Some proteins in blood plasma
Автор: Boqiyeva I.V.
Журнал: Экономика и социум @ekonomika-socium
Рубрика: Основной раздел
Статья в выпуске: 4-2 (95), 2022 года.
Бесплатный доступ
The article provides information on the formation of cavities and apoptotic bodies in the cytoplasm.
Apoptosis, implantation, organogenesis, ceramidase, sphingosine, embryogenesis
Короткий адрес: https://sciup.org/140291754
IDR: 140291754
Текст научной статьи Some proteins in blood plasma
Plasma proteins can be broadly classified into two groups: those, including albumin, that are synthesized by the liver, and the immunoglobulins, which are produced by plasma cells of the bone marrow, usually as part of the immune response.
A number of plasma proteins have the ability to bind certain molecules (their ligands) with a high affinity and specificity. These proteins can then act as a reservoir for the ligand and help control its distribution and availability by transporting it to tissues. Binding to a protein can also render a toxic substance less harmful to the tissues. Major binding proteins and their ligands are shown in Table 1 .
Albumin. Albumin serves as an osmotic regulator and is a major transport protein
Albumin is the predominant plasma protein. It has no known enzymatic or hormonal activity, and accounts for approximately 50% of the protein found in human plasma. Its normal concentration is 35-45 g/L. With a molecular weight of about 66 kDa, albumin has highly polar nature and dissolves easily in water. At pH 7.4, it is an anion with 20 negative charges per molecule; this gives it a high capacity for non-selective binding of many ligands. It also plays a critical role in maintaining colloid osmotic pressure of the plasma.
Table 1. Transport proteins and their ligands
|
Proteins |
Ligands |
|
Cation binding |
|
|
Albumin |
Divalent and trivalent cations, e.g. С1Л Fe^ |
|
Ceruloplasmin |
or |
|
Transferrin |
|
|
Hormone binding |
|
|
Thyroid-binding globulin (TBG) |
Thyroxine (T4), tri-iodothyronine (T3) |
|
Cortisol-binding globulin (CBG) |
Cortisol |
|
Sex hormone-binding globulin (SHBG) |
Androgens (testosterone), estrogens (estradiol) |
|
Hemoglobin/protoporphyrin binding |
|
|
Albumin |
Heme, bilirubin, biliverdin |
|
Haptoglobin |
Hemoglobin dimers |
|
Fatty acid binding |
|
|
Albumin |
Nonesterified fatty acids, steroids |
The rate of albumin synthesis (14-15 g daily) depends on nutritional status, especially on the extent of amino acid deficiencies. Its half-life is about 20 days. Importantly, although the albumin level reflects the nutritional status in the longer term, in hospitalized patients the short-term changes in plasma albumin concentration are usually due to changes in hydration/
Albumin is the primary plasma protein responsible for the transport of hydrophobic fatty acids, bilirubin, and drugs
Albumin can bind (and thus solubilize) a range of substances that include the long-chain fatty acids, sterols, and several synthetic compounds. The transport of long-chain fatty acids underpins much of the body's distribution of energy-rich substrates. There are numerous fatty acid binding sites on the albumin molecule, with variable affinities. The highest affinity sites are believed to lie in the globular segments within specialized clefts of the albumin molecule ( Fig. 1 ).
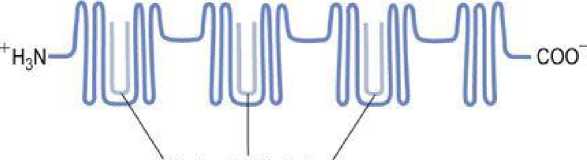
Hydrophobic clefts
FIG.1 Molecular model of human albumin.
The hydrophobic clefts are globular segments of albumin that bind fatty acids with high affinity.
In addition to binding fatty acids, albumin binds unconjugated bilirubin ( Chapter 30 ). The sites within the albumin molecule are also capable of binding a variety of drugs, including salicylates, barbiturates, sulfonamides, penicillin and warfarin. This is of great pharmacologic relevance. Such interactions are weak and the ligands become easily displaced by substances competing for a binding site. Somewhat surprisingly, albumin is not essential for human survival and rare congenital defects have been described where there is hypoalbuminemia or complete absence of albumin (analbuminemia).
Proteins that transport metal ions. Transferrin transports iron
The binding of ferric ions (Fe3+) to transferrin protects against the toxic effects of these ions. In inflammatory reactions, the iron–transferrin complex is degraded by the reticuloendothelial system without a corresponding increase in the synthesis of either of its components; this results in low plasma concentrations of transferrin and iron.
Ferritin is the major iron storage protein found in almost all cells of the body Ferritin acts as the reserve of iron in the liver and bone marrow. The concentration of ferritin in plasma is proportional to the amount of stored iron; therefore measurement of plasma ferritin is one of the best indicators of iron deficiency.
Ceruloplasmin is the major transport protein for copper
Ceruloplasmin helps export copper from the liver to peripheral tissues, and is essential for the regulation of the oxidation reduction reactions, transport, and utilization of iron ( Fig.2 ). Increased concentrations of ceruloplasmin occur in active liver disease and in tissue damage.
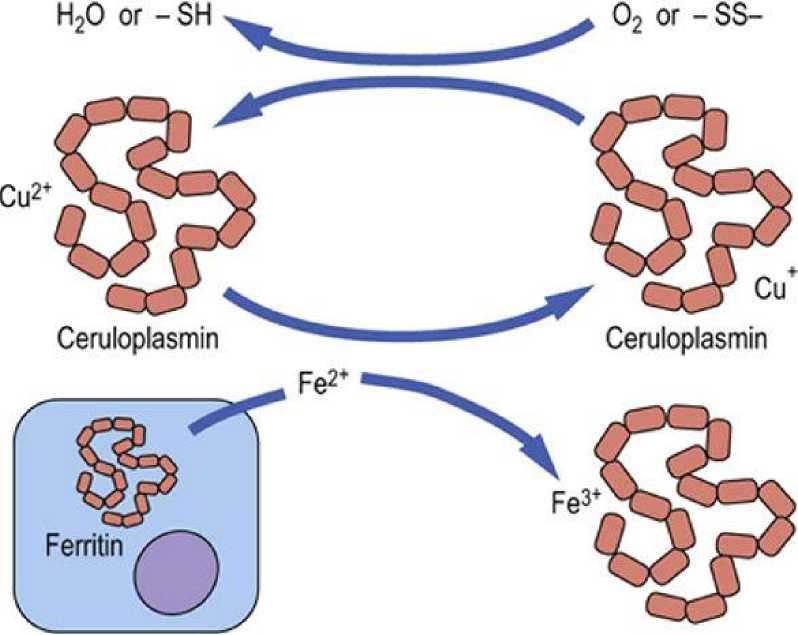
Transferrin
FIG.2 . Plasma ferroxidase activity of ceruloplasmin.
Oxidation of Fe2+ by ceruloplasmin permits the binding and transport of iron by plasma transferrin. The cuprous ion (Cu2+) bound to ceruloplasmin is regenerated by reaction with oxygen or with oxidized thiol groups.
Список литературы Some proteins in blood plasma
- Boqiyeva Ibodatxon Vohobjonovna.Interaction of the structure of individual proteins and the structure of Multimolecular proteins, isofunctional proteins. "Экономика и социум"2021.- №2(81) часть 1.Ст. 108-111.
- Boqiyeva Ibodatxon Vohobjonovna. History of development and modern trends in biochemistry. "Экономика и социум". 2021.- №9(88) часть 1.-С.
- Bokiyev Mirzoxidbek Muzafarjon o'g'li, Khaldarov S.A. Causes, symptoms of the development of diabetes mellitus in gant. "Экономика и социум". 2021.- №11(90) часть 1.131-134 Ст.
- https://doctorlib.info/medical/biochemistry/6.html#:~:text=Plasma%20contains%20many%20proteins%20broadly,bilirubin%2C%20and%20free%20fatty%20acids.


