Скольжение бактерий: способ пассивного распространения без использования жгутиков и пилей (обзор)
Автор: Цыганов Иван Вадимович, Нестерова Лариса Юрьевна, Ткаченко Александр Георгиевич
Журнал: Вестник Пермского университета. Серия: Биология @vestnik-psu-bio
Рубрика: Микробиология
Статья в выпуске: 4, 2021 года.
Бесплатный доступ
Бактерии используют несколько типов перемещения в окружающей среде, среди которых скольжение является пассивным способом движения. Оно осуществляется за счет толкающей силы, возникающей при делении клеток, а также дополнительных факторов, изменяющих поверхностные свойства бактерий. В данном обзоре описывается механизм скольжения, виды микроорганизмов, способные к скольжению, а также отличие скользящей подвижности, от других типов движения бактерий. Кроме того, дается классификация типов скольжения, основанная на отличиях поверхностно активных веществ, используемых клетками для движения.
Скольжение, подвижность бактерий, типы скольжения
Короткий адрес: https://sciup.org/147236786
IDR: 147236786 | УДК: 579.22 | DOI: 10.17072/1994-9952-2021-4-263-274
Текст научной статьи Скольжение бактерий: способ пассивного распространения без использования жгутиков и пилей (обзор)
For citacion: Tsyganov I V., Nesterova L. Yu., Tkachenko A. G. [Sliding bacteria: a method of passive spread without using of flagella and pili (review)]. Bulletin of Perm University. Biology. Iss. 4 (2021): pp. 263274. (In Russ.).
Acknowledgments : the work was carried out with the financial support of the Ministry of Science and Higher Education of the Russian Federation (AAAA19-119112290009-1).
Дикая природа, а также антропогенная среда представлены большим разнообразием биотических и абиотических поверхностей, колонизация которых бактериями необходима для их успешного выживания. Для распространения в разнообразных средах бактерии в ходе эволюции выработали несколько механизмов перемещения, как с помощью специальных органелл, так и в их отсутствие. Скольжение (sliding) – это один из механизмов пассивного распространения бактерий за счет действия экспансивной силы, возникающей при делении клеток и их давлении друг на друга, а также за счет изменения свойств клеточной поверхности, снижающей силу трения между клетками и средой [Henrichsen, 1972; Daffé, Draper, 1997; Hölscher, Kovács, 2017]. Впервые данный вид клеточной подвижности был описан в 1972 г. Херниксеном, и характеристика, данная этому типу движения, остается актуальной до настоящего времени. Помимо скольжения были также описаны ещё 5 видов подвижности: плавание (swiming), роение (swarming), подтягивание (twiching), ползание (gliding) и выбрасывание (darting). Последний тип подвижности в настоящее время не выделяется, а включен в такие виды движения, как скольжение или ползание – sliding и gliding [Pollitt, Diggle, 2017]. Несмотря на то, что определения, данные почти 50 лет назад, являются достаточно лаконичными и точными, в настоящий момент существуют трудности с характеристикой типов движения и некорректным использованием терминов. Это связано с тем, что на данный момент все типы бактериальной транслокации исследованы далеко не полно, а скольжение объективно является наименее исследованным. Учитывая это, важно понимать разницу между механизмами скольжения (sliding) и другими типами бактериальной подвижности. Цель представленного обзора – обобщение современных данных, касающихся скольжения бактерий разных видов и его сравнение с другими типами подвижности.
Различные виды подвижности бактерий
Среди известных механизмов движения бактерий плавание, роение и подтягивание реализуются за счет работы органелл: жгутиков [Henrichsen, 1972; Manson, 1992; Harshey, 2003; Ермилова, Залуцкая,
Лапина, 2004] или пилей [Mattick, 2002, Ермилова, Залуцкая, Лапина, 2004; Turnbull, Whitchurch 2014].
Указанные типы подвижности являются наиболее изученными.
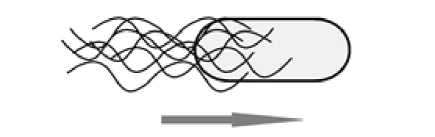
Рис. 1 . Плавание бактерий.
Стрелкой указано направление движения [Swimming of bacteria.
The arrow indicates the direction of movement]
Плавание осуществляется за счет работы одного или нескольких жгутиков (рис. 1). Вращение органелл против или по часовой стрелке определяет направление движения и смену курса [Harshey 2003; Magariyama et al. 1995; Magariyama, Sugiyama, Kudo, 2001]. Регулирование данного процесса осуществляется при помощи рецепторных белков, реагирующих на различные химические сигналы (хемотакис) [Manson, 1992; Bourret, Stock, 2002; Wong-Ng, Celani, Vergassola, 2018], интенсивность света (фототаксис) [Porter, Armitage, 2002; Schuergers, Mullineaux, Wilde, 2017], магнитное поле Земли (магнитотаксис)
[Blakemore, 1982; Müller, Schüler, Pfeiffer, 2020].
Роение также осуществляется за счет работы жгутиков [Harshey, 2015, Fraser, Hughes, 1999], но в отличие от плавания, данный тип перемещения – коллективный. При инициации роения бактерии претер-
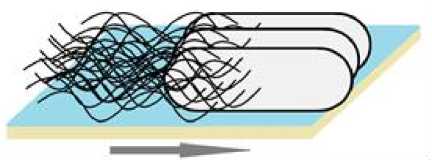
Рис. 2 . Роение бактерий.
Стрелкой указано направление движения [Swarming of bacteria.
The arrow indicates the direction of movement]
певают морфологические изменения: удлинение клеток и увеличение числа жгутиков [Eberl, Molin, Givskov 1999; Verstraeten et al., 2008; Alberti, Harshey, 1990]. С помощью жгутиков бактерии объединяются в «плоты» [Verstraeten et al, 2008], которые перемещаются по полужидкой поверхности (рис. 2).
Клетки, выпавшие из движущегося роя, прекраща- ют роиться. При этом, в отличие от плавания, клетки не избегают участков с неблагоприятными условиями, а движутся через них [Jiang, Gest, Bauer, 1997; Kearns, 2010].
Подтягивающий тип движения реализуется не за счет жгутиков, а благодаря пилям IV типа – филаментам, диаметром около 6 нм и длиной в несколько мик- рометров [Mattick, 2002; Ермилова, Залуцкая, Лапина, 2004]. Пили, расположенные на дистальном конце клетки, связываются с субстратом, после чего происходит втягивание филаментов и резкое продвижение клетки (рис. 3).
Ранее считалось, что вытягивание пилей не приводит к движению, вероятно из-за того, что они слишком гибкие и не способны толкать бактерии [Semmler, Whitchurch, Mattick, 1999], но впоследствии было продемонстрировано, что некоторые нитчатые цианобактерии модифицируют свои пили, чтобы двигаться за счет толкания, а не подтягивания вперед [Khayatan, Meeks, Risser, 2015]. Подтягивание, также как и роение, является коллективным видом перемещения [Merz, So, Sheetz, 2000; Wall, Kaiser, 1999]. В ходе экспериментов было показано, что при расхождении от места инокуляции, бактерии, как при роении, формировали «плоты», в которых клетки двигались синхронно, связываясь между собой при помощи пилей [Henrichsen, 1972; Wall, Kaiser, 1999]. Отдельные бактерии, поблизости от которых не было движущихся сообществ, демонстрировали снижение частоты подтягиваний [Semmler, Whitchurch, Mattick, 1999; Wall, Kaiser, 1999].
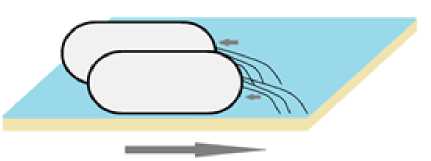
Рис. 3 . Подтягивание бактерий.
Большой стрелкой указано направление движения [Tightening of bacteria.
The big arrow indicates the direction of movement]
Механизмы бактериального перемещения при помощи органелл движения изучены достаточно подробно. В то же время механизмы движения, связанные со скольжением бактерий, на сегодняшний день остаются малоизученными. В связи с особенностями перевода, два разных типа перемещения, определяемые в англоязычной литературе «gliding» и «sliding» по-русски могут звучать одинаково и часто переводятся словом «скольжение», что приводит к путанице. В связи с необходимостью различать указанные типы движения, а также учитывая вновь полученные данные, движение по типу «gliding» в данном обзоре будет обозначено как «ползание», а «sliding» ‒ как «скольжение». Несмотря на то, что на первый взгляд данные виды подвижности схожи, они имеют некоторые особенности. Ползание характерно для таких таксономических групп, как миксобактерии, флавобактерии и микоплазмы. У миксобактерий присутствуют пили IV типа [Wu, Wu, Kaiser, 1997], поэтому им также доступно подтягивание. Ранее считалось, что ползание осуществляется за счет секреции слизи [Kaiser, 2003], которая уменьшает трение о поверхность, облегчая подтягивание [Wolgemuth et al., 2002; Ермилова, Залуцкая, Лапина, 2004]. Однако недавно проведенные исследования позволили открыть связь между ползанием и белками, формирующими протонный канал в мембране миксобактерий, гомологичными статору жгутиков у E.coli [Nan et al., 2011; Sun et al., 2011]. Поскольку у миксобактерий нет жгутиков, множество незакрепленных каналов движутся вдоль тела клетки по спирали, когда бактерия находится в жидкой среде [Nan et al., 2015]. По- пав на поверхность твердых сред, движение каналов замедляется в нескольких местах на вентральной стороне клетки, образуя динамические кластеры [Nan et al., 2013]. Во время движения клетки места скопления каналов не меняют своего положения относительно поверхности среды [Mignot et al., 2007; Sun et al., 2011; Nan et al., 2015; Nan, Zusman, 2016]. Впоследствии были обнаружены белки ползания, которые движутся вместе с каналами, и в местах контакта с субстратом – кластерах – прижимаются к клеточной стенке, деформируя поверхность бактерии и генерируя обратную поверхностную волну, толкающую клетку вперед [Nan et al., 2011, 2013; Jakobczak et al., 2015]. В это время клетка дополнительно поворачивается по часовой стрелке (рис. 4) [Faure et al., 2016].
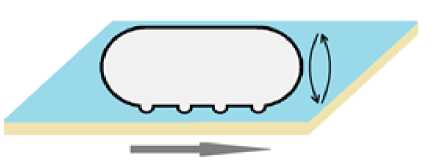
Рис. 4 . Ползание бактерий.
Стрелкой указано направление движения [Gliding of bacteria.
The arrow indicates the direction of movement]
Поскольку данные места скопления носят временный характер, а каналы постоянно продолжают двигаться, пептидогликановый слой не разрушается [Nan, 2017]. В данной модели движения продуцируемая слизь нужна не столько для облегчения ползания бактерии, сколько для плотного контакта со средой, позволяющего клетке отталкиваться [Nan et al., 2011; Balagam et al., 2014]. Механизмы подвижности флавобактерий и микоплазм в настоящее время не изучены полностью, тем не менее, известно, что одним из условий их движения также является временное прикрепление к поверхности, реализуемое при помощи белков [Nan, Zusman 2016].
Типы скольжения
Скольжение на данный момент является самым малоизученным типом движения. Механизм движения по типу скольжения принципиально отличается от всех описанных ранее типов перемещения.
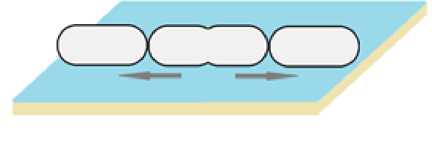
Рис. 5 . Скольжение бактерий.
Стрелкой указано направление движения [Sliding of bacteria.
The arrow indicates the direction of movement]
Скольжение – единственный вид пассивного распространения бактерий [Henrichsen, 1972; Daffé, Draper, 1997; Kearns, 2010]. Это значит, что клетки не расходуют дополнительную энергию на движение. Перемещение является следствием процессов деления (рис. 5) [Henrichsen, 1972; Daffé, Draper, 1997].
Тем не менее, некорректно будет отрицать существование такого типа движения, поскольку при скольжении образуется особая фенотипическая картина колонии – монослой клеток, нехарактерный для полностью неподвижных видов [Kearns, 2010; Kobayashi, Kanesaki, Yoshikawa, 2016]. Наслоения клеток при делении не происходит по причине уменьшения силы трения о поверхность среды за счет выделения ими поверхностно активных веществ или особого строения клеточной стенки некоторых бактерий [Henrichsen, 1972; Daffé, Draper, 1997; Kobayashi, Kanesaki, Yoshikawa, 2016]. По типу веществ, используемых для слайдинга, скользящие виды делят на три группы (табл. 1). К первой относят бактерии, которые для облегчения скольжения выделяют во внешнюю среду различные сурфактанты. Во вторую группу включают виды, которым для скольжения необходимо секретировать дополнительные компоненты, например, полисахариды. В третью группу входят виды, которые для скольжения не нуждаются в продукции поверхностно-активных соединений (рис. 6) [Hölscher, Kovács, 2017].
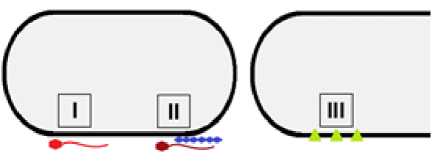
Рис. 6 . Типы скольжения бактерий [Types of bacteria sliding]
Первым из указанных типов скольжения обладают такие хорошо изученные виды, как Pseudomonas aeruginosa, Pseudomonas fluorescens, Serratia marcescens и Legionella pneumophila и многие другие. Скользящая подвижность у P. аeruginosa была обнаружена во время исследований, проведенных на мутанте с делециями генов fliC и pilA, ответственных за синтез жгутиков и пилей, соответственно [Murray, Kazmierczak, 2008]. Несмотря на отстствие органелл движения, клетки распространялись от места инокуляции. Впоследствии движение было идентифицировано как скольжение, а также было установлено, что для скольжения необходима продукция клетками рамнолипидов [Murray, Kazmierczak, 2008]. Кроме того, были установлены ещё несколько регуляторов скольжения. Двухкомпонентная система GacAS косвенно влияет на экспрессию рамнолипидов, а также регулирует роение [Murray, Kazmierczak, 2008]. Другой регулятор – RetS – также участвует в скольжении, но на данный момент его роль не определена [Kuchma et al., 2007]. Регулятор BifA отвечает за синтез цикло-ди-ГМФ, но кроме того, установлено, что сверхэкспрессия гена bifA приводит к усилению скольжения [Merritt et al., 2007]. Вероятно, это неполный список регуляторов скольжения, и в будущем будут открыты другие факторы, влияющие на подвижность. Другой представитель псевдомонад, Pseudomonas syringae, также способен к скольжению, если у мутантных штаммов отсутствуют жгутики и пили. Но для скольжения он использует липопептид сирин-гафактин [Nogales et al., 2015]. Движение Serratia marcescens осуществляется по типу скольжения благодаря липопептидному поверхностно активному веществу серраветину. Это было доказано в экспериментах, в которых мутанты, неспособные к продукции данного липопептида, прекращали распространяться по поверхности [Matsuyama, Bhasin, Harshey, 1995]. Кроме того, показано, что в присутствии экзогенного серраветина подвижность восстанавливалась [Matsuyama, Bhasin, Harshey, 1995]. Скольжение у Legionella pneumophila было открыто, когда мутанты, лишенные генов, ответственных за синтез флагел-лина и пиллина, демонстрировали образование «волн» на полужидком агаре [Stewart, Rossier, Cianciotto, 2009]. На данный момент не установлен сурфактант, обеспечивающий скользящее движение. Тем не менее, известно, что его действие связано с системой секреции 2-го типа, а также зависит от белка внешней мембраны TolC [Stewart, Rossier, Cianciotto, 2009; Stewart, Burnside, Cianciotto, 2011].
Скольжение бактерий, относящихся ко второму типу, зависит не только от поверхностно активных веществ, но также от экзополисахаридов. Известными представителями этой группы являются Bacillus subtilis и Sinorhizobium meliloti. При изучении B. subtilis было обнаружено, что данный вид способен к скольжению благодаря флагеллюмину. Бактерии образовывали дендриты, исходящие от точки инокуляции [Kinsinger, Shirk, Fall, 2003; Fall, Kearns, Nguyen, 2006], но если в среду поступало достаточное количество ионов калия, дендритный тип скольжения сменялся равномерным распространением по всей площади поверхности [Kinsinger, Shirk, Fall, 2003]. Предположительно, калий способствует усилению выработки сурфактанта [Kinsinger, Shirk, Fall, 2003; Kinsinger et al., 2005]. Кроме того, была показана необходимость экзополисахаридов для скольжения B. subtilis. Штаммы, не обладающие опероном epsA-O, продукты которого участвуют в биосинтезе экзополисахаридов, теряли возможность скользить [Grau et al., 2015; van Gestel, Vlamakis, Kolter, 2015]. Примечательно, что этот же кластер генов участвует в образовании биопленок [Vlamakis et al., 2013]. Для скольжения S. meliloti необходим регулятор транскрипции ExpR. Было установлено, что данный регулятор отвечает также за регуляцию продукции экзополи-сахарида второго типа (EPSII) галактоглюкана [Nogales et al., 2012], а штамм с делецией гена, кодирующего регулятор, терял способность к скольжению [Seminara et al., 2012]. С целью увлажнения поверхности и облегчения скольжения, бактерии продуцируют ризобактин. Мутация в соответствующем гене также приводила к прекращению скольжения [Nogales et al., 2012]. Важным открытием была демонстрация скольжения для S. meliloti в естественной среде обитания. Данный вид известен способностью к симбиозу с бобовыми. Внутри корневого волоса S. meliloti проникает в апоплазму через инфекционные нити [Gage, Margolin, 2000], формируя кластеры, которые удлиняются со временем. Предполагается, что колонизация инфекционной нити происходит за счет скольжения, так как клетки лишены жгутиков [Fournier et al., 2008].
Особенности скольжения у различных микроорганизмов [Holscher, Kovacs, 2017]
[Features of sliding in various microorganisms [Holscher, Kovacs, 2017]]
|
Тип скольжения |
Микроорганизмы |
Регуляторы скольжения |
Гены/Опероны |
|
I |
Pseudomonas aeruginosa |
Серраветин GacAS RetS цикло-ди-ГМФ |
gacAS retS bifA |
|
Pseudomonas syringae |
Сирингафактин FleQ |
fleQ |
|
|
Serratia marcescens |
Серингафактин |
swrW |
|
|
Legionella pneumophila |
Неизвестный сурфактант TolC |
tolC |
|
|
II |
Bacillus subtilis |
KinB/C |
epsA-O |
|
Sinorhizobium meliloti |
ExpR |
expR |
|
|
III |
Salmonella enterica serovar Typhimurium |
PhoPQ PagM |
phoPQ рagM |
|
Mycobacterium smegmatis |
Гликопептидолипиды |
ptsAB gtf1 gtf2 gtf3 |
|
|
Mycobacterium abscessus |
Гликопептидолипиды, неизвестный регулятор |
ptsAB gtf1 gtf2 gtf3 mab_3083c |
Третий тип скольжения не зависит от наличия поверхностно активных веществ, но также осуществляется под давлением, создаваемым делением клеток. С помощью этого вида скольжения перемещаются Salmonella enterica serovar Typhimurium [Park, Pontes, Groisman, 2015] и различные виды микобактерий, в частности Mycobacterium smegmatis [Martínez, Torello, Kolter, 1999; Recht et al., 2000]. Про скольжение S. enterica известно только, что для перемещения клеткам необходимо наличие поверхностного белка PagM, содержание которого регулируется системой PhoP/PhoQ, индуцируемой низким уровнем ионов магния.
Микобактерии долгое время считались неподвижными из-за отсутствия жгутиков, но вскоре было обнаружено, что клетки начинают распространяться по поверхности полужидких сред после продолжительной инкубации [Martínez, Torello, Kolter, 1999]. При этом они формировали монослой, в котором клетки были расположены произвольно. Кроме того, распространение колонии сопровождалось делением клеток, что позволило определить этот процесс как скольжение. В ходе исследования грубых и гладких форм M. smegmatis было установлено, что грубый фенотип имеет нарушения в скольжении.
Данный факт свидетельствует в пользу того, что концентрация гликопептидолипидов (GPL), определяющая фенотип микобактерий, вступает в прямую корреляцию со способностью к скольжению. Глико- пептидолипиды являются уникальными гликоконьюгатами микобактерий. GPL состоят из смеси 3-гидрокси и 3-метокси длинноцепочечных (С26-С34) жирных кислот, амидированных трипептидом (D-Phe-D-alloThr-D-Ala), кончающимся аминоспиртом (L-аланиол). Аланиол гликозируется О-метилированным моно- или дирамносильным остатком [Schorey, Sweet, 2008]. GPL находятся на внешней стороне клеточной стенки микобактерий и контактируют с поверхностью среды. Анализ липидных экстрактов доказал связь между наличием GPL и скольжением [Martínez, Torello, Kolter, 1999]. В последующем исследовании с использованием транспозонового мутагенеза выявлено несколько мутантов, которые утратили способность к скольжению [Recht et al., 2000]. Все они характеризовались грубой морфологией колоний, а также отсутствием гликопептидолипидов. Почти все вставки транспозонов были локализованы в гене mps, кодирующем нерибосомальную пептидную синтетазу, участвующую в биосинтезе GPL, давая таким образом прямое доказательство важности GPL для скольжения [Recht et al., 2000]. Тем не менее, судя по последним данным, за способность к скольжению микобактерий ответственны не только гликопептидолипиды. Так, мутантный штамм Mycobacterium abscessus mab_3083c::Tn, с гладким морфотипом, скользил быстрее штамма дикого типа, но не отличался по уровню содержания гликопептидолипидов [Liu et al., 2021]. Кроме того, установлено, что биогенные полиамины, присутствующие в окружающей среде, способны менять поверхностный заряд бактерий M. smegmatis, не влияя на гидрофобность клеточной стенки. В присутствии данных поликатионов у микобактерий понижалась скорость скольжения, усиливалась агрегация клеток, а также увеличивалась масса биопленок [Нестерова, Цыганов, Ткаченко, 2020]. Данные факты свидетельствует о том, что, возможно, существуют другие молекулы, ответственные за способность к скольжению, а также то, что механизм скольжения нуждается в дальнейшем исследовании.
Связь между скольжением и образованием биопленок
В отличие от других типов подвижности, когда, например, мутанты с дефектом роения демонстрировали лучшую способность к биопленкообразованию [Verstraeten et al., 2008], при повышении уровня GPL, усиливалось как скольжение, так и биопленкообразование [Hölscher, Kovács, 2017]. Мутанты M. smegmatis с дефектами в mps и GPL мембранных белков, кодируемых gap , и имевшие недостаток GPL, становились неподвижными, в отличие от их родительских форм, способных синтезировать GPL [Agustí et al., 2008]. Некоторые из этих мутантов хуже образовывали биопленки на поливинилхлоридных пластинах. Схожие результаты наблюдались и у pstA / pstB мутантов M. avium . В 2000 г. Рехтом [Recht et al., 2000] была предложена модель скользящего типа движения, согласно которой GPL с выступающими жирно-ацильными хвостами, расположенными на поверхности клеточной стенки, создают гидрофобную поверхность, которая снижает трение между бактерией и гидрофильной поверхностью. В отличие от них, мутанты с дефектом GPL имеют гидрофильную поверхность, что приводит к снижению подвижности [Martínez, Torello, Kolter, 1999; Recht et al., 2000]. В ходе генетического анализа скольжения и биоплен-кообразования микобактерий было показано, что у mps и tmtpC мутантов M. smegmatis, не способных синтезировать или экспортировать GPL, гидрофильная часть гликопептидолипида была обращена наружу. Это приводило к возрастанию трения клетки о гидрофильную поверхность агара, а также снижению способности прикрепления к гидрофобной поверхности поливинилхлоридной пластинки и, следовательно, образования биопленки [Recht et al., 2000].
Процессы скольжения и биопленкообразования микобактерий находятся под контролем сигнальных нуклеотидов (вторичных мессенджеров), таких как гуанозин тертафосфат (ppGpp) и циклический дигуанозинмонофосфат (c-di-GMP). У микобактерий ppGpp и c-di-GMP синтезируются и расщепляются бифункциональными белками RelMsm и DcpA соответственно. Алармон (p) ppGpp необходим для длительного выживания M. smegmatis во время голодания [Mathew et al., 2004]. c-di-GMP контролирует экспрессию генов транспорта и метаболизма липидов через фактор транскрипции LtmA M. smegmatis [Li, He, 2012], а также регулирует патогенность и состояние покоя у M. tuberculosis [Hong et al., 2013]. Известно, что штаммы M. smegmatis с делецией генов rel и dcpA демонстрируют измененные свойства клеточной поверхности, имеют пониженное количество гликопептидолипидов (GPL) и полярных липидов в клеточных стенках по сравнению с диким типом M. smegmatis , а также обладают дефектами в формировании биопленок [Gupta, Kasetty, Chatterji 2015; Gupta et al., 2016]. Следовательно, можно сделать вывод, что вторичные мессенджеры (p)ppGpp и c-di-GMP у M. smegmatis принимают участие в регуляции процессов скольжения и биопленкообразования.
Заключение
Несмотря на то, что данный обзор в первую очередь посвящен скольжению, немалая его часть отведена описанию других типов бактериальной транслокации. Это обусловлено необходимостью описания кардинальных различий среди механизмов, лежащих в основе скольжения. Различные виды бактерий, способные к скольжению по полужидким поверхностям, могут быть отнесены к трем группам: скользящие при помощи выделяемых сурфактантов, использующие сурфактанты и поверхностно активные вещества, и виды, не использующие для скольжения секрецию веществ. При этом, деление между группами 1 и 2 условно, поскольку в будущем могут быть обнаружены другие соединения, способствующие скольжению. Кроме того, упомянутые в обзоре регуляторы скольжения у некоторых штаммов лишь способствуют облегчению реализации других механизмов перемещения бактерий – роения или подтягивания. В этом случае наблюдать скольжение возможно лишь на мутантах, не обладающих соответствующими органеллами. Тем не менее, данный факт указывает на возможность эволюционной связи различных механизмов бактериальной транслокации. Поскольку на сегодняшний день скольжение остается наименее изученным из всех типов движения, нельзя исключить описание этого типа перемещения у других видов микроорганизмов, а также более детального исследования биохимических процессов, лежащих в его основе.
Список литературы Скольжение бактерий: способ пассивного распространения без использования жгутиков и пилей (обзор)
- Ермилова Е.В., Залуцкая Ж.М., Лапина Т.В. Подвижность и поведение микроорганизмов. СПб.: Изд-во СПбГУ, 2004. 187 с.
- Нестерова Л.Ю., Цыганов И.В., Ткаченко А.Г. Биогенные полиамины влияют на антибиотикочув-ствительность и поверхностные свойства клеток Mycobacterium smegmatis // Прикладная биохимия и микробиология. 2020. T. 56, № 4. C. 342-351.
- Agusti G. et al. Surface spreading motility shown by a group of phylogenetically related, rapidly growing pigmented mycobacteria suggests that motility is a common property of mycobacterial species but is restricted to smooth colonies // Journal of Bacteriology. 2008. Vol. 190, № 20. Р. 6894-6902.
- Alberti L, Harshey R.M. Differentiation of Serratia marcescens 274 into swimmer and swarmer cells // Journal of Bacteriology. 1990. Vol. 172, № 8. Р. 4322-4328.
- Balagam R. et al. Myxococcus xanthus gliding motors are elastically coupled to the substrate as predicted by the focal adhesion model of gliding motility // PLOS Computational Biology. 2014. Vol. 10, № 5. Р. e1003619.
- Blakemore R.P. Magnetotactic bacteria // Annual Review of Microbiology. 1982. № 36. Р. 217-238.
- Bourret R.B., Stock A.M. Molecular information processing: lessons from bacterial chemotaxis // Journal of Biological Chemistry. 2002. Vol. 277, №. 12. Р. 9625-9658.
- Daffe M., Draper P. The envelope layers of mycobacteria with reference to their pathogenicity // Advances in microbial physiology. 1997. Vol. 39. Р. 131-203.
- Eberl L., Molin S., Givskov M. Surface motility of Serratia liquefaciens MG1 // Journal of Bacteriology. 1999. Vol. 181, № 6. Р. 1703-1712.
- Fall R., Kearns D.B., Nguyen T. A defined medium to investigate sliding motility in a Bacillus subtilis flagella-less mutant // BMC Microbiology. 2006. Vol. 6. Р. 31.
- Faure L.M. et al. The mechanism of force transmission at bacterial focal adhesion complexes // Nature. 2016. Vol. 539, № 7630. Р. 530-535.
- Fournier J. et al. Mechanism of infection thread elongation in root hairs of Medicago truncatula and dynamic interplay with associated rhizobial colonization // Plant Physiology. 2008. Vol. 148, № 4. Р. 1985-1995.
- Fraser G.M., Hughes C. Swarming motility // Current Opinion in Microbiology. 1999. Vol. 2, № 6. Р. 630-635.
- Gage D.J., Margolin W. Hanging by a thread: invasion of legume plants by rhizobia // Current Opinion in Microbiology. 2000. Vol. 3, № 6. Р. 613-617.
- Grau R.R.et al. A Duo of Potassium-Responsive Histidine Kinases Govern the Multicellular Destiny of Bacillus subtilis // mBio. 2015. Vol. 6, № 4. Р. e00581.
- Gupta K.R., Kasetty S., Chatterji D. Novel functions of (p)ppGpp and Cyclic di-GMP in mycobacterial physiology revealed by phenotype microarray analysis of wild-type and isogenic strains of Mycobacterium smegmatis // Applied and Environmental Microbiology. 2015. Vol. 81, № 7. Р. 2571-2578.
- Gupta K.R. et al. Regulation of Growth, Cell Shape, Cell Division, and Gene Expression by Second Messengers (p)ppGpp and Cyclic Di-GMP in Mycobacterium smegmatis // Journal of Bacteriology. 2016. Vol. 198, № 9. Р. 1414-1422.
- Harshey R.M. Bacterial motility on a surface: many ways to a common goal // Annual Review of Microbiology. 2003. № 57. Р. 249-273.
- Harshey R.M., Partridge J.D. Shelter in a Swarm // Journal of Molecular Biology. 2015. Vol. 427, № 23. Р. 3683-3694.
- Henrichsen J. Bacterial surface translocation: a survey and a classification // Bacteriological Reviews. 1972. Vol. 36, № 4. Р. 478-503.
- Hölscher T., Kovacs Ä.T. Sliding on the surface: bacterial spreading without an active mot or // Environmental Microbiology. 2017. Vol. 19, № 7. Р. 2537-2545.
- Hong Y. et al. Cyclic di-GMP mediates Mycobacterium tuberculosis dormancy and pathogenicity // Tuberculosis (Edinburgh). 2013. Vol. 93. P. 625-634.
- Jakobczak B. et al. Contact and Protein Transfer-Dependent Stimulation of Assembly of the Gliding Motility Machinery inMyxococcusXanthus // PLOS Genetics. 2015. Vol. 11, № 7. P. e1005341.
- Jiang Z.Y., Gest H., Bauer C.E. Chemosensory and photosensory perception in purple photosynthetic bacteria utilize common signal transduction components // Journal of Bacteriology. 1997. Vol. 179, № 18. P. 5720-5727.
- Kaiser D. Coupling cell movement to multicellular development in myxobacteria // Nature Reviews Microbiology. 2003. Vol. 1, № 1. P. 45-54.
- Kearns D.B. A field guide to bacterial swarming motility // Nature Reviews Microbiology. 2010. Vol. 8, № 9. P. 634-644.
- Kinsinger R.F., Shirk M.C., Fall R. Rapid surface motility in Bacillus subtilis is dependent on extracellular surfactin and potassium ion // Journal of Bacteriology. 2003. Vol. 185, №. 18. P. 5627-5631.
- Kinsinger R.F. et al. Genetic requirements for potassium ion-dependent colony spreading in Bacillus subtilis // Journal of Bacteriology. 2005. Vol. 187, № 24. P. 8462-8469.
- Khayatan B., Meeks J.C., Risser D.D. Evidence that a modified type IV pilus-like system powers gliding motility and polysaccharide secretion in filamentous cyanobacteria // Molecular Microbiology. 2015. Vol. 98, № 6. P. 1021-1036.
- Kobayashi K., Kanesaki Y., Yoshikawa H. Genetic Analysis of Collective Motility of Paenibacillus sp. NAIST15-1 // PLOS Genetics. 2016. Vol. 12, № 10. P. e1006387.
- Kuchma S.L. et al. BifA, a cyclic-Di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA1 // Journal of Bacteriology. 2007. Vol. 189, № 2. P. 81658178.
- Li W., He Z.G. LtmA, a novel cyclic di-GMP-responsive activator, broadly regulates the expression of lipid transport and metabolism genes in Mycobacterium smegmatis // Nucleic Acids Research. 2012. Vol. 40, № 22. P. 11292-11307.
- Liu T.Y. et al. Mab_3083c Is a Homologue of RNase J and Plays a Role in Colony Morphotype, Aggregation, and Sliding Motility of Mycobacterium abscessus // Microorganisms. 2021. Vol. 9, № 4. P. 676.
- Magariyama Y., Sugiyama S., Kudo S. Bacterial swimming speed and rotation rate of bundled flagella // FEMS Microbiology Letters. 2001. Vol. 199, № 1. P. 125-129.
- Magariyama Y. et al. Simultaneous measurement of bacterial flagellar rotation rate and swimming speed // Biophysical Journal. 1995. Vol. 69, № 5. P.2154-2162.
- Manson M.D. Bacterial motility and chemotaxis // Advances in Microbial Physiology. 1992. Vol. 33. P. 277-346.
- Martínez A., Torello S., Kolter R. Sliding motility in mycobacteria // Journal of Bacteriology. 1999. Vol. 181, № 23. P. 7331-7338.
- Mathew R. et al. Deletion of the rel gene in Mycobacterium smegmatis reduces its stationary phase survival without altering the cell-surface associated properties // Current Science. 2004. Vol. 86. P. 149-153.
- Mattick J.S. Type IV pili and twitching motility // Annual Review of Microbiology. 2002. Vol. 56. P. 289-314.
- Matsuyama T., Bhasin A., Harshey R.M. Mutational analysis of flagellum-independent surface spreading of Serratia marcescens 274 on a low-agar medium // Journal of Bacteriology. 1995. Vol. 177, № 4. P. 98799891.
- Matsuyama T. et al. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens // Journal of Bacteriology. 1992. Vol. 174, № 6. P. 17691776.
- Merritt J.H. et al. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function // Journal of Bacteriology. 2007. Vol. 189, № 22. P. 8154-8164.
- Merz A.J., So M., Sheetz M.P. Pilus retraction powers bacterial twitching motility // Nature. 2000. Vol. 407, № 6800. P. 98-102.
- Mignot T. et al. Evidence that focal adhesion complexes power bacterial gliding motility // Science. 2007. Vol. 315, № 5813. P. 853-856.
- Müller F.D., Schüler D., Pfeiffer D. A Compass To Boost Navigation: Cell Biology of Bacterial Magnetotaxis // Journal of Bacteriology. 2020. Vol. 202, № 21. P. e00398-20.
- Murray T.S., Kazmierczak B.I. Pseudomonas aeruginosa exhibits sliding motility in the absence of type IV pili and flagella // Journal of Bacteriology. 2008. Vol. 190, № 8. P. 2700-2708.
- Nan B. Bacterial Gliding Motility: Rolling Out a Consensus Model // Current Biology. 2017. Vol. 27, № 4. P. R154-R156.
- Nan B. et al. Myxobacteria gliding motility requires cytoskeleton rotation powered by proton motive force // Proceedings of the National Academy of Sciences USA. 2011. Vol. 108, № 6. P. 2498-2503.
- Nan B. et al. Flagella stator homologs function as motors for myxobacterial gliding motility by moving in helical trajectories // Proceedings of the National Academy of Sciences USA. 2013. Vol. 110, № 16. P. E1508-1513.
- Nan B. et al. The polarity of myxobacterial gliding is regulated by direct interactions between the gliding motors and the Ras homolog MglA // Proceedings of the National Academy of Sciences USA. 2015. Vol. 112, № 2. P. E186-193.
- Nan B., Zusman D.R. Novel mechanisms power bacterial gliding motility // Molecular Microbiology. 2016. Vol. 101, № 2. P. 186-193.
- Nogales J. et al. ExpR is not required for swarming but promotes sliding in Sinorhizobium meliloti // Journal of Bacteriology. 2012. Vol. 194, № 8. P. 2027-2035.
- Nogales J. et al. FleQ coordinates flagellum-dependent and -independent motilities in Pseudomonas sy-ringae pv. tomato DC3000 // Applied and Environmental Microbiology. 2015. Vol. 81, № 21. P. 7533-7545.
- Park S.Y., Pontes M.H., Groisman E.A. Flagella-independent surface motility in Salmonella enterica serovar Typhimurium // Proceedings of the National Academy of Sciences USA. 2015. Vol. 112, № 6. P.1850-1855.
- Pollitt E.J.G., Diggle S.P. Defining motility in the Staphylococci // Cellular and Molecular Life Sciences. 2017. Vol. 74, № 16. P. 2943-2958.
- Porter S.L., Armitage J.P. Phosphotransfer in Rhodobacter sphaeroides chemotaxis // Journal of Molecular Biology. 2002. Vol. 324, № 1. P. 35-45.
- Recht J. et al. Genetic analysis of sliding motility in Mycobacterium smegmatis // Journal of Bacteriology. 2000. Vol. 182, № 15. P. 4348-4351.
- Schorey J.S., Sweet L. The mycobacterial glycopeptidolipids: structure, function, and their role in pathogenesis // Glycobiology. 2008. Vol. 18, № 11. P. 832-841.
- Schuergers N., Mullineaux C.W., Wilde A. Cyanobacteria in motion // Current Opinion in Plant Biology. 2017. Vol. 37. P. 109-115.
- Seminara A. et al. Osmotic spreading of Bacillus subtilis biofilms driven by an extracellular matrix // Proceedings of the National Academy of Sciences USA. 2012. Vol. 109, № 4. P. 1116-1121.
- Semmler A.B., Whitchurch C.B., Mattick J.S. A re-examination of twitching motility in Pseudomonas aeruginosa // Microbiology. 1999. Vol. 145. P. 2863-2873.
- Stewart C.R., Burnside D.M., Cianciotto N.P. The surfactant of Legionella pneumophila Is secreted in a TolC-dependent manner and is antagonistic toward other Legionella species // Journal of Bacteriology. 2011. Vol. 193, № 21. P. 5971-5984.
- Stewart C.R., Rossier O., Cianciotto N.P. Surface translocation by Legionella pneumophila: a form of sliding motility that is dependent upon type II protein secretion // Journal of Bacteriology. 2009. Vol. 191, № 5. P. 1537-1546.
- Sun M. et al. Motor-driven intracellular transport powers bacterial gliding motility // Proceedings of the National Academy of Sciences USA. 2011. Vol. 108, № 18. P. 7559-7564.
- Turnbull L., Whitchurch C.B. Motility assay: twitching motility // Methods in Molecular Biology. 2014. Vol. 1149. P. 73-86.
- van Gestel J., Vlamakis H., Kolter R. From cell differentiation to cell collectives: Bacillus subtilis uses division of labor to migrate // PLOS Biology. 2015. Vol. 13, № 4. P. e1002141.
- Verstraeten N. et al. Living on a surface: swarming and biofilm formation // Trends in Microbiology. 2008. Vol. 16, № 10. P. 496-506.
- Vlamakis H. et al. Sticking together: building a biofilm the Bacillus subtilis way // Nature Reviews Microbiology. 2013. Vol. 11, № 3. P. 157-168.
- Wall D., Kaiser D. Type IV pili and cell motility // Molecular Microbiology. 1999. Vol. 32, № 1. P. 1-10.
- Wolgemuth C. et al. How myxobacteria glide // Current Biology. 2002. Vol. 12, № 5. P. 369-377.
- Wong-Ng J., Celani A., Vergassola M. Exploring the function of bacterial chemotaxis // Current Opinion in Microbiology. 2018. Vol. 45. P. 16-21.
- Wu S.S., Wu J., Kaiser D. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced // Molecular Microbiology. 1997. Vol. 23, № 1. P. 109-121


