Simulation of ethylene oxide production from ethylene cholorhydrin
Автор: Abd Allah Elrafie Ahmed, Kasif Abdel elhameed M.O., Mohamed Yasir Awad Alla, Mahmoud Ayat abdel elkhalig H.
Журнал: Вестник Воронежского государственного университета инженерных технологий @vestnik-vsuet
Рубрика: Химическая технология
Статья в выпуске: 1 (91), 2022 года.
Бесплатный доступ
This research has been performed in the Ethylene Oxide production process. It is a flammable and colorless gas at temperatures above 11 °C. It is an important commodity chemical for the production of solvents, antifreeze, textiles, detergents, adhesives, polyurethane foam, and pharmaceuticals. Small amounts of Ethylene Oxide [EO] are used in manufacturing fumigants and sterilants for spices and cosmetics, as well as hospital sterilization for surgical equipment. Modern Ethylene oxide [EO] productions employ either air or oxygen (O2)to oxidize ethylene (C2H4) with a silver catalyst on an alumina oxide carrier[Ag/Al2O3]catalyst packed in a fixed-bed reactor (plug-flow reactor)but the oxygen-base reaction process is more desirable here we used oxygen. Mainly two reactions occur, partial oxidation of ethylene to ethylene oxide and total oxidation of ethylene to carbon dioxide and water. The design models of the process in this research based on a three-part system. They are: the reaction system, absorption system and Ethylene Oxide [EO] purification system. The largest cost in production of ethylene oxide is ethylene therefore, it’s important to optimize the selectivity towards ethylene oxide and thus reduce the consumption of Ethylene. The aim of this work is to create a simulation model of the Ethylene Oxide production process from Ethylene using Aspen Hysys V9. Also to knowing the optimum operational conditions (temperature -pressure -flow rate) for the oxidation reactions of Ethylene. The simulation was running three times with various operational conditions to make a good result. The conclusion was that during operational time the activation energy increased for both reactions which have to be compensated with increasing reactor temperature. At the same time the selectivity for producing Ethylene Oxide decreases, i.e. more carbon dioxide and water are formed. The simulation models yield Ethylene Oxide with purity of 99.2%.
Simulation, municipal solid, gasification, fixed bed reactor, pyrolysis, gibbs energy, rgibbs model, gasifier
Короткий адрес: https://sciup.org/140293762
IDR: 140293762
Текст научной статьи Simulation of ethylene oxide production from ethylene cholorhydrin
DOI:
Ethylene : Ethylene is widely used in chemical industry, and its worldwide production (over 109 million ton in 2006) exceeds that of any other organic compound. It mostly used to produce three chemical compounds: Ethylene Oxide, Ethylene dichloride, Ethyl benzene, and a variety kinds of Polyethylene. Moreover, it is an ideal base material for many other petrochemicals, as it is readily available at high purity, low cost, and usually reacts with other low cost components, such as Oxygen and water. Currently, Ethylene is produced in the petrochemical industry by thermal cracking of a lkanes such as Ethane, Propane, Butane, Naphtha and gas oil. The choice of feedstock is an important economic issue as it influences other costs as well. In this process, feed stocks are heated to 700 – 900 ο C. This process converts large hydrocarbons into smaller ones and introduces un saturation. The reactor effluent is quickly quenched to avoid further reaction, then compressed, and finally sent to a separation unit for the recovery of Ethylene and other products such as Methane, Ethane, propane, Propylene, Butylenes, and Pyrolysis gasoline. Ethylene Oxide (EO) is a flammable and colorless gas at temperatures above 11 °C, which smells like ether at toxic
levels. It is an important commodity chemical for the production of solvents, antifreeze, textiles, detergents, adhesives, polyurethane foam, and pharmaceuticals. Small amounts of EO are used in manufacturing fumigants and sterilants for spices and cosmetics, as well as hospital sterilization for surgical equipments. Modern EO productions employ either air or Oxygen (О2) to oxidize Ethylene (С2Н4) with Ag/Аl2 О3 catalyst packed in a fixed-bed reactor (plug-flow reactor). The Oxygenbased reaction process is more desirable because of four major benefits: (i) higher productivity and selectivity; (II) lower initial capital costs; (III) less expensive catalyst required; and (IV) less air pollutants resulting from the purge gas .Industrial production of Ethylene Oxide:
-
1. Wurtz-process:
-
2. Direct oxidation process:
Materials and Methods
Simulation: Simulation is a situation in which a particular set of conditions is created artificially in order to study or experience something that could really exist in reality. It is the act if pretending that something is real when it is not. A computer simulation is an attempt to model a real-life or hypothetical situation on a computer so that it can be studied to see how the system works.
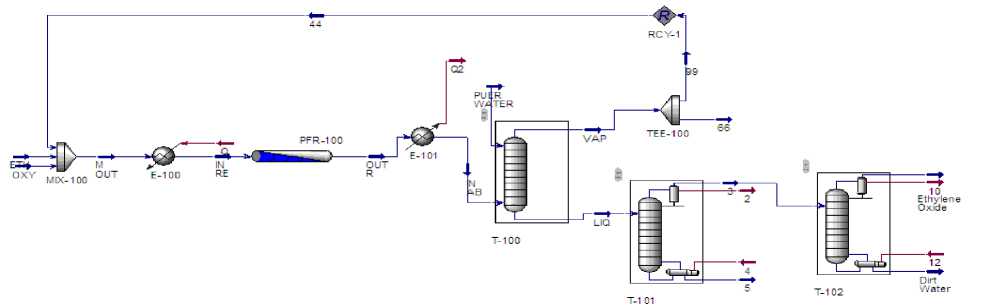
Figure 1.Simulation flow diagram of Ethylene Oxide production
Table 1.
Main shortcut from figure (1)
|
Name |
Object Type |
Name |
Object Type |
|
MIX-100 |
Mixer |
TEE-100 |
Tee |
|
E-100 |
Heater |
RCY-1 |
Recycle |
|
PFR-100 |
Plug Flow Reactor |
T-101 |
Distillation |
|
E-101 |
Cooler |
T-102 |
Distillation |
|
T-100 |
Absorber |
Results and Discussions
The Effect of Operating Condition: In this section, we see the influence of operating condition (temperature, pressure and flow rate) in Ethylene Oxide [EO] production
The Effect of flow Rate : The Effect of Ethylene flow Rate on Ethylene Oxide Production:
-
Figure (1) shows the Ethylene Oxide molar flow increases with Ethylene molar flow increases.
The Effect of Oxygen Flow Rate on Ethylene Oxide Production: Oxygen flow rate has positive effect in the production of Ethylene Oxide as we show figure (2). The reaction for production Ethylene Oxide is exothermic (shown in figure (3)). The temperature has negative effect in the production of Ethylene Oxide.
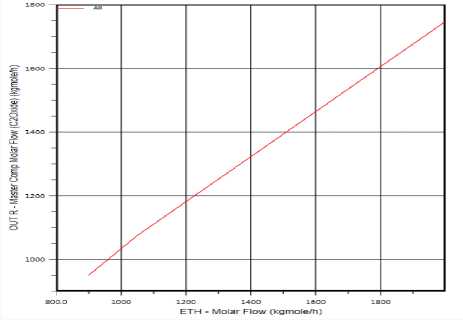
Figure 2. The effect of Ethylene flow rate on Ethylene Oxide production
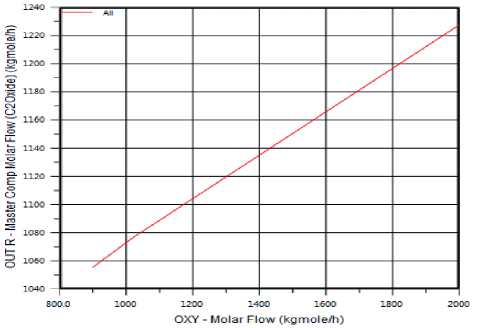
Figure 3. The effect of Oxygen flow rate on Ethylene Oxide production
The Effect of Temperature on Ethylene Oxide production: The reaction between Ethylene and Oxygen has main product (Ethylene oxide) increases when temperature decreases (shown in figure 4) and by product (СО 2 &Н 2 O) increases with temperature, has high value at 250 °C and decreases above it.
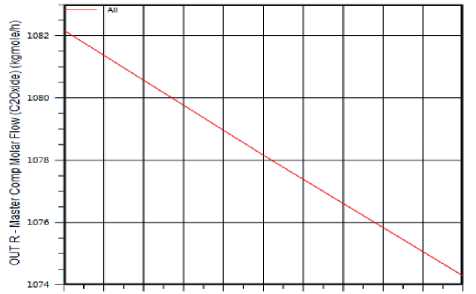
TOO О 120 0 140 0 160 0 180 0 200 0 220 0 240 0 260 0 280 0 300 0
IN RE - Temperature (C)
Figure 4. The effect of Temperature on Ethylene Oxide production
The Effect of Pressure on Ethylene Oxide production: Figure (6) shows the pressure has negative effect the flow rate of product decreases with pressure increases. The reaction between Oxygen and Ethylene occurs under a pressure of approximately 2000 kPa and a temperature of ap-
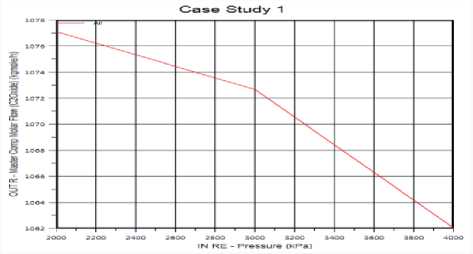
Figure 7. The effect of Pressure on ethylene oxide production
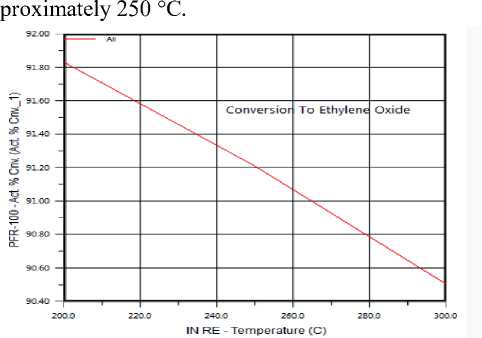
Figure 5. The effect of Temperature on Ethylene partial oxidation
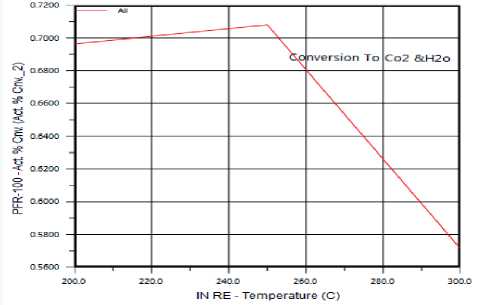
Figure 6. The effect of Temperature on Ethylene total oxidation
Conclusions
The oxidation process was simulated by the aspen (Hysys) software version.9 and it gave good results for operating condition. One of the most important results obtained from this work is that, it is very important to make analysis for the process operating condition. From this results, it can be seen that the optimum temperature to yield high amount of Ethylene Oxide is 200 ° C. But It has been proven that, the process was favored at a pressure of approximately 2000 kPa and a temperature of approximately 250 °C over a silver catalyst on an alumina oxide carrier. The results of simulation show that, high amount of Carbon dioxide and water contained at 250 °C. The selectivity towards Ethylene Oxide was decreasing over time and this is a result from deactivation of the catalyst. When this deactivation takes place, more Ethylene is more oxidized form Carbon dioxide and water. The simulation models yield Ethylene Oxide with purity of 99.2 %.
Recommendations
-
1. Using Aspen HYSYS program to develop any process will be very helpful, because it is very accurate and very helpful in equipment design and selection of the optimum operating conditions.
-
2. Detail studies must be taken for accurate selection of operation conditions and equipment specifications.
-
3. The simulation models need further and more study.
-
4. The simulation models need to add the СО 2 section [СО 2 absorption, СО 2 desorption] in order to: maintain an acceptable СО2 concentration in the circulated reactor gas, avoid catalyst deactivation and improved selectivity towards Ethylene Oxide production.
-
5. The stream from the bottom of the second distillation column can be send to the glycol section because it contains Ethylene Oxide and water.
Список литературы Simulation of ethylene oxide production from ethylene cholorhydrin
- Bayat M., Hamidi M., Dehghani Z., Rahimpour M.R. Dynamic optimal de sign of an industrial ethylene oxide (EO) reactor viadifferential evolution algorithm. Journal of Natural Gas Science and Engineering. 2013. no. 12. pp. 56-64.
- Perzon H.A Simulation Model of a reactor for Ethylene Oxide production. 2015.
- Kursawe D.C.A. Partial Oxidation of Ethene to Ethylene Oxide in Microchannel Reactors. 2009.
- Trupti Ambar, Tyagee Chavan, Manali Kavale, Walke S.M. Simulation of Process Equipment by using Hysys. International Journal of Engineering Research and Applications (IJERA). 2012. pp. 41-42.
- Yusuff A.S., Adeyi A.A., Oseh J.O. Ethylene oxide Selectivity Enhancement from Oxidation of Ethylene on Silver Catalyst By Mathematical Modeling. International Journal of Scientific & Engineering Research. 2015. vol. 6. no. 6. pp. 1626-1642.
- Leow W.R., Lum Y., Ozden A., Wang Y. et al. Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science. 2020. vol. 368. no. 6496. P. 1228-1233. doi: 10.1126/science.aaz8459
- Bononi M., Quaglia G., Tateo F. Identification of ethylene oxide in herbs, spices and other dried vegetables imported into Italy. Food Additives & Contaminants: Part A. 2014. vol. 31. no. 2. pp. 271-275. doi: 10.1080/19440049.2013.872808
- Marsh G.M., Keeton K.A., Riordan A.S., Best E.A. et al. Ethylene oxide and risk of lympho-hematopoietic cancer and breast cancer: a systematic literature review and meta-analysis. International Archives of Occupational and Environmental Health. 2019. vol. 92. no. 7. pp. 919-939. doi: 10.1007/s00420-019-01438-z
- Zhang D., Hang P., Liu G. Recycle optimization of an ethylene oxide production process based on the integration of heat exchanger network and reactor. Journal of Cleaner Production. 2020. vol. 275. pp. 122773. doi: 10.1016/j.jclepro.2020.122773
- Perzon H. A Simulation Model of a reactor for Ethylene Oxide production. 2015.
- Schonfeldt N. Surface active ethylene oxide adducts. Elsevier, 2013.
- Shintani H. Ethylene oxide gas sterilization of medical devices. Biocontrol science. 2017. vol. 22. no. 1. pp. 1-16. doi: 10.4265/bio.22.1
- Sreejith L.S., Sasi R. Residual Ethylene Oxide in Medical Devices: Effects and Estimation Methods, an Overview. Trends in Biomaterials & Artificial Organs. 2020. vol. 34. no. 1.
- Nawaz Z. Heterogeneous Reactor modeling of an industrial multitubular packed-Bed ethylene oxide reactor. Chemical Engineering & Technology. 2016. vol. 39. no. 10. pp. 1845-1857. doi: 10.1002/ceat.201500603
- Bandehali S., Moghadassi A., Parvizian F., Hosseini S.M. et al. Advances in high carbon dioxide separation performance of poly (ethylene oxide)-based membranes. Journal of Energy Chemistry. 2020. vol. 46. pp. 30-52. doi: 10.1016/j.jechem.2019.10.019
- Zeng G., Zhang Q., Wang X., Wu K.H. Association between blood ethylene oxide levels and the risk of cardiovascular diseases in the general population. Environmental Science and Pollution Research. 2021. vol. 28. no. 45. pp. 64921-64928.
- Bessaire T., Stroheker T., Eriksen B., Mujahid C. et al. Analysis of ethylene oxide in ice creams manufactured with contaminated carob bean gum (E410). Food Additives & Contaminants: Part A. 2021. vol. 38. no. 12. pp. 2116-2127. doi: 10.1080/19440049.2021.1970242
- Danner A.K., Leibig D., Vogt L.M., Frey H. Monomer-activated copolymerization of ethylene oxide and epichlo-rohydrin: In situ kinetics evidences tapered block copolymer formation. Chinese Journal of Polymer Science. 2019. vol. 37. no. 9. pp. 912-918. doi: 10.1007/s10118-019-2296-y
- Wei X., Chen L., Chaves B.D., Ponder M.A. et al. Modeling the effect of temperature and relative humidity on the ethylene oxide fumigation of Salmonella and Enterococcus faecium in whole black peppercorn. LWT. 2021. vol. 140. pp. 110742. doi: 10.1016/j.lwt.2020.110742
- Pandya B.M., Shah B.H. Efficient use of Ethylene Oxide in Vinyl Sulphone Industry. International Journal of Engineering Research. 2013. vol. 2. no. 2. pp. 62-65.


