Solution of the diffusion problem in thermocyclic nitrocementation of steel
Автор: Bakhracheva Yuliya Sagidullovna
Журнал: НБИ технологии @nbi-technologies
Рубрика: Технико-технологические инновации
Статья в выпуске: 2 (21), 2016 года.
Бесплатный доступ
It is shown that cyclic heating and cooling considerably accelerate the kinetics of chemical thermal processing of steels. The author studies the laws of formation of the strengthened layers at thermocyclic nitrocementation of steel 20 Kh. Production tests of machine parts after chemical thermal and chemical-thermocyclic processings were performed. The structure, phase composition and intensity of wear process of a blanket depending on a number of cycles are investigated.
Thermocyclic nitrocementation, steel, diffusive layer, microhardness, wear resistance
Короткий адрес: https://sciup.org/14968423
IDR: 14968423 | УДК: 669.539.382.2 | DOI: 10.15688/jvolsu10.2016.2.2
Текст научной статьи Solution of the diffusion problem in thermocyclic nitrocementation of steel
DOI:
Application of chemical heat treatment to superficial hardening of steel products material.
The majority of traditional technologies of chemical heat treatment are carried out at long endurance therefore the most important task is acceleration of diffusive saturation. It is also necessary to provide good mechanical and operational properties of steel. Chemical and thermocyclic processing will be applied for this purpose.
The research of features of formation of structure and properties of diffusive layers in the conditions of saturation is conducted became carbon and nitrogen at cyclic change of temperature. The knowledge of qualitative and quantitative regularities of formation of structural and phase states at thermocyclic influence is the cornerstone of improvement of the technologies of nitrocementation allowing to increase durability and wear resistance of products at operation.
Experiment
In this work, we investigate the influence on the wear resistance temperature and number of cycles on the distribution of carbon and nitrogen through the thickness of the diffusion layer. As well as the formation of structural sites, depending on the carbon content in thermocyclic nitrocementation of steel 20 Kh in the range of 900-600 °C [5; 6].
The chemical composition of this steel is given in Table 1.
It was implemented two modes with number of cycles 6 and 10. For comparison classic mode was carried out at a constant temperature. The carbon potential of the process atmosphere in the upper temperature boundary amounted to 1,0 ± 0,5 % in all experiments. The modes of nitrocementation are shown in Table 2.
The research was carried out on microscope “Neophot-2” at magnifications X400, X1000, X2000. The distribution of carbon and nitrogen through the thickness of the diffusion layer was determined by chemical analysis of the chips layer by layer removed from cylindrical samples with a diameter of 30 mm and length 120 mm the thickness of the release layer was 0,05 mm [1; 4].
Results and discussion
The experimental distribution curves of the carbon layer thickness for the studied modes are shown in Fig. 1.
Comparison of experimental curves of the distribution of the carbon content on the depth of the diffusion layer shows an increase of mass transfer of carbon at thermal nitrocementation, compared with the traditional process at a constant temperature. The curve on the distribution of nitrogen varies insignificantly with changes in the number of cycles.
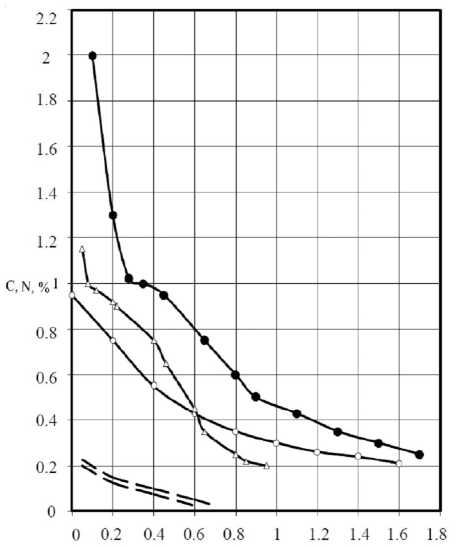
Fig 1. A curve showing the distribution of carbon in depth of the diffusion layer:
• – ten cycles; Δ – six cycles;
ο – isothermal nitrocementation, the dotted lines show the interval of nitrogen content in the diffusion layer of all performed experiments
We can assume that the different content of carbon leads to different mechanisms of formation of diffusion layer in each of the plots. Each area of the experimental distribution curve of carbon in the layer depth should be described by its equation.
We also conducted a study of the distribution of microhardness across the thickness of the diffusion layer. The results of measurement of microhardness by the depth diffusion layer for the studied modes of chemical-heat treatment is shown in Fig. 2.
Table 1
Chemical composition of steel 20 Kh
|
Grade of steel |
Chemical composition, % |
|||||
|
С |
Mn |
Si |
Cr |
S |
P |
|
|
20 Kh |
0,21 |
0,58 |
0,30 |
0,90 |
0,015 |
0,018 |
Table 2
The modes of nitrocementation of steel 20 Kh
|
№ experiment |
Temperature, оС |
The total time of the process, h |
Tempering temperature, оС |
Number of cycles |
|
|
heating |
cooling |
||||
|
1 |
900 |
– |
7,5 |
900 |
– |
|
2 |
900 |
650 |
4,3 |
900 |
6 |
|
3 |
900 |
650 |
7,5 |
900 |
10 |
Fig. 3 shows the influence of technological mode of nitrocementation on the wear rate.
In the case of single-component thermochemical treatment diffusion coefficient is determined by solving the Fick equation. For one-dimensional case, the Fick equation can be represented as:
∂ C = D ∂ 2 C ∂ t ∂ x 2 ,
where: C – the concentration of the introducing element; D – the diffusion coefficient of the element; x – path diffusion element; t – time of diffusion.
In the case of multicomponent diffusion, this equation takes the form:
∂ Ci ∂ 2 C
∂ t = ∑ j Dij ∂ x 2
That is, the diffusion coefficients Dij are the matrix form. Study of diffusion coefficients enables to obtain additional information about the implementation of elements.
When nitrocementation (two-component diffusion) equation (2) can be converted into a system of differential equations consisting of two equations
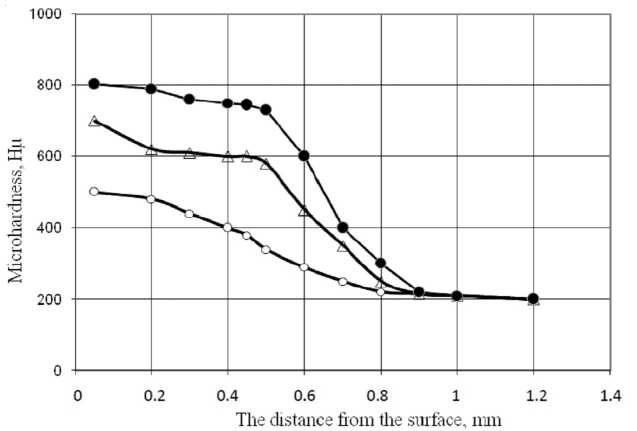
Fig. 2. The distribution of microhardness over the depth of the diffusion layer: • – ten cycles; Δ – six cycles; ο – isothermal nitrocementation
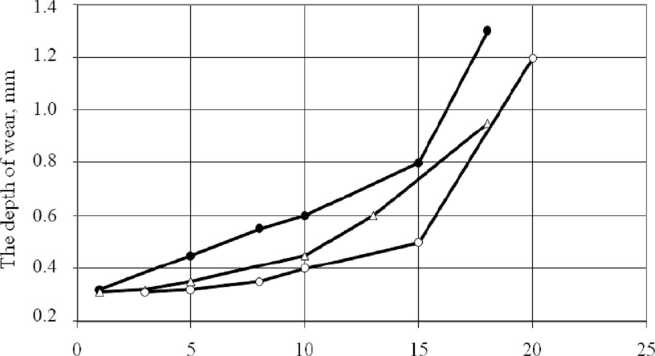
Time, h
Fig. 3. The dependence of the depth of the layer worn away with time: • – ten cycles; Δ – six cycles; ο – isothermal nitrocementation
|
d C 1 5 1 |
= D |
+ D 12 5 x2 12 |
d 2 C 2 5 x2 |
|
d С 2 |
= D 21 |
2 d C 1 1 n |
5 2 C 2 |
|
6 t |
2 + D 22 0 x |
d x 2 |
,
where: C 1, C 2 – respectively the concentration of carbon and nitrogen in the sample as a function of x and t ; D 12 – the diffusion coefficient of carbon in the gradient of nitrogen concentration; D 21 – the diffusion coefficient of nitrogen in the gradient of the carbon concentration; D 11 – the diffusion coefficient of carbon under its own concentration gradient; D 22 – the coefficient of diffusion of nitrogen under the action of its own concentration gradient.
In the literature similar solutions for the system iron-carbon-nitrogen is virtually absent. Typically, the process of nitrocementation is represented as a mathematical sum of two independent processes - carburizing and nitriding. However, the calculations showed that in the austenite (solid solution of carbon in g-iron) carbon and nitrogen displace each other and thereby increase of its thermodynamic activity [2].
The solution of equation (3) with selected boundary conditions was carried out comparatively for four plots, wherein the slope of the curve and the concentration gradient of elements across the thickness of the layer for all the studied modes. The analysis of the data shows that the effective diffusion coefficient of carbon at thermal nitrocementation have larger values than the conventional process, i. e. a cyclic change of temperature accelerates the diffusion of carbon during nitrocementation (Table 3).
As a result of thermal-cycling effects on the material diffusion mobility of atoms of saturation in the steel is increased in 2,5-3 times.
The greatest wear is observed on the samples processed according to the traditional mode at a constant temperature. The least worn specimens were observed after 10 cycles of nitrocementation. Processing steel on thermocyclic modes of nitrocementation can significantly improve the load capacity of friction surfaces. Due to the fact that wear resistance is related to the intensity of structural-phase transformations in deformed micro volumes, of great importance is the study of not only the original structure, but also complex properties emerging in the process of contact interaction. As the wear of the friction surfaces is structurally sensitive characteristic, metallographic investigation of the surface was conducted before and after friction tests [2; 3].
Metallographic investigation of the surface friction showed that after the test the surface after the carbonitriding temperature Cycling did not undergo any significant changes (Fig. 4, a ). The surface of wear after treatment by serial technology shows traces of plastic deformation of the metal (Fig. 4, b ).
Table 3
Data for calculation of diffusion coefficient of carbon in steel nitrozomocevina
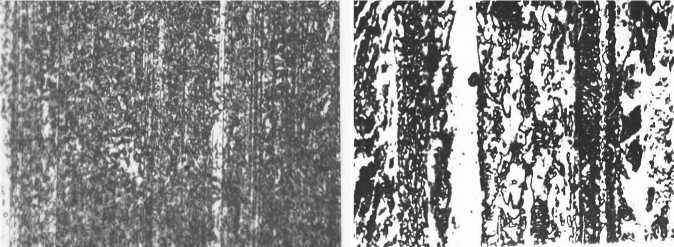
аб
Fig. 4. The structure of the surface wear at magnification X1000:
|
Regime |
The diffusion coefficients of carbon in steel, D ×10 –11 , m 2 /s |
|||
|
Part 1 |
Part 2 |
Part 3 |
Part 4 |
|
|
Ten cycles |
1,09 |
0,74 |
0,69 |
0,54 |
|
Six cycles |
0,87 |
0,68 |
0,33 |
– |
|
Isothermal nitrocementation |
0,49 |
0,42 |
0,39 |
0,34 |
( a ) after the thermocyclic nitrocementation 10 cycles; ( b ) after the isothermal nitrocementation
Conclusion
-
1. The experimental results show that the increase of mass transfer of carbon during the thermocyclic nitrocementation is compared with isothermal nitrocementation.
-
2. The results of the study show that the thermocyclic nitrocementation provides increased durability.
Список литературы Solution of the diffusion problem in thermocyclic nitrocementation of steel
- Bakhracheva Yu.S. Fracture toughness prediction by means of indentation test. International Journal for Computational Civil and Structural Engineering, 2013, no. 9, pp. 21-24.
- Bakhracheva Yu.S. The Method for lifetime estimation through the mechanical properties in Tension. Science Journal of Volgograd State University. Technology and Innovations, 2014, no. 11, pp. 27-32 DOI: 10.15688/jvolsu10.2014.2.4
- Baron A.A., Bakhracheva Yu.S. A method for impact strength estimation // Mechanika. 2007. № 66. P. 31.
- Khokhlov V.M. Wear laws at elastic interaction. Russia Engineering Research, 1996, no. 16, pp. 11-12.
- Semenova L.M., et al. Laws of formation of diffusion layers and solution of the diffusion problem in temperature-cycle carbonitriding of steel. Metal Science and Heat Treatment, 2013, no. 55, pp. 34-37.
- Shapochkin V.I., et al. Effect of nitrogen content on the structure and properties of nitrocarburized steel. Metal Science and Heat Treatment, 2011, no. 52, pp. 413-419.


