Soil microbiome of plaggic anthrosol and calcic cryosols in Central Yakutia
Автор: Polyakov V.I., Petrov A.A., Abakumov E.V., Kimeklis A.K., Gladkov G.V., Andronov E.E.
Журнал: Бюллетень Почвенного института им. В.В. Докучаева @byulleten-esoil
Рубрика: Статьи
Статья в выпуске: 119, 2024 года.
Бесплатный доступ
Soil microbiome makes a significant contribution to the implementation of ecosystem services, which are necessary for the sustainable functioning of ecosystems. Soils of central Yakutia develop under dynamic physical and chemical conditions (long-term freezing/thawing processes, redistribution of nutrients), which ensures the formation of a specific microbial community in natural and anthropogenically transformed areas. The object of the study was the natural, fallow, and agricultural soils of central Yakutia. The method of high-throughput sequencing of 16S rRNA gene fragment on Illumina MiSEQ sequencer was used to analyze the microbial community. As a result, in fallow lands a decrease in nutrients was revealed if compared to the lands involved in agricultural turnover. Based on the composition of the microbiome it was observed that the most common phyla are Acidobacteria, Actinobacteria, Verrucomicrobiota, Pseudomonadota (Alphaproteobacteria, Gammaproteobacteria), Bacterioidota, Chloroflexi, Planctomycetota. The presence of a core set of microorganisms for the studied soils was recorded, up to 17.8% of phylotypes are unique and up to 25.7% are common to fallow lands and background plots. Microbial communities vary depending on geographical locations and on types of natural resource use. The most distinct microbial communities are formed in hydromorphic soils with the development of gley processes, as well as in agricultural soils.
16s amplicons, soil biodiversity, high-throughput sequencing, permafrost affected soils
Короткий адрес: https://sciup.org/143183306
IDR: 143183306 | DOI: 10.19047/0136-1694-2024-119-6-29
Текст научной статьи Soil microbiome of plaggic anthrosol and calcic cryosols in Central Yakutia
7/9 Universitetskaya nab., St. Petersburg 199034, Russian Federation, *, e-mail: , ***, e-mail: ,
, e-mail: ,
, e-mail: 2Arctic and Antarctic Research Institute,
3 Podbelskoye highway, St. Petersburg 196608, Russian Federation, ******, e-mail:
The central part of Yakutia has been a major agricultural center for a long time, but today, most of the land is fallow and subject to selfrestoration processes. As a result of cryogenic processes, landscape transformation, degradation of soil cover, removal of nutrients and regular changes in soil microbiome occur. The soil microbiome plays a significant role in vegetation development, and soil nutrient redistribution, and influences the quality of life of the population (Jansson, Hof-mockel, 2019; Suman et al., 2022). Therefore, the study of soil microbiomes is an important step toward sustainable agriculture, especially in soils affected by permafrost processes (Santos, Olivares, 2021). Soils of the central part of Yakutia are represented by permafrost Calcic Cryosols, which are formed under conditions of low temperatures and short growing seasons (Ivanova et al., 2013). The unique geological and geochemical features of central Yakutia determine the specific composition of microflora that develops under conditions of dynamic change of physical and chemical environmental factors in a wide range of low temperatures (Ivanova et al., 2014). However, natural and anthropogenically transformed soils are extremely poorly studied and there is still no complete picture of the quantitative and qualitative composition of soil microbiota in permafrost landscapes of Yakutia (Kuzmina et al., 2021). From the previously obtained data, it was revealed that the greatest influence on the composition of soil microbiota in the soils of central Yakutia is exerted by the presence of moisture in the soil profile. A characteristic feature of these soils is the content of a high number of bacteria throughout the entire profile of the studied soils (Kuzmina et al., 2021). Climatic conditions, the presence of carbonates in the parent rocks, and the proximity of a large river lead to intensive agriculture in the region (Okoneshnikova, 2015). Among oth- er regions located in the Arctic sector of the Russian Federation, Yakutia is the least urbanized territory, indicating a relatively high proportion of the rural population engaged in agriculture and animal husbandry. In Yakutia, there were 19 446.5 thousand ha of agricultural lands in 2021, but the share is annually decreasing, as far as in 2005 the area of agricultural lands amounted to 24 632.1 thousand ha. The gradual transition of lands into fallows negatively affects the state of soil cover, which leads to soil degradation and loss of fertility (Ahmad et al., 2022; Mitin et al., 2024). As a result of agro-landscape degradation, the soil cover is transformed into natural ecosystems (Desyatkin et al., 2021). Along with the change of leading soil-forming processes, the soil microbiome is changing as well (Santos, Olivares 2021; Zverev et al., 2022). The ecosystem services performed by the soil microbiome are vital for soil carbon sequestration and nutrient supply to plants, so the importance of the soil microbiome in soil conservation cannot be overestimated (Jansson, Hofmockel, 2019).
The soil microbiome plays an important role in ecosystem functioning and is largely responsible for the balance of carbon and other nutrients, the microbiome plays a key role in climate regulation, including the production or consumption of greenhouse gases (Jansson, Hof-mockel, 2019). From a fertility perspective, the soil microbiome can influence plant growth, and be used as part of plant defense against pests (Dubey et al., 2019). During secondary succession, plant communities change, and with it the soil microbiome changes (Lin et al., 2021). Afforestation of the area and formation of phytocenoses on the site of former pastures and arable land leads to carbon storage in plant biomass, but the degradation of agricultural soils leads to a decrease in soil organic carbon that was stored here as a result of anthropogenic activities (Suman et al., 2022). Under climate change, pastures and fallow lands will be increasingly exposed to natural events such as fires, droughts, and floods (Jansson, Hofmockel, 2019). Over the last few years, fires have become more frequent in Yakutia, starting in late spring and lasting until late summer (Polyakov et al., 2022). During the upper fires, the upper soil cover, which is exposed to the direct impact of fire, suffers the greatest damage, the litter and the upper humus-accumulative horizon are burned out, and microorganisms eliminate (Chebykina et al., 2022; Desyatkin et al., 2024). Fires result in de- creased rates of soil carbon sequestration, which is associated with decreased biodiversity of the soil microbiome and disruption of the integrity of internal carbon polymer production links that contribute to soil aggregate formation and carbon sequestration (Jansson, Hofmockel, 2019). The soil microbiome provides key ecosystem services, regulation of water, and nutrients, and is involved in carbon balance, carbon sequestration energy redistribution, and humification (Jansson, Hof-mockel, 2019; Suman et al., 2022; Santos, Olivares, 2021). Studying the soil microbiome of Yakutia is important in terms of climate change and food security of the largest region in Russia. Under the conditions of permafrost degradation, natural phenomena, and soil transition to fallow state, it is necessary to study the soil microbiome and its response to landscape transformation, this determines the purpose of this study.
MATERIALS AND METHODS
Study area. Soil sampling was carried out during fieldwork at the end of the summer of 2021 in 3 replicates from each spot. Soils were selected from arable and fallow lands as well as zonal terrestrials. The area of study is shown in Fig.1.
Yakutsk is located in the Tuimaada valley on the left bank of the Lena River, in its middle reaches. It is the largest city located in the permafrost zone. Soil sampling took place on the Prilensky plateau. It is composed of Cambrian and Ordovician gypsiferous and saline limestones and dolomites (Polyakov et al., 2022; Okoneshnikova, Ivanova 2020). The vegetation cover is represented by taiga pine and larch forests. The climate is sharply continental with long frosty, low-snow winters. The temperature drops to -45 °C in winter. Summers are moderately warm (15–17 °C), during which most precipitation falls. Precipitation is about 350–450 mm per year (Polyakov et al., 2022; Okonesh-nikova, Ivanova, 2020).
Soil sampling was conducted in the west of the city. Soil samples were collected from the fallow land (Y1), hayfield formed on fallow land (Y7), land used in modern agriculture (Y13), and zonal soil formation variants (Y3, Y9, Y11) (Fig. 2).
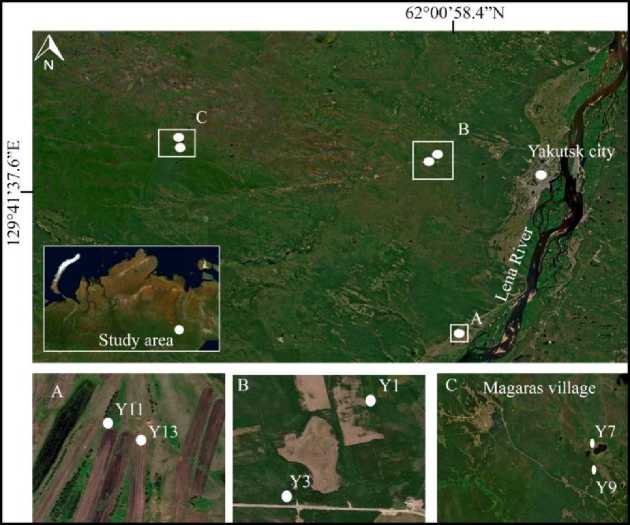
Рис.1. Область исследования в центральной части Якутии.
Fig. 1. The study area of the central part of Yakutia.
The description of soil profiles is presented in Table 1.
Soil samples were frozen at the time of collection and delivered to the Applied Ecology Laboratory of Saint-Petersburg state University. Samples were stored at +4 °C to analyze the main agrochemical parameters and pH. For all samples, the main nutrition parameters were determined: pH, available phosphorus and potassium, ammonium and nitrate nitrogen. The chemical parameters are presented in Table 2.
Microbiome analysis. For the microbiome analysis upper horizon of each soil was chosen. DNA was isolated from six soil samples in three replicates using the NucleoSpin Soil Kit (Macherey-Nagel GmbH & Co. KG, Germany) and a Precellus 24 homogenizer (Bertin, USA). The quality control of the isolation was carried out by PCR and agarose gel electrophoresis.

Рис. 2. Изученные почвенные профили. А – залежные почвы Y1; B – зональные палевые почвы Y3; С – залежные почвы, используемые под сенокос Y7; D – зональные палевые почв Y9; E – зональные палевые почвы Y11; F – сельскохозяйственные почвы Y13.
Fig. 2. The studied soil profiles. A – fallow land Y1; B – zonal Calcic Cryoslol Y3; C – hayfield formed on fallow land Y7; D – zonal Calcic Cryosol Y9; E – zonal Calcic Cryosol Y11; F – arable land Y13.
Таблица 1. Общая информация о строении почвенного профиля
Table 1. The general information of studied soils
|
Soil ID |
Horizon* |
Depth, cm |
Description |
Color |
Location |
Coordinates |
Soil name** |
|
Y1 |
Ah |
0 – 6 |
Horizon with accumulation of organic matter |
7.5YR 3/4 |
Fallow lands, began to overgrow by Betula platyphylla |
N 62°06’ 07.6” E 129°16’39.9” |
Plaggic Anthrosol*** (Loamic) |
|
Abhp |
6 – 30 |
Buried ploughing horizon with accumulation of organic matter, inclusion of coal |
7.5YR 7/2 |
||||
|
Y3 |
Ah |
4 – 15 |
Horizon with accumulation of organic matter |
7.5YR 6/1 |
Background forest with domination of Larix dahurica . |
N 62°05’27.7” E 129°15’19.6” |
Calcic Cryosol (Loamic) |
|
Y7 |
Ahp |
0 – 24 |
Ploughing horizon with accumulation of organic matter |
7.5YR 4/1 |
Fallow land, today it is used as a hayfield. |
N 62°08’11.5” E 128°11’41.5” |
Plaggic Anthrosol (Loamic) |
Продолжение таблицы 1
Table 1 continued
|
Soil ID |
Horizon* |
Depth, cm |
Description |
Color |
Location |
Coordinates |
Soil name** |
|
Y9 |
Ah |
5 – 13 |
Horizon with accumulation of organic matter |
7.5YR 6/1 |
Background forest with domination of Larix dahurica . |
N 62°07’33.2” E 128°11’38.3” |
Calcic Cryosol (Loamic) |
|
A/Bp |
26 – 55 |
Transit ploughing horizon |
7.5YR 7/2 |
||||
|
Y11 |
Ah |
0 – 6 |
Horizon with accumulation |
7.5YR 4/2 |
Background forest with domination of Betula platyphylla. |
N 62°41’06.1” E 129°22’46.4” |
Calcic Cryosol (Loamic) |
|
Y13 |
Ahp |
0 – 25 |
Ploughing horizon with accumulation of organic matter |
7.5YR 5/3 |
Modern arable land with oats. |
N 61°41’04.9” E 129°22’50.9” |
Plaggic Anthrosol (Loamic) |
|
Bt |
25 – 40 |
Horizon with illuvial concentration |
7.5YR 6/4 |
Note. * Guidelines for soil description (2006); ** WRB (2015); *** Fallow lands.
Таблица 2. Распределение важнейших агрохимических характеристик почв, мг/кг
Table 2. The main agrochemical parameters of studied soils, mg/kg
|
Soil ID |
рН |
P 2 O 5 |
K 2 O |
NH 4 |
NO 3 |
|
Y1 |
5.72 |
40 |
39 |
8.07 |
0.80 |
|
Y3 |
5.23 |
29 |
174 |
18.30 |
2.74 |
|
Y7 |
5.47 |
346 |
43 |
6.95 |
2.73 |
|
Y9 |
5.60 |
40 |
53 |
12.60 |
2.46 |
|
Y13 |
6.08 |
210 |
180 |
33.10 |
25.5 |
|
Y11 |
6.95 |
313 |
35 |
7.78 |
5.18 |
|
Min |
5.23 |
29 |
35 |
6.95 |
0.8 |
|
Max |
6.95 |
346 |
180 |
33.10 |
25.5 |
|
Mean |
5.84 |
163 |
87 |
14.46 |
6.56 |
|
Standart deviation |
0.61 |
145 |
69 |
10.07 |
9.37 |
|
p -value |
<0.02 |
||||
Due to the well-developed methods of taxonomical annotation and relatively representative sequencing, the v4 variable region (f515/r806) of the 16S rDNA gene was selected for future analysis. Sequencing of the variable region was performed on the Illumina MiSEQ sequencer using primers f515 (GTGCCAGCMGCCGCGGTAA) and r806 (GGA CTACVSGGGTATCTAAT) (Bates et al., 2010). The general processing of sequences was carried out on the dada2 (v1.14.1) package (Callahan, 2016). Reads were filtered by length (240 bp for forwards and 180 for reverse) and expected error rate (maxEE = 2) no N was allowed. Reads were paired by the “consensus” method, and annotated using Bayesian Naive classifier using SILVA 138 database as the training set (Quast, 2013). The main diversity analysis of the results was carried out using the phyloseq (v1.30.0) package in R (v3.6.3) (Mcmurdie, Holmes, 2013). Differential abundance of taxa in pairwise comparisons was estimated using DESeq2 (v1.26.0) (Love, Andres, 2017). The Difference in the abundances (marking phylotype as “variable”) was determined by two thresholds (baseMean >=10 and log2FoldChange >=2) and p-adj (Langfelder, Horvath, 2008). The WGCNA method was used to reveal the main phylotypes, the presence of which in microbiomes was influenced by the chemical parameters of the soil.
RESULTS AND DISCUSSION
The distribution of biogenic elements in the studied soils is presented in Fig. 3.
The highest content of nutrients, as expected, is observed in agricultural soils, this is due to the systematic application of mineral and organic fertilizers to the soil. The fallow lands are characterized by a significant decrease in the content of the main agrochemical indicators, it is associated with the processes of degradation of soil cover, removal of nutrients from soils, and transformation of fallow land soils in the direction of zonal soil formation.
For comparative assessment of the soil microbiome structure, NMDS analysis of beta diversity (Bray-Curtis) was carried out (Fig. 4). Samples were not grouped either by type or geographic location. The greatest differences were observed in the sample Y7 hayfield formed on fallow land, and in the soil sample Y13 taken from modern arable land. According to the NMDS diversity, samples of fallow soil Y1 and zonal soils Y3 and Y9 are in the same cluster, which indicates that the microbiome of fallow soils currently corresponds to the microbiome of zonal soils of Central Yakutia. The transition of Y1 to fallow took place about 30 years ago, since that time, the process of soil cover degradation and soil formation according to zonal type occurred. Significant differences in the microbiome of Y7 hayfield formed on fallow land can be caused by the fact that haying operations have been carried out on the presented soils for a long time. The investigated soils are also characterized by signs of staling starting from 24 cm, which may influence the change in microbiome composition. The microbiome of soils Y11 and Y13 have significant differences compared to other samples, which is related to the underlying parent rocks. These soils have alluvial origin because they are located in the bed of an ancient river.
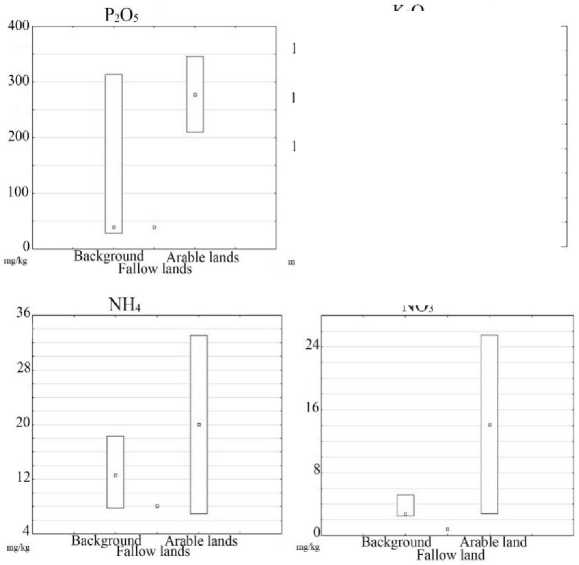
Рис. 3. Распределение важнейших агрохимических характеристик почв.
Fig. 3. The distribution of main agrochemical parameters of studied soils.
The most represented phyla in the microbiomes by relative representation (in descending order) are Acidobacteriota, Actinobacteriota, Verrucomicrobiota, Pseudomonadota (Alphaproteobacteria), Bacteroi-dota, Planctomycetota (Fig. 5). It is necessary to note that the samples described above are characterized by changes in relative representation already at the level of individual phylum. The hayfield formed on fallow land Y7 shows the most specific taxonomic profile. The relative representation of Archaea and Chloroflexi is higher, and the proportion of Verrucomicrobiota and Acidobacteriota is lower. The high difference in hayfield formed on fallow land Y7, apparently, is the processes of gleying, which take place in the underlying horizons, which leads to the suppression of microorganisms. When comparing fallow soil Y1
and modern agricultural soil, we can note that in fallow soil there is a decrease in such major phyla as Actinobateriota , Verrucomicrobiota , Chloroflexi , Crenarhaeota and Myxococcota. Among the background soils, the greatest difference is noted in sample Y11. Different types of land use are characterized by the presence of a core set of microorganisms (Fig. 6). At the same time, background soils do not differ qualitatively in contrast to fallow soils. Up to 17.8% of phylotypes are unique to each soil type. Up to 25.7% of phylotypes are common to fallow soils and background plots.
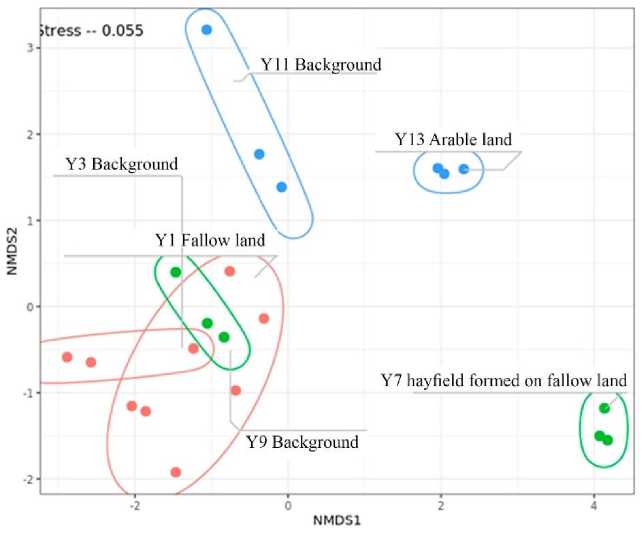
Рис. 4. NMDS для бета-разнообразия (Брей-Кертис). Эллипсы ограничивают повторы в пределах одного образца почвы. Цвет образцов соответствует месту отбора: синий – A (Y11, Y13), красный – B (Y1, Y3), зеленый – C (Y7, Y9).
Fig. 4. NMDS for beta diversity (Bray-Curtis). Ellipses confine replicates within one soil sample. The color of the samples corresponds with the sampling site: blue – A (Y11, Y13), red – B (Y1, Y3), green – C (Y7, Y9).
|
Acidobacteriota- |
16.3 |
27.4 |
4.2 |
15.5 |
14.4 |
22.7 |
|
Actinobacteriota - |
13.9 |
9.7 |
15.2 |
10.5 |
19.9 |
8.8 |
|
Verrucomicrobiota |
10 9 |
9.5 |
1.7 |
17.7 |
13.3 |
23.1 |
|
Alphaproteobacteria ■ Bacteroidota ■ |
14.7 |
16.3 |
11.4 |
14.5 |
7 |
7.8 |
|
12.1 |
7.5 |
12.1 |
10.4 |
8.1 |
5 8 |
|
|
Gammaproteobacteria ■ Gemmatimonadota ■ |
9.9 |
10 |
11.8 |
11.8 |
6.4 |
7 |
|
4.9 |
4.8 |
1.6 |
5.1 |
3.6 |
4 4 |
|
|
Chloroflexii |
2.4 |
1.7 |
11.6 |
1.5 |
4.8 |
2.4 |
|
Planctomycetota - |
3.4 |
3.4 |
1.2 |
3.7 |
2.4 |
3.7 |
|
Patescibacteria |
3.4 |
5.5 |
2.5 |
3 |
2.3 |
0.7 |
|
Firmicutes- |
1.7 |
0.3 |
4.4 |
0.4 |
2.4 |
7.7 |
|
Crenarchaeota - |
0 |
0 |
6.2 |
8.4 |
1.3 |
|
|
Desulfobacterota- |
2.6 |
0.5 |
4.8 |
1.6 |
0.1 |
0.6 |
|
Myxococcota- |
1.4 |
0.7 |
1.3 |
1.7 |
2.7 |
1.1 |
|
RCP2-54- |
0.4 |
0.7 |
0.8 |
0.2 |
0.6 |
0.7 |

Рис. 5. Мажорные филумы – относительное обилие, от наибольшего (оранжевый) к наименьшему (синий). Представители филума Pseudomonadota показаны на уровне классов.
Fig. 5 Major phyla – relative abundance, from the highest (orange) to the lowest (blue). Pseudomonadota representatives are shown on the class level.
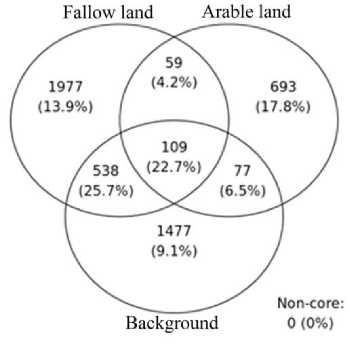
Рис. 6. Диаграмма Венна, показывающая количество уникальных и общих представителей микробных сообществ среди исследованных почв с различными типами использования земель.
Fig. 6. Venn diagram illustrating quantity of the unique and common phylotypes of soil microbial communities for the sampling sites with different types of land use.
Alpha diversity indices show the general pattern except for the sample Y13 (arable land) – there indices characterizing evenness of diversity are higher (Fig. 7). Non-reclamated soils are characterized by relatively low indices of uniformity of diversity, which may be associated with the lack of organic and mineral fertilizers applied, as well as degradation of soil cover. However, when comparing fallow soils with natural soils, it is noted that the alpha diversity of microorganisms in fallow soils is higher than in natural soils. A significant decrease in the alpha-diversity index in Y13 can be related to the pH value close to neutral, while other samples have an acidic and slightly acidic reaction.
WGCNA analysis was used to identify phylotypes that would be characteristic of the core, yet dynamically respond in similar ways to environmental factors (Fig. 8).
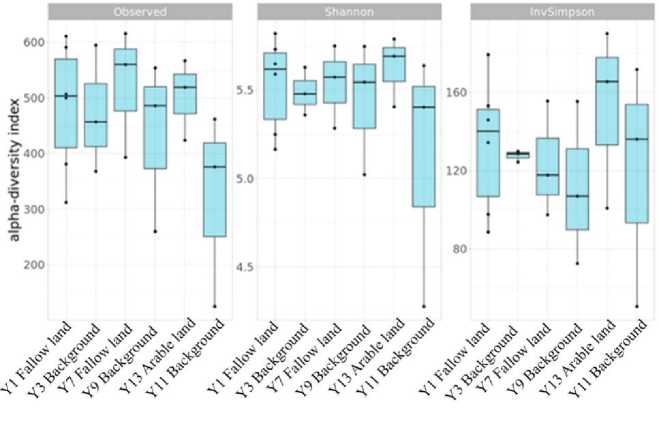
Рис. 7. Альфа-индексы разнообразия, слева направо: Наблюдаемый, Шеннона и инвертированный Симпсона.
Fig. 7. Alpha diversity indices, from left to right: Observed, Shannon and Inverted Simpson.
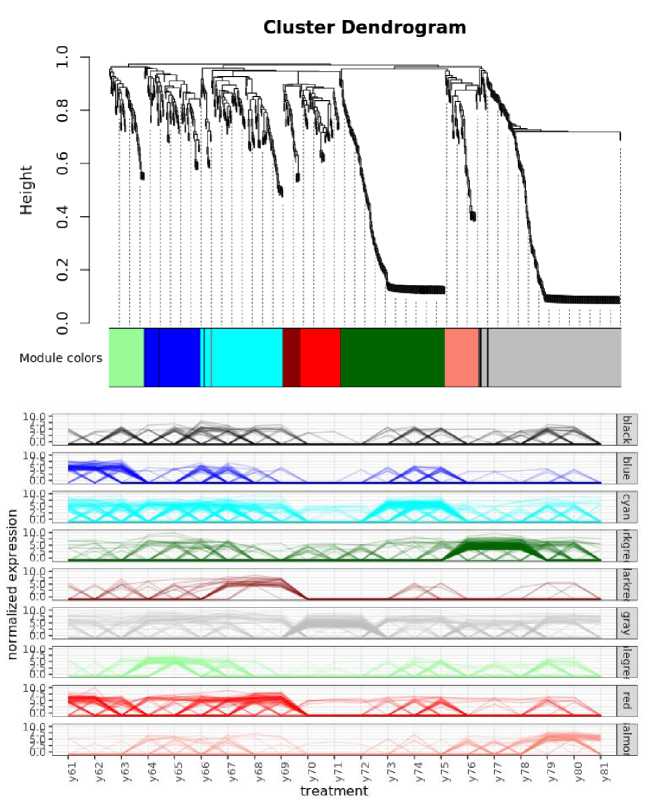
Рис. 8. Анализ WGCNA. Сверху – кластеризация совместно изменяющихся филотипов (использовался неориентированный граф) и выделение значимых кластеров филотипов. Снизу – динамика изменений отдельных кластеров.
Fig. 8. WGCNA analysis. Top – clustering of jointly changing phylotypes (undirected graph was used) and selection of significant clusters of phylotypes. Below – the dynamics of changes in the individual clusters.
A cluster of 223 phylotypes was identified, which varied in abundance together and were present in most of the samples. It should be noted that this cluster is taxonomically uneven. It is characterized by the presence of groups of microorganisms ( Udaeobacter, Pirullulacea, Acidobacteriota) difficult to cultivate and typical of soils of the far north and characterized by complex reactions to such soil properties as pH and complex carbon sources (Fig. 9).
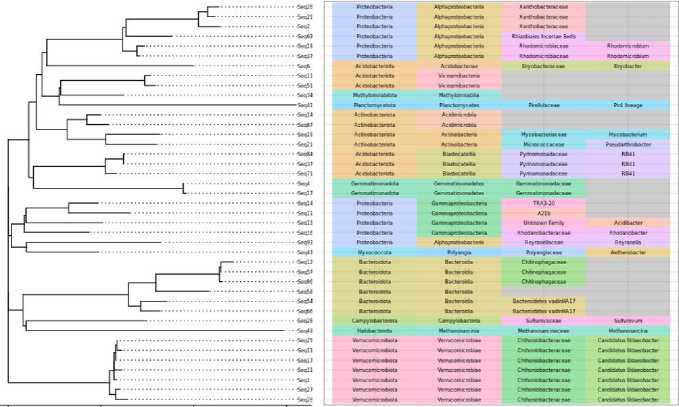
0.0 0.2 0.4 0.6 Phylum Class Family Genus
Рис. 9. Филогенетическое положение основных филотипов в “сером” кластере – кластере, содержащем основные филотипы.
Fig. 9. Phylogenetic position of major phylotypes in the “gray” cluster, a cluster containing core phylotypes.
Spearman correlation analysis was used to analyze the statistical relationship between the main agrochemical indicators and major phyla (Table 3). From the obtained data we can note that Acidobacteriota and Actinobacteriota have a strong statistical relationship with pH and NO3 (r = 1), Verrucomicrobiota c P2O5 (r = 0.98), Alphaproteobacteria c K2O and NH4 (r = 1), etc. However, these levels of the statistical rela- tionship differ depending on soil formation conditions, so, for example, on soils affected by fires according to Mantel's test there was no statistical relationship between pH reaction and agrochemical parameters, but there was a strong positive correlation between major phyla and soil substrate-induced respiration. Thus, we can conclude that these indicators only indirectly indicate that although there is a strong positive relationship between phyla, but they do not fully determine the composition of the soil microbiome.
Таблица 3. Коэффициент ранговой корреляции Спирмена основных агрохимических параметров и мажорных филогенетических групп
Table 3. Spearmen correlation of main agrochemical parameters and major phyla
|
Major phyla |
рН |
P 2 O 5 |
K 2 O |
NH 4 |
NO 3 |
|
Acidobacteriota |
1 |
0.19 |
0.65 |
0.65 |
1 |
|
Actinobacteriota |
1 |
0.60 |
0.29 |
0.65 |
1 |
|
Verrucomicrobiota |
0.10 |
0.98 |
0.56 |
0.71 |
0.56 |
|
Alphaproteobacteria |
0.13 |
0.11 |
1 |
1 |
0.13 |
|
Bacteroidota |
0.54 |
0.78 |
0.93 |
0.54 |
0.12 |
|
Gammaproteobacteria |
0.10 |
0.88 |
0.93 |
0.43 |
0.20 |
|
Chloroflexi |
0.72 |
0.07 |
0.93 |
0.50 |
0.55 |
|
Planctomycetota |
0.51 |
0.50 |
0.46 |
0.97 |
0.77 |
|
Patescibacteria |
0.10 |
0.07 |
0.56 |
0.56 |
0.17 |
|
Firmicutes |
0.13 |
0.01 |
0.24 |
0.17 |
0.41 |
|
Crenarchaeota |
0.45 |
0.09 |
0.73 |
1 |
0.16 |
|
Desulfobacterota |
0.56 |
0.51 |
0.17 |
0.03 |
0.10 |
|
Myxococcota |
0.41 |
0.83 |
0.41 |
0.41 |
1 |
|
RCP2-54 |
0.67 |
0.26 |
0.77 |
0.37 |
0.43 |
The obtained data show the specificity of the microbiome of soils of the far north. One of the characteristic features of these soils is the growth of microbiome diversity in agrocenoses with changes in the representation of specific groups of microorganisms between samples.
The studied soils are characterized by non-significant differences between fallow, natural and agricultural soils. However, according to the beta diversity index (Fig. 4), fallow soils that are subject to natural self-restoration are in the same cluster as background soils. This indicates a gradual degradation of fallow soils and their transformation towards zonal Calcic Cryosol. Such dynamics were noted by us during the micromorphological analysis of soils, fallow soils subjected to selfrestoration have less developed soil structure, which indicates the process of land degradation (Polyakov et al., 2022). Up to 25.7% of phylo-types are common to fallow soils and background plots, this indicates the fact that fallow soils have been undergoing a significant transformation towards the zonal soil series over the last 30 years, which is confirmed by the beta diversity. Among the background soils, the greatest difference is noted in sample Y11, this is due to the neutral reaction of pH, as well as the formation of soils in the bed of an ancient river. The difference between Y11 and Y13 could be due to the fact that Y13 is annually planted with crops (potatoes) and fertilizers are applied.
The city of Yakutsk and its surroundings are located in the permafrost zone, which affects the composition of the soil microbiome (Ivanova et al., 2014). Thus, three groups of microorganism’s characteristics of the northern soil microbiome (Udaeobacter, Pirullulacea, and Acidobacteriota) were found. A similar picture is characteristic of the agrosoils of Yamal, here the predominance of these phyla is also noted (Abakumov et al., 2021a). The relatively high proportion of Chloroflexi indicates the process of water-logging of soils since this phylum is an autotrophic anaerobic component of the microbiome (Abakumov et al., 2021a; Abakumov et al., 2021b). Field and background soils are characterized by an increased proportion of nitrogenfixing microorganisms (Alphaproteobacteria), while in agricultural soils their proportion decreases, indicating that there is a deficit of available nitrogen in agroecosystems (Abakumov et al., 2021b). The dominance of Acidobacteriota indicates arid conditions, which is confirmed by our study, and the significant reduction of this component in Y7 tand he predominance of Chloroflexi is a consequence of excessive moistening conditions (Ivanova et al., 2021). Presence of Crenarchaeo-ta may accompany soil-forming processes in the fallow sites of the sharply continental climate (Nicol et al., 2005). Unusual is the fact that the alpha diversity index revealed the highest number of detected phyla in fallow soil (Y1) and hayfield, formed on fallow land Y7, in typical conditions, however, the highest alpha diversity is supposed to be found in natural (background) soils (Abakumov et al., 2021a). The similar conclusion was reached by researchers in Yamal; apparently, some components of the microbiome, associated with arable soil, remain to some extent in fallow soils, but zonal components of the microbiome are also acquired.
CONCLUSIONS
The soil microbiome is represented by the dominant major phyla Acidobacteriota, Actinobacteriota, Verrucomicrobiota, Alphaproteobacteria, Bacterioidota, Gammaproteobacteria, Chloroflexi, and Planc-tomycetota. A cluster of 223 phylotypes was identified which co-vary in abundance and yet were present in the majority of the samples. The studied cluster is characterized by the presence of groups of microorganisms ( Udaeobacter, Pirullulacea, Acidobacteriota), which are characteristic groups of microorganisms for the far north. Up to 25.7% of amplicon sequence variants are common to fallow soils and background plots, this may indicate the fact that fallow soils have undergone significant transformation towards zonal soil series over the last 30 years, which is confirmed by the beta diversity analysis. Degradation of fallow land soil cover leads to the reduction of the alpha diversity index, however, when comparing fallow soils with natural soils, it is noted that the alpha diversity of microorganisms in fallow soils is higher than in natural soils.
Список литературы Soil microbiome of plaggic anthrosol and calcic cryosols in Central Yakutia
- Abakumov E., Kimeklis A., Gladkov G., Andronov E., Morgun E., Microbiomes of natural and abandoned agricultural soils of the Central part of Yamal region, IOP Conf. Series: Earth and Environmental Science, No. 941, 2021a, ID 012029, https://doi.org/10.1088/1755-1315/941/1/012029.
- Abakumov E., Zverev A., Kichko A., Kimeklis A., Andronov E., Soil microbiome of different-aged stages of self-restoration of ecosystems on the mining heaps of limestone quarry (Elizavetino, Leningrad region), Open Agriculture, 2021b, Vol. 6, No. 1, pp. 57-66, https://doi.org/10.1515/opag-2020-0207.
- Ahmad F., Saeed Q., Shah S.M.U., Gondal M.A., Mumtaz S., Chapter 11 - Environmental sustainability: Challenges and approaches, In: Natural Resources Conservation and Advances for Sustainability, Elsevier, 2022, pp. 243-270, https://doi.org/10.1016/B978-0-12-822976-7.00019-3.
- Bates S.T., Berg-Lyons D., Caporaso J.G., Walters W.A., Knight R., Fierer N., Examining the global distribution of dominant archaeal populations in soil, ISME Journal, 2010, No. 5, pp. 908-917, https://doi.org/10.1038/ismej.2010.171.
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P., DADA2: High-Resolution Sample Inference from Illumina Amplicon Data, Nat. Methods, 2016, No. 13, pp. 581-583, https://doi.org/10.1038/nmeth.3869.
- Chebykina E., Polyakov V., Abakumov E., Petrov A., Wildfire Effects on Cryosols in Central Yakutia Region, Russia, Atmosphere, 2022, No. 13, ID 1889, https://doi.org/10.3390/atmos13111889.
- Desyatkin R., Filippov N., Desyatkin A., Konyushkov D., Goryachkin S., Degradation of Arable Soils in Central Yakutia: Negative Consequences of Global Warming for Yedoma Landscapes, Front. Earth Sci., 2021 No. 9, ID 683730, https://doi.org/10.3389/feart.2021.683730.
- Desyatkin R.V., Nikolaeva M.Ch., Ivanova A.Z., Desyatkin A.R., Okoneshnikova M.V., Filippov N.V., The impact of 2021 large forest fires on vegetation and soils, on the territory of distribution of light soil-forming rocks in Central Yakutia, Dokuchaev Soil Bulletin, 2024, Vol. 118, pp. 231-275, https://doi.org/10.19047/0136-1694-2024-118-231-275.
- Dubey A., Malla M.A., Khan F., Soil microbiome: a key player for conservation of soil health under changing climate, Biodivers Conserv, 2019, No. 28, pp. 2405-2429, https://doi.org/10.1007/s10531-019-01760-5.
- Food and Agriculture Organization of The United Nation. World reference base for soil resources 2014. Rome, Italy, 2015, 203 p.
- Food and agriculture organization of the United Nations. Guidelines for soil description. Rome, Italy, 2006, 98 p.
- Ivanova E., Gladkov G., Kimeklis A., Kichko A., Karpova D., Andronov E. Abakumov E., The structure of the prokaryotic communities of the initial stages of soil formation in Antarctic Peninsula, IOP Conf. Series: Earth and Environmental Science, 2021, No. 862, ID 012056, https://doi.org/10.1088/1755-1315/862/1/012056.
- Ivanova T.I., Kuz’mina N.P., Savvinov D.D., Microbial cenoses of alas soils on the Lena-Amga interfluve in central Yakutia, Eurasian Soil Science, 2013, Vol. 46, No. 4, pp. 417-430, https://doi.org/10.1134/S1064229313040054.
- Ivanova T.I., Kuzmina N.P., Savvinov D.D., Microbial communities of frozen soils of the Tuimaada valley in Central Sakha, Biology Bulletin, 2014, Vol. 41, No. 6, pp. 500-511, https://doi.org/10.1134/S106235901406003X.
- Jansson, J.K., Hofmockel, K.S., Soil microbiomes and climate change. Nature Reviews Microbiology, 2019, No. 18, pp. 35-46, https://doi.org/10.1038/s41579-019-0265-7.
- Kuzmina N.P., Ermolaeva S.V., Chevychelov A.P., Microbiological activity of permafrost forest soils in Central Yakutia, IOP Conf. Series: Earth and Environmental Science, 2021, No. 862, ID 012057, https://doi.org/10.1088/1755-1315/862/1/012057.
- Langfelder P., Horvath S., WGCNA: An R Package for Weighted Correlation Network Analysis, BMC Bioinformatics, 2008, No. 9, ID 559, https://doi.org/10.1186/1471-2105-9-559.
- Lin Q., Baldrian P., Li L., Novotny V., Heděnec P., Kukla J., Umari R., Meszárošová L., Frouz J., Dynamics of Soil Bacterial and Fungal Communities During the Secondary Succession Following Swidden Agriculture IN Lowland Forests, Front. Microbiol., 2021, No. 12, ID 676251, https://doi.org/10.3389/fmicb.2021.676251.
- Love M., Andres S., DESeq2 data package, 2017.
- McMurdie P.J., Holmes S., Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data, PLOS ONE, 2013, No. 8, ID e61217, https://doi.org/10.1371/journal.pone.0061217.
- Mitin S.G., Sysoev G.V., Starostin I.A., Eshchin A.V., Technical and technological support for the involvement of fallow lands in agriculture, Dokuchaev Soil Bulletin, 2024, Vol. 118, pp. 276-308, https://doi.org/10.19047/0136-1694-2024-118-276-308.
- Nicol GW, Tscherko D, Embley TM, Prosser JI., Primary succession of soil Crenarchaeota across a receding glacier foreland, Environ Microbiol., 2005, Vol. 7(3), pp. 337-347, https://doi.org/10.1111/j.1462-2920.2005.00698.x.
- Okoneshnikova M.V., Gumusnoye sostoyaniye merzlotnykh poymennykh pochv doliny sredney Leny (Humus state of permafrost floodplain soils of the Middle Lena valley), Nauka i obrazovaniye, 2015, No. 3(79), pp. 94-97.
- Okoneshnikova M.V., Ivanova A.Z., Pochvy i tekhnogennyye poverkhnostnyye obrazovaniya odnoy iz promyshlennykh baz goroda Yakutska (Soils and technogenic surface formations of one of the industrial bases of the city of Yakutsk), Bulletin of the North-Eastern Federal University. M.K. Ammosov, 2020, No. 6(80), pp. 5-19.
- Polyakov V., Petrov A., Abakumov E., Micromorphological Characteristics of Fallow, Pyrogenic, Arable Soils of Central Part of Yakutia, Soil Systems, 2022, No. 6, ID 68, https://doi.org/10.3390/soilsystems6030068.
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O., The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools, Nucleic Acids Research, 2013, No. 41, pp. 590-596, https://doi.org/10.1093/nar/gks1219.
- Santos L.F., Olivares F.L., Plant microbiome structure and benefits for sustainable agriculture, Current Plant Biology, 2021, No. 26, ID 100198, https://doi.org/10.1016/j.cpb.2021.100198.
- Suman J., Rakshit A., Ogireddy S.D., Singh S., Gupta C., Chandrakala J., Microbiome as a Key Player in Sustainable Agriculture and Human Health, Front. Soil Sci., 2022, No. 2, ID 821589, https://doi.org/10.3389/fsoil.2022.821589.
- Zverev A., Kimeklis A., Kichko A., Microbial features of mature and abandoned soils in refractory clay deposits, BMC Microbiol, 2022, No. 22, ID 237, https://doi.org/10.1186/s12866-022-02634-7.


