Bojar, R.A. (1986) The in vitro antimicrobial effect of azelaic acid. Brit. J. Dermatology, 115, 551-556.
- Mayer, A.M. (2006) Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry, 67, 2318-2331.
- Moran, P.J. and Thompson, G.A. (2001) Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol., 125, 1074-1085.
- Nelson, E.B. (2004) Microbial dynamics and interactions in the spermosphere. Annu. Rev. Phytopathol., 42, 271-309.
- Page, E.R., Kemanian, A.R., Fuerst, E.P., and Gallagher, R.S. (2007) Spatially variable patterns of wild oat emergence in eastern Washington. Crop Protection, 26, 232-236.
- Page, E.R., Gallagher, R.S., Kemanian, A.R., Zhang, H., and Fuerst, E.P. (2007) Modeling sitespecific wild oat (Avena fatua) emergence across a variable landscape. Weed Sci., 54, 838-846.
- Parr, A. J, Waldron, K.W., Ng, A., and Parker, M.L. (1976) The wall-bound phenolics of Chinese Water Chestnut (Eleocharis dulcis). J. Sci. Food Agric., 71, 501-507.
- Shirley, B.W. (1998) Flavonoids in seeds and grains: physiological function, agronomic importance and the genetics of biosynthesis. Seed Sci. Res., 8, 415-422.
- Simpson, G. M. (1990) Seed Dormancy in Grasses, Cambridge Univ Press, UK, pp. 67-70.


