Сорбция фульвокислоты на подфракциях ила, выделенных из минеральных горизонтов торфянисто-подзолисто-глееватой почвы
Автор: Колчанова К.А., Толпешта И.И., Изосимова Ю.Г.
Журнал: Бюллетень Почвенного института им. В.В. Докучаева @byulleten-esoil
Рубрика: Спецвыпуск по результатам молодежной конференции
Статья в выпуске: S1, 2024 года.
Бесплатный доступ
Изучение взаимодействий органического вещества с различными по размеру и составу минералогическими фракциями почвы способствует созданию прогнозных моделей по закреплению органического углерода в почвах и его устойчивости к биодеградации. В работе изучали сорбцию фульвокислоты (ФК), полученной из горизонта Н торфянисто-подзолисто-глееватой почвы на подфракциях ила, выделенных из горизонтов ELG и Ecng той же почвы: 0-0.2 мкм (I), 0.2-0.06 мкм (II), 0.06-0.02 мкм (III) и
Сорбция, фульвокислота (фк), подфракции ила, минералогия
Короткий адрес: https://sciup.org/143183582
IDR: 143183582 | УДК: 631.4 | DOI: 10.19047/0136-1694-2024-SPYC-37-72
Текст статьи Сорбция фульвокислоты на подфракциях ила, выделенных из минеральных горизонтов торфянисто-подзолисто-глееватой почвы
1 Leninskie Gori, Moscow 119234, Russian Federation, *, e-mail:
Взаимодействие органического вещества с минеральными компонентами твердой фазы почв является важнейшим процес- сом, регулирующим цикл и баланс углерода в биосфере. Сорбированное на минералах органическое вещество становится более устойчивым к микробному и ферментативному разложению (Kleber et al., 2015). Сорбция предотвращает вымывание органического вещества из почвенного профиля и ограничивает его миграцию (Hong et al., 2019; Chen, 2017). Сорбированные на поверхностях минералов гуминовые кислоты значительно модифицируют их поверхность и меняют их свойства (Kögel-Knabner et al., 2008; Jones, Singh, 2014; Anveri-Katz et al., 2017).
Исследования с использованием микроскопии показали, что органическое вещество на минеральных поверхностях сорбируется не сплошным слоем, а в виде пятен (Ransom et al., 1997,1998; Bennet et al., 2012). Это свидетельствует о том, что на поверхности минералов существуют разные сорбционные центры, на которых органическое вещество может сорбироваться посредством разных механизмов. Силоксановые поверхности каолинита в основном гидрофобные, а на силоксановых поверхностях монтмориллонита и особенно иллита и вермикулита значительная доля площади обладает гидрофильными свойствами в местах локализации постоянного отрицательного заряда кристаллической решетки. Алюми-нольные и силанольные группы боковых поверхностей слоистых алюмосиликатов обеспечивают гидрофильные взаимодействия. Площадь боковых поверхностей глинистых минералов значительно меньше, чем площадь планарных поверхностей (Tournassat et al., 2015).
Чем больше общая площадь поверхности, доступная к сорбции, тем больше органического вещества потенциально может сорбироваться на минералах (Wei et al., 2021; Yu et al., 2013; Saidy et al., 2013). Илистая фракция почвы, обладающая большой удельной площадью поверхности, является основным сорбентом органического вещества в почвах. Часто наблюдается прямая зависимость между количеством сорбированного органического вещества и содержанием илистой фракции (Mayer, Xing, 2001; Six et al., 2002). Однако в пределах илистой фракции, несмотря на меньшую площадь поверхности, крупные подфракции (0.2–2 мкм) могут содержать больше органического углерода, чем мелкие (<0.2 мкм).
В работах Kahle и Gonzalez с соавторами показано, что крупная подфракция ила содержит более ароматическое и более конденсированное органическое вещество, в более мелких подфракциях ОВ более лабильное и менее гумусированное (Kahle et al., 2004; Gonzalez, Laird, 2003), что может указывать на разные механизмы связывания ОВ и минеральных частиц.
В экспериментах по сорбции гуминовых кислот и водорастворимого органического вещества показано, что более крупные молекулы сорбируются минералами в большей степени, чем меньшие по размеру (Specht et al., 2000; Zhang et al., 2012; El-sayed et al., 2019; Chen et al., 2019; Kaiser, Zech, 2000). Высокомолекулярные соединения, гидрофобные фракции и ароматические компоненты селективно сорбируются на силоксановых поверхностях каолинита, а гидрофильные фракции с малой молекулярной массой сорбируются на боковых поверхностях кристаллитов каолинита (Chen et al., 2019; Hong et al., 2019). Гумусовые кислоты с молекулярным весом более 100 кДа в основном представлены алифатическими структурами с низким содержанием карбоксильных групп, а фракции с размерами менее 30 кДа в основном представлены ароматическими компонентами (Khalaf et al., 2003; El-sayed et al., 2019). Показано, что алифатические фракции гуминовой кислоты, выделенной из торфа, имеют большее сродство к поверхности каолинита по сравнению с ароматическими (Ghosh et al., 2009). Wang и Xing показали, что и на каолините, и на монтмориллоните преимущественно сорбируются алифатические фракции гуминовой кислоты. Адсорбированная на обоих минералах гуминовая кислота оказалась более конденсированная, чем исходная. Авторы предполагают, что более конденсированные структуры при невысокой концентрации сорбируемого вещества могут образоваться на поверхности минерала в результате сильных взаимодействий с поверхностью (Wang, Xing, 2005). В экспериментах с водорастворимым органическим веществом озерной воды установлено, что в широком диапазоне рН на каолините сорбируются преимущественно более крупные гидрофобные молекулы ОВ, а маленькие по размеру молекулы с высоким содержанием карбоксильных функциональных групп практически не сорбируются на минерале (Specht et al., 2000).
Органические вещества закрепляются на минеральной матрице почвы посредством различных механизмов, таких как электростатические взаимодействия, гидрофобные взаимодействия, лигандный обмен, через катионные мостики, а так же с помощью водородных и Ван-дер-Ваальсовых связей (Feng et al., 2005;
Lutzow et al., 2006; Kleber et al., 2007; Zhang et al., 2012; Chen et al., 2017). Сорбция органического вещества минеральной матрицей почвы зависит от состава и содержания органического вещества, величины рН, ионной силы, минерального состава и преимущественно осуществляется на глинистых минералах, содержащихся в илистой фракции почв. Идентификация механизмов сорбции органического вещества часто осложняется полиминеральностью и наличием в составе ила подфракций разного размера. Несмотря на накопленные к настоящему времени сведения о сорбционном фракционировании компонентов органического вещества на минеральных поверхностях, до сих пор не существует четкого представления о количественном вкладе различных минералов в трансформацию ОВ. Цель нашей работы заключалась в определении закономерностей и выявлении механизмов сорбции фульво-кислоты разными по размеру и минеральному составу подфракциями ила.
ОБЪЕКТЫ И МЕТОДЫ
Сорбат. Фульвокислоту (ФК) выделяли из горизонта H тор-фянисто-подзолисто-глееватой почвы (ТПГ), отобранной на территории Центрального лесного государственного природного биосферного заповедника. По WRB почва относится к Dystric Albic Gleyic Histic Retisol (WRB, 2014). Почвенный профиль состоит из оторфованых горизонтов Т 1 и Т 2 , перегнойного Н и минеральных горизонтов ELG, Eih и Ecng, которые развиты в пределах верхнего наноса, представленного средним пылеватым суглинком, подстилаемым плохосортированной тяжелосуглинистой мореной.
Образец, отобранный из горизонта Н, высушивали, перетирали и просеивали через сито с диаметром отверстий 1 мм. Для выделения ФК образец заливали раствором 0.1 н. NаOH (соотно- шение 1 : 5) и проводили 4 последовательные экстракции в течение 24 часов. Полученный щелочной экстракт подкисляли HСl до pH 2 для осаждения гуминовых кислот. Через 24 часа ФК, растворенную в надосадочной жидкости, отделяли центрифугированием (5 000 об./мин, 5 мин) и очищали по методике Форсита (Forsyth, 1947) с дополнительной обработкой HF по методике IHSS (Swift, 1996). Рабочий раствор ФК готовили, растворяя 0.3 г ФК в 1 л дистиллированной воды. ФК содержит функциональные группы с рКа1 4.1 и рКа2 5.7. Функциональные группы с рКа2 значительно преобладают над функциональными группами с рКа1 (Kolchanova et al., 2021).
Сорбенты. Фульвокислоту сорбировали на подфракциях ила, выделенных из горизонтов ELG и Ecng ТПГ почвы. Илистую фракцию (<1 мкм) выделяли из горизонтов ELG и Ecng по методике Айдиняна (1960) методом седиментации без предварительной химической обработки с двукратным разминанием. Илистая фракция была обработана 10%-ной H 2 O 2 на водяной бане при температуре 80 °С для удаления органического вещества. После обработки перекисью водорода илистую фракцию промывали дистиллированной водой до pH 6–7 и высушивали. Из обработанной Н 2 О 2 илистой фракции удалили несиликатные соединения железа по методике Мера и Джексона. Далее илистую фракцию переводили в Са-форму, промывая ее 1 М CaCl 2 . После удаления избытка реактива илистая фракция была высушена и разделена на четыре подфракции: 1.0–0.2 мкм (I), 0.2–0.06 мкм (II), 0.06–0.02 мкм (III) и <0.02мкм (IV) по методике, описанной в работе Laird (1994).
Схема проведения сорбционного эксперимента. В качестве сорбата использовалась фульвокислота, выделенная из горизонта H торфянисто-подзолисто-глееватой почвы. Навеску (0.3 г) подфракции ила помещали в пластиковую центрифужную пробирку объемом 50 мл, заливали 50 мл рабочего раствора фульво-кислоты с концентрацией Сорг 61.01 мг/л и с рН 4.64 единиц, встряхивали на ротаторе в течение 24 часов при 230 об./мин. Затем суспензию фильтровали через мембранный фильтр с размером пор 0.45мкм (OMNIPORE JHWP02500, диаметр 25 мм). В полученном растворе определяли содержание Сорг и N, рН, амфифильное и молекулярно-массовое распределения, выполненные методами, описанными ниже. Количество сорбированного углерода рассчитывали по разности концентрации углерода в рабочем растворе и в растворе после взаимодействия ФК с подфракциями ила. Эксперимент проводили в двукратной повторности.
Методы. Минеральный состав илистой фракции определяли в образцах без удаления из них органического вещества и несиликатных соединений железа методом ренгендифрактометрии на приборе MiniFlex-600 (Rigaku, Япония) с использованием CuK α излучения, фильтрованного K β фильтром, с напряжением на трубке 30 кВ и током 15 мА, и детектором D/teX Ultra. Минеральный состав подфракций ила определяли методом рентгендифрактомет-рии на приборе ДРОН-3 с использованием CuK α -излучения, фильтрованного Ni в диапазоне 2°–62° 2θ с шагом 0.05 и временем экспозиции 10 с. Напряжение и ток на трубке составляли 35 кВ и 20 мА соответственно. Съемку илистой фракции в целом и подфракций ила проводили в воздушно-сухом состоянии, после насыщения этиленгликолем и после прокаливания в течение 2 ч при температурах 350 °С и 550 °С. Содержание глинистых минералов определяли по методике Корнблюма с поправкой на LP-фактор.
Определение удельной поверхности проводили на анализаторе Quadrasorb SI / Kr (Quantachrome Instruments, Бойнтон-Бич, Флорида, США). Адсорбция проводилась при температуре жидкого азота (77.35 К). Адсорбатом служил азот чистотой 99.999%. Для калибровки объема измерительных ячеек использовали гелий марки 6.0 (99.9999%). Расчет проводился по многоточечной изотерме БЭТ в диапазоне P/P0 от 0.05 до 0.30.
Реакцию среды (рН) водной суспензии измеряли электродом (LE438 IP 67) на приборе F2‐ Standard (Mettler Toledo) при соотношении твердая : жидкая фазы – 1 : 50.
Молекулярно-массовое распределение получали на хроматографе Bio-Rad (BioLogic LP system) на колонке фирмы Pharma-cia (1.5 см × 100 см), заполненной гелем Sephadex G-50 (GE
Healthcare); скорость элюирования составляла 0.5 мл/мин, а время элюирования – 350 мин, элюентом служила дистиллированная вода. Свободный объем колонки определяли по голубому декстрану, а конечный объем по K 2 Cr 2 O 7 . Молекулярная масса рассчитывалась по эмпирической формуле Детермана (1970): lgM = 4.79 – 0.42 (V e /V 0 ); где V e – объем выхода элюата, а V 0 – свободный объем колонки, определяемый по голубому декстрану).
Амфифильные свойства изучали методом гидрофобной хроматографии на колонке Bio-Rad (9 мм × 10 см) на геле OCTYLAGAROSE (Sigma-Aldrich). Гидрофильные фракции элюировались 10% (NH 4 ) 2 SO 4 в 0.03 M ТRIS-HCl (pH 7.2) (буфер А) в течение 80 мин, затем ступенчатым градиентом при переходе от сульфата (буфер А) к чистому 0.03 M ТRIS-HCl (буфер Б) в течение 80 мин. Промывка колонки чистым ТRIS-HCl составляла 40 мин. Для элюирования гидрофобной фракции использовался 0.03 М TRIS-HCl с добавлением детергента 0.3% додецил сульфата Na (буфер С) в течение 250 мин. Скорость элюирования и общее время элюирования составили 1мл/мин и 450 мин соответственно. Коэффициент гидрофобности (hph) рассчитывали как отношение содержания гидрофобных компонентов к гидрофильным.
Содержание органического углерода и азота определяли на анализаторе High Temperature TOC/TNb Analyzer liquidTOC (Ele-mentar Analysensysteme, GmbH, Langenselbold, Germany).
РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
Содержание и минеральный состав илистой фракции и подфракций ила. В подзолистом горизонте ELG содержится 8% илистой фракции, что почти вдвое меньше, чем в нижележащем горизонте (табл. 1). Распределение частиц по размерам в пределах илистой фракции оказалось отличным в двух исследованных горизонтах. В илистой фракции подзолистого горизонта преобладают частицы размером от 1.0 мкм до 0.2 мкм (56%), а на долю частиц >0.06 мкм приходится 80%. В илистой фракции нижележащего горизонта сумма фракций >0.06 составляет 60%, а в ее составе преобладают частицы от 0.06 мкм до 0.2 мкм (35%).
Таблица 1. Содержание илистой фракции и подфракций ила
Table 1. Content of clay fraction and clay subfractions
|
Образец |
Размерные фракции |
||||
|
<1 мкм |
1.0-0.2 мкм |
0.2-0.06 мкм |
0.06-0.02 мкм |
<0.02 мкм |
|
|
% |
% от содержания ила |
||||
|
ELG |
8 |
56 |
24 |
10 |
10 |
|
Ecng |
17 |
25 |
35 |
19 |
21 |
В илистой фракции обоих изученных горизонтов диагностированы иллит, каолинит, минералы с лабильной решеткой в количестве 28%, 62%, 10% и 48%, 40%, 12% для горизонтов ELG и Ecng соответственно. Лабильные минералы в составе ила обоих горизонтов преимущественно представлены вермикулитом. В подзолистом горизонте обнаружены почвенные хлориты, образованные по вермикулиту (HIV). В составе ила обоих горизонтов присутствуют смешаннослойные минералы, состоящие из иллитовых и вермикулитовых слоев. Увеличение диффузного рассеяния в сторону малых углов на рентгендифрактограммах илистых фракций горизонта Ecng после обработки препаратов этиленгликолем (рентгенограммы не приводятся) свидетельствует о том, что в составе смешаннослойной фазы могут содержаться монтмориллонитовые слои. Прокаливание образцов ила из горизонта Ecng при 550 °С не привело к полному сжатию решетки, что можно объяснить присутствием в образце смешаннослойных минералов с хлоритовыми слоями.
Минеральный состав илистой фракции дифференцирован по размерам подфракций (табл. 2, рис. 1). Каолинит в сумме с хлоритом в обоих горизонтах преимущественно содержатся в составе крупных подфракций ила (0.06–1 мкм). В обоих горизонтах относительное содержание иллита возрастает с уменьшением размера фракций ила. Наименьшее содержание иллита (7%) обнаружено во фракции 1.0–0.2 мкм в составе ила, выделенного из горизонта Ecng. Относительное содержание лабильных минералов в горизонте ELG увеличивается с уменьшением размера подфракций ила, что в целом согласуется с литературными данными. Показа- но, что с уменьшением размера частиц в подфракциях ила уменьшается содержание каолинита и вермикулита и увеличивается содержание иллита и смектита (Ndzana, 2018; Zhi et al., 2016; Laird, 1991; Gonzalez, 2003).
Таблица 2. Минеральный состав подфракций ила Table 2. Mineral composition of clay subfractions
|
Горизонт |
Подфракция, мкм |
Содержание минералов, % от суммы трех компонентов |
||
|
каолинит + хлорит |
иллит |
лабильные минералы |
||
|
ELG |
1.0–0.2 |
67 |
28 |
5 |
|
0.2–0.06 |
51 |
30 |
19 |
|
|
0.06–0.02 |
18 |
59 |
23 |
|
|
<0.02 |
3 |
60 |
37 |
|
|
Ecng |
1.0–0.2 |
65 |
7 |
28 |
|
0.2–0.06 |
38 |
36 |
26 |
|
|
0.06–0.02 |
18 |
66 |
16 |
|
|
<0.02 |
2 |
93 |
5 |
|
В горизонте Ecng наблюдалась обратная зависимость. В самой мелкой подфракции обоих горизонтов по увеличению межплоскостного расстояния с 1.3–1.4 нм в исходном состоянии до 1.6 нм в образце, насыщенном этиленгликолем (рис. 1), диагностируется монтмориллонит. В элювиальном горизонте в первых трех подфракциях диагностирован почвенный хлорит. Хлорит однозначно диагностируется только в самой крупной фракции ила горизонта Ecng (рис. 1).
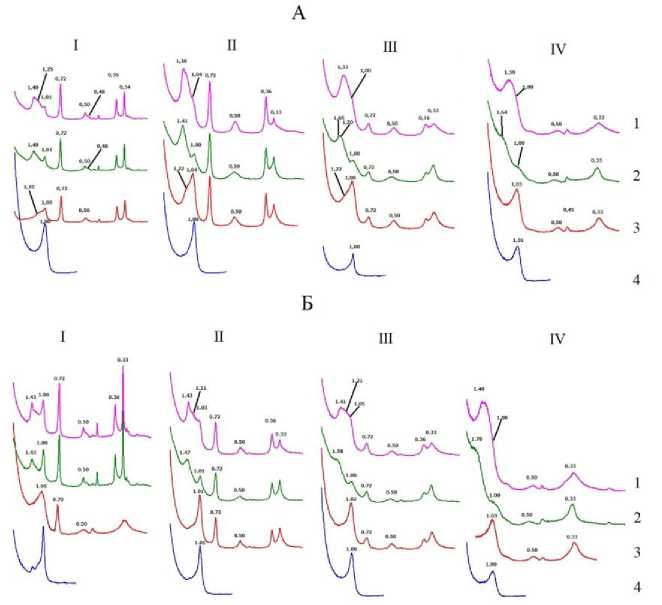
Рис.1. Рентгендифрактограммы подфракций ила горизонтов ELG ( A ) и Ecng ( Б ) 1.0–0.2 мкм (I), 0.2–0.06 мкм (II), 0.06–0.02 мкм (III) и <0.02 мкм (IV) в воздушно-сухом состоянии (1), насыщенные этиленгликолем (2) и прокаленные при температурах 350 °С (3) и 550 °С (4). Числа на кривых – d/n, нм.
Fig. 1. X-ray diffraction patterns of clay subfractions of the ELG ( A ) and Ecng ( Б ) horizons 1.0–0.2 µm (I), 0.2–0.06 µm (II), 0.06–0.02 µm (III) and <0 .02 µm (IV) in an air-dry state (1), saturated with ethylene glycol (2) and calcined at temperatures of 350 °С (3) and 550 °С (4). The numbers on the curves are d/n, nm.
С уменьшением размера подфракций в обоих горизонтах увеличивается содержание смешаннослойных минералов. На рентгенограммах воздушно-сухих образцов подфракции IV (<0.02мкм) нет острых симметричных дифракционных максимумов, а отражение от иллита (d(001) ≈ 1 нм) перекрывается отражениями от смешаннослойных минералов и монтмориллонита (рис. 1, IV). По увеличению межплоскостного расстояния до 1.6– 1.8 нм после насыщения образцов IV подфракции этиленгликолем можно сделать вывод о наличии в образцах монтмориллонита как в составе самостоятельной фазы, так и в составе смешаннослойного иллита-монтмориллонита. Широкие с небольшой интенсивностью отражения с максимумом 7.7–7.2 нм на рентгенограммах образцов IV фракции свидетельствует о присутствии в образце небольших количеств каолинита либо в составе кристаллитов небольшой толщины, либо в составе смешаннослойной фазы. На рентгенограмме образцов IV фракции, прокаленных при 550 °С, эти отражения отсутствуют. В литературе имеются сведения о том, что с уменьшением размера частиц уменьшается окристалли-зованность минералов (Zhi et al., 2016), что также приводит к уширению пиков на ренгенограммах и снижению их интенсивности.
Удельная площадь внешней поверхности подфракций ила увеличивается с уменьшением размера подфракций (табл. 3). Для первых трех подфракций площади поверхности оказались близкими в обоих горизонтах. В обоих горизонтах максимальный общий объем пор характерен для II размерной подфракции, а минимальный – для подфракции I. Средний размер пор в обоих горизонтах оказался практически одинаковым для подфракций одного размера. При этом в IV подфракции в обоих горизонтах размер пор оказался в 2 раза меньше, чем в более крупных подфракциях. Поры первых двух размерных подфракций представлены мезо- и макропорами. В III и IV подфракциях обнаружены микропоры, средний размер и объем которых в 2 раза больше в IV подфракции, по сравнению с III подфракцией.
Таблица 3. Площадь поверхности подфракций ила, выделенных из горизонтов ELG и Ecng
Table 3. Surface area of clay subfractions isolated from the ELG and Ecng horizons
|
Подфракции ила (мкм) |
Площадь поверхности (мг/м2) |
Объем пор (г/см3)/сред-ний размер пор (нм) |
Объем микропор (см3/г) |
Площадь поверхности (м2/г) |
|
|
микропор |
остальных пор |
||||
|
ELG 1.0–0.2 |
26.1 |
0.093/6.1 |
0 |
0 |
26.1 |
|
ELG 0.2–0.06 |
72.7 |
0.215/6.8 |
0 |
0 |
72.7 |
|
ELG 0.06–0.02 |
124.4 |
0.177/6.1 |
0.029 |
42.1 |
82.3 |
|
ELG <0.02 |
160 |
0.144/3.8 |
0.064 |
110.1 |
49.9 |
|
Ecng 1.0–0.2 |
31 |
0.112/6 |
0 |
0 |
31 |
|
Ecng 0.2–0.06 |
73.5 |
0.200/6 |
0 |
0 |
73.5 |
|
Ecng 0.06–0.02 |
110.5 |
0.167/6.6 |
0.024 |
34.7 |
75.8 |
|
Ecng <0.02 мкм |
134.3 |
0.146/3.6 |
0.057 |
91 |
43.3 |
Сорбция ФК пофдракциями ила и ее механизмы. В пересчете на единицу массы больше ФК сорбируют подфракции III и IV, обладающие большей площадью поверхности (табл. 4), что согласуется с литературными данными (Kennedy, 2014; Mayer, Xing, 2001; Six et al., 2002; Wei et al., 2021). В пересчете на единицу площади поверхности наблюдается обратная зависимость: чем крупнее фракция, тем больше на ней сорбируется ФК.
Таблица 4. Количество сорбированной ФК и ее гидрофильных и гидрофобных компонентов на подфракциях ила Table 4. Amount of sorbed FA and its hydrophilic and hydrophobic components on clay subfractions
|
Образец |
Сорбировано С орг |
Сорбировано С (гфл) |
Сорбировано С (гфб) |
||||
|
мг/г |
мг/м2 |
мг/г |
мг/м2 |
мг/г |
мг/м2 |
||
|
Горизонт ELG |
|||||||
|
1.0–0.2мкм |
1 |
0.97 |
0.037 |
0.042 |
0.001 |
0.927 |
0.036 |
|
2 |
0.95 |
0.036 |
0.036 |
0.001 |
0.909 |
0.035 |
|
|
0.2–0.06мкм |
1 |
1.73 |
0.024 |
0.072 |
0.001 |
1.660 |
0.023 |
|
2 |
2.06 |
0.028 |
0.081 |
0.001 |
2.055 |
0.028 |
|
|
0.06–0.02мкм |
1 |
2.66 |
0.021 |
1.232 |
0.011 |
1.426 |
0.011 |
|
2 |
2.98 |
0.024 |
1.427 |
0.012 |
1.550 |
0.012 |
|
|
<0.02мкм |
1 |
2.34 |
0.015 |
0.867 |
0.006 |
1.469 |
0.009 |
|
2 |
2.45 |
0.015 |
0.890 |
0.005 |
1.551 |
0.010 |
|
Продолжение таблицы 4
Table 4 continued
|
Образец |
Сорбировано С орг |
Сорбировано С (гфл) |
Сорбировано С (гфб) |
||||
|
мг/г |
мг/м2 |
мг/г |
мг/м2 |
мг/г |
мг/м2 |
||
|
Горизонт Ecng |
|||||||
|
1.0–0.2мкм |
1 |
1.62 |
0.052 |
0.039 |
0.001 |
1.577 |
0.052 |
|
2 |
1.47 |
0.047 |
0.080 |
0.003 |
1.370 |
0.047 |
|
|
0.2–0.06мкм |
1 |
2.09 |
0.028 |
0.092 |
0.001 |
2.000 |
0.028 |
|
2 |
1.90 |
0.026 |
0.036 |
0.001 |
1.863 |
0.026 |
|
|
0.06–0.02мкм |
1 |
2.37 |
0.021 |
0.484 |
0.004 |
1.885 |
0.021 |
|
2 |
2.64 |
0.024 |
0.552 |
0.005 |
2.090 |
0.024 |
|
|
<0.02мкм |
1 |
2.76 |
0.021 |
0.210 |
0.002 |
2.545 |
0.021 |
|
2 |
3.05 |
0.023 |
0.298 |
0.003 |
2.750 |
0.023 |
|
После взаимодействия с фульвокислотой в равновесной жидкой фазе увеличивается значение рН. С уменьшением размера подфракции ила разница между исходным и равновесным значениями рН ФК становится больше (табл. 5), что может свидетельствовать о различных механизмах сорбции ФК на минеральных частицах разного размера. После взаимодействия с сорбентами в равновесном растворе увеличивается отношение C / N, вероятно, вследствие преимущественной сорбции азотсодержащих соединений. Прослеживается тенденция к уменьшению этого отношения с уменьшением размера подфракций (табл. 5). Можно предполагать, что сорбированное органическое вещество на разных по размеру подфракциях ила имеет разный состав. В работах, посвященных изучению состава органического вещества, сорбированного на разных по размеру минеральных компонентах, показано, что более крупная подфракция ила содержит более ароматическое и более конденсированное органическое вещество, а в более мелких подфракциях преобладает лабильное и менее гумусированное органическое вещество (Kahle et al., 2004; Gonzalez, Laird, 2003).
Сорбция ФК на подфракциях ила не привела к изменению молекулярно-массового распределения оставшейся в равновесном растворе ФК. На кривых молекулярно-массового распределения ФК до и после сорбции наблюдался один пик, соответствующий молекулярной массе 20 кДа (рис. 2).
На кривых амфифильного распределения исходной ФК гидрофильная фракция представлена широким пиком относительно небольшой интенсивности, а более гидрофобные компоненты – группой частично перекрывающих друг друга пиков (рис. 3). Доля гидрофобных компонентов в исходном растворе ФК превышает долю гидрофильных, а величина коэффициента гидрофобности hph равна 3.17 (табл. 5).
После сорбции во всех вариантах опыта изменилось соотношение гидрофильных и гидрофобных фракций в растворе ФК. На всех размерных фракциях сорбируется больше гидрофобных компонентов ФК, чем гидрофильных (табл. 4, рис. 3).
Таблица 5. Свойства ФК до и после сорбции на подфракциях ила горизонта ELG и Ecng
Table 5. Properties of FA before and after sorption on clay subfractions of the ELG and Ecng horizons
|
Образец |
pH |
C орг (мг/л) |
N (мг/л) |
C / N |
hph |
|
|
ФК |
4.26 |
61.01 |
0.99 |
60 |
3.17 |
|
|
ELG |
||||||
|
1.0–0.2 мкм |
1 |
5.60 |
55.19 |
0.87 |
64 |
2.85 |
|
2 |
5.55 |
55.33 |
0.84 |
66 |
2.85 |
|
|
0.2–0.06 мкм |
1 |
5.91 |
50.61 |
0.61 |
83 |
2.57 |
|
2 |
5.87 |
48.67 |
0.96 |
60 |
2.45 |
|
|
0.06–0.02 мкм |
1 |
6.18 |
45.05 |
Не опр. |
- |
5.25 |
|
2 |
6.16 |
43.14 |
Не опр. |
- |
6.14 |
|
|
<0.02 мкм |
1 |
6.73 |
46.98 |
Не опр. |
- |
4.00 |
|
2 |
6.77 |
46.29 |
Не опр. |
- |
4.00 |
|
Продолжение таблицы 5
Table 5 continued
|
Образец |
pH |
C орг (мг/л) |
N (мг/л) |
C/N |
hph |
|
|
ФК |
4.26 |
61.01 |
0.99 |
60 |
3.17 |
|
|
Ecng |
||||||
|
1.0–0.2 мкм |
1 |
5.96 |
51.30 |
0.72 |
71 |
2.57 |
|
2 |
5.93 |
52.30 |
0.52 |
100 |
2.70 |
|
|
0.2–0.06 мкм |
1 |
6.31 |
48.45 |
0.58 |
84 |
2.45 |
|
2 |
6.34 |
49.61 |
0.63 |
79 |
2.45 |
|
|
0.06–0.02 мкм |
1 |
6.44 |
46.79 |
0.53 |
88 |
3.00 |
|
2 |
6.48 |
45.15 |
0.70 |
65 |
3.00 |
|
|
<0.02 мкм |
1 |
6.60 |
44.47 |
0.70 |
63 |
2.33 |
|
2 |
6.64 |
42.71 |
0.53 |
81 |
2.33 |
|
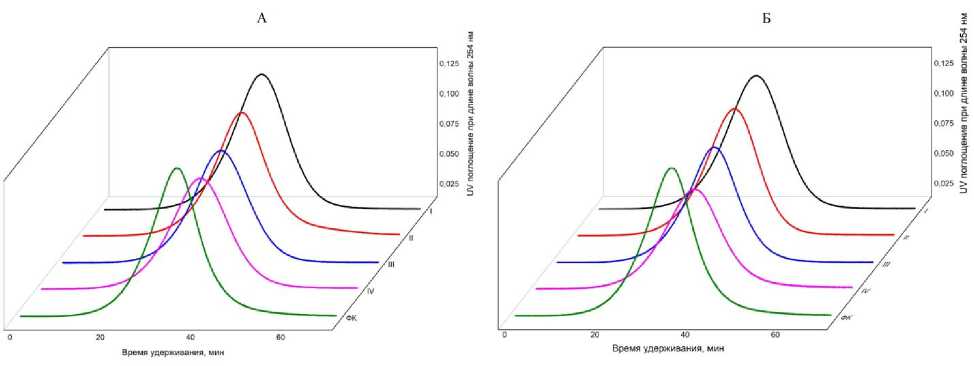
Рис. 2. Молекулярно-массовые распределения ФК до и после сорбции на подфракциях ила I, II, III и IV, выделенных из горизонтов ELG (А) и Ecng (Б).
Fig. 2. Molecular weight distributions (HPSEC chromatograms) of FA before and after sorption on clay subfractions I, II, III and IV, isolated from the ELG (A) and Ecng (Б) horizons.
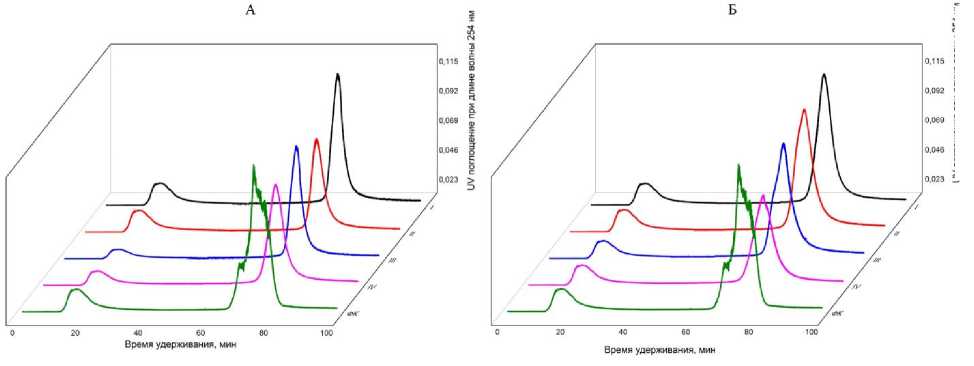
Рис. 3. Амфифильное распределение ФК до и после сорбции на подфракциях ила I, II, III и IV, выделенных из горизонтов ELG (А) и Ecng (Б) .
Fig. 3. Amphiphilic distribution of FA before and after sorption on clay subfractions I, II, III and IV, isolated from the ELG (A) and Ecng (Б) horizons.
UV поглощение при длине волны 254 нм
Наблюдается общая закономерность: чем ниже коэффициент гидрофобности в растворе после сорбции, тем больше сорбируется органического вещества (таблицы 4 и 5). Однако подфракция III илистой фракции горизонта ELG выпадает из общей закономерности. После сорбции на этой подфракции, оставшаяся в растворе ФК характеризуется значительно более высоким коэффициентом гидрофобности, чем исходная ФК, но при этом именно на этой подфракции наблюдается максимальная сорбция органического углерода в пересчете на единицу массы (табл. 4).
Для обоих горизонтов наблюдается классическая pH-зависимая сорбция ФК: чем ниже значение pH, тем больше органического углерода сорбируется на минеральной поверхности (рис. 4).
Такая зависимость обусловлена сорбцией ФК на рН-зависимых боковых поверхностях глинистых минералов, которая осуществляется за счет гидрофильных компонентов ФК. Так как в ФК, использованной в эксперименте, преобладают функциональные группы с рКа 2 5.7, то с увеличением от 5.7 до 6.8 единиц количество депротонированных карбоксильных групп должно увеличиваться. Liu с соавторами показали, что силанольные (≡Si–OH) и алюминольные (≡Al–OH 2 OH) группы каолинита имеют рКа 6.9 и рКа 5.7 соответственно (Liu et al., 2013). Вследствие того, что в подфракциях I и II преобладает каолинит, в диапазоне рН от 5.6 до ≈ 6 эти функциональные группы могут быть частично протонированы, что приводит к увеличению сорбции ФК в этом диапазоне рН. Увеличение рН до 7 способствует депротонированию алюминольных и силанольных группировок и возникновению дополнительного отрицательного заряда поверхности минерала, и отталкиванию от нее депротонированных функциональных групп ФК.
Наиболее высокие равновесные значения рН наблюдались в экспериментах с IV подфракцией, на которой сорбируется наименьшее количество органического вещества в пересчете на единицу поверхности (рис. 4).
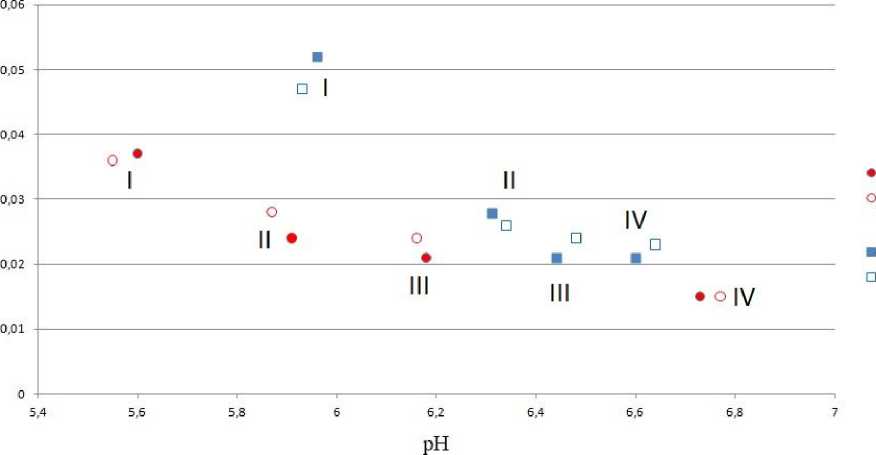
Рис. 4. Зависимость сорбции ФК в мг/м2 от равновесного значения pH для подфракций ила, выделенного из горизонтов ELG (1) и Bcng (2). I–IV подфракции ила.
Fig. 4. Dependence of FA sorption in mg/m2 on the equilibrium pH value for sludge subfractions isolated from the ELG (1) and Bcng (2) horizons. I–IV clay subfractions.
В этой подфракции содержится более 60% иллита, а содержание каолинита не превышает 3% в обоих горизонтах (табл. 2). Алюминольные группы мусковита характеризуются несколько меньшими величинами рКа1, чем у каолинита и варьируют от 3.9 до 5.52 единиц (Kriaa et al., 2008; Liu et al., 2018). Следовательно, в изученном диапазоне рН с увеличением рН возрастает количество депротонированных отрицательно заряженных алюминольных групп, что уменьшает сорбцию ФК (рис. 4).
Наблюдаемое увеличение рН в равновесной жидкой фазе можно объяснить сорбцией ФК на минералах путем лигандного обмена на ОН-. Учитывая тот факт, что увеличение рН в равновесном растворе, по сравнению с исходным раствором ФК, в наибольшей степени наблюдалось для подфракции IV, можно предположить, что этот механизм вносит большой вклад в сорбцию ФК на самых мелких подфракциях ила.
Еще одним механизмом гидрофильных компонентов ФК может быть образование мостиковой связи между Са2+, присутствующим на поверхности минералов в обменной форме и депро-тонированными функциональными группами. Наибольшее количество гидрофильной фракции в пересчете на единицу массы сорбировалось IV фракцией горизонта ELG, в которой содержалось набольшее количество лабильных минералов (табл. 2 и 4).
Гидрофобные фракции ФК сорбировались преимущественно на каолините. Небольшой постоянный отрицательный заряд кристаллической решетки не оказывает значительного влияния на гидрофобные свойства силоксановой поверхности каолинита. Чем больше в составе подфракции каолинита, тем больше на ней сорбируется гидрофобных компонентов (рис. 5). Плотность постоянного отрицательного заряда на силоксановой поверхности иллита значительно больше, чем у каолинита ввиду большей степени изоморфных замещений в тетраэдрической сетке. Следовательно, на силоксановых поверхностях иллита доля гидрофобных участков будет меньше, чем на поверхности каолинита. В пересчете на единицу поверхности сорбция гидрофобных компонентов уменьшалась с увеличением содержания иллитов в подфракциях ила, т. е. от более крупных подфркаций к более мелким (рис. 5).
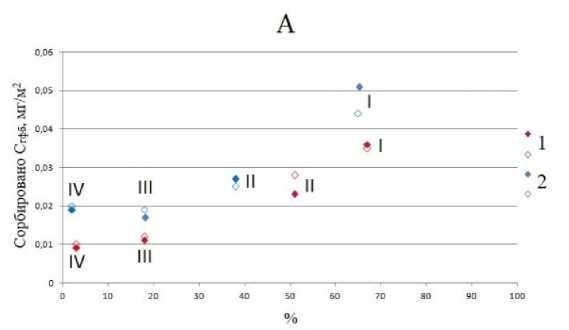
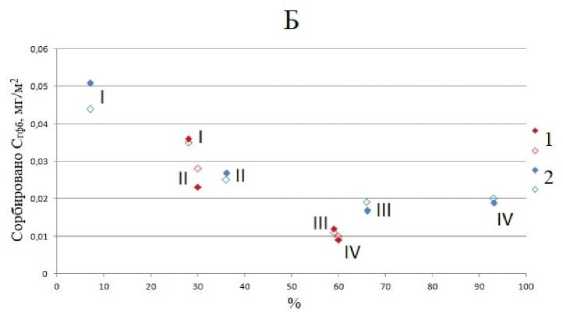
Рис. 5. Зависимость сорбции гидрофобной фракции ФК в мг/м2 от содержания каолинита (А) и иллита (Б) в % для подфракций ила, выделенного из горизонтов ELG (1) и Bcng (2). I–IV – подфракции ила.
Fig. 5. Dependence of sorption of the hydrophobic fraction of FA in mg/m2 on the content of kaolinite ( A ) and illite ( Б ) in % for subfractions of clay, isolated from the ELG (1) and Bcng (2) horizons. I–IV are clay subfractions.
В III и IV подфракциях ила обнаружены микропоры. С увеличением количества микропор увеличивается площадь поверхности образца и количество сорбционных мест, что должно приводить к увеличению сорбции органического вещества. Из рисунка 6
видно, что количество сорбированного органического вещества в пересчете на единицу не зависит от площади микропор и увеличивается с увеличением площади мезо- и макропор.
Полученные результаты свидетельствуют о том, что в условиях проведенного эксперимента молекулы ФК не сорбируются в микропорах. Средний размер пор в IV подфракции составляет 3.6– 3.8 нм (табл. 3), а значение pH составляет 6.7 (табл. 5). Из литературы известно, что такие белки как химотрипсин, миоглобин, лизоцим и папаин с близкой к сферической или компактной эллипсоидной структурой, обладают близкой к ФК молекулярной массой – 14–25кДа (Страйер, 1984; Blocklehurst et al.,1981; Марри и др., 1993). Диаметр таких молекул при значениях рН около 4 составляет около 4.5–5 нм. При повышении значения pH молекула фульвокислоты претерпевает конформационные изменения: карбоксильные и фенольные группы молекулы депротонируются, и из-за возникающих электростатических сил отталкивания увеличивается объем молекулы. В работе (Avena, 1999) доказано, что размер молекулы фульвокислоты с молекулярной массой 23кДа увеличивается при увеличении значений pH от 4 до 6.5 при низком значении ионной силы. Если предположить, что размеры молекул фульвокислоты, использованной в нашем эксперименте, были близки к размерам, приведенным в цитируемой литературе, то они сорбировались на поверхности, не проникая в мелкие поры. Таким образом, площадь поверхности, доступная для сорбции на IV подфракции ила, уменьшается за счет исключения порового пространства микропор. Показано, что более крупные молекулы гуминовой кислоты сорбировались преимущественно на поверхности глинистых минералов, в то время как более мелкие молекулы ФК проникали в поровое пространстве минералов (Zhang, 2012). Для различных почв Maer с соавторами установили, что в мезопорах размером <8 нм содержится менее 10% от общего органического углерода, вероятно из-за частичной блокировки пор крупными молекулами органического вещества (Mayer et al., 2004).
A
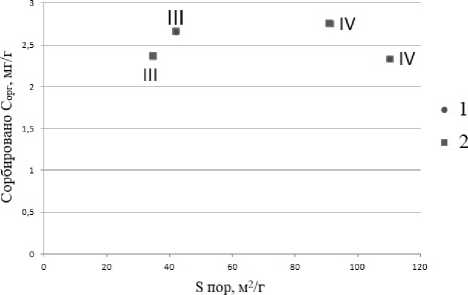
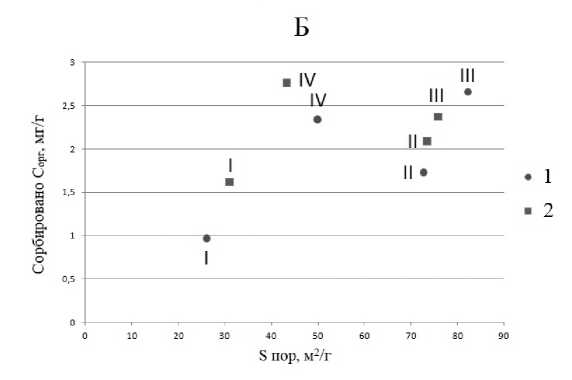
Рис. 6. Зависимость сорбции C орг в мг/г от площади поверхности микропор ( А ) для III и IV подфракций ила и от площади поверхности остальных пор ( Б ).
Fig. 6. Dependence of C org sorption in mg/g on the surface area of micropores ( A ) for clay subfractions III and IV and on the surface area of the remaining pores ( Б ).
Из полученных экспериментальных данных о свойствах сорбентов и сорбата до и после взаимодействия с подфракциями ила можно заключить, что сорбционная способность подфракций ила зависит от pH, площади поверхности, наличия и размера пор, минерального состава подфракции и свойств сорбируемого органического вещества.
ЗАКЛЮЧЕНИЕ
Выделенные из горизонтов ELG и Ecng торфянисто-подзолисто-глееватой почвы подфракции ила дифференцированы по минеральному составу. Каолинит в обоих горизонтах преимущественно содержится в составе крупных подфракций ила (0.06– 1 мкм), а содержание иллита возрастает с уменьшением размера фракций ила. В горизонте ELG с уменьшением размера подфракций ила увеличивается относительное содержание лабильных минералов. С уменьшением размера подфракций в обоих горизонтах увеличивается содержание смешаннослойных минералов и монтмориллонита, как в составе иллита-монтмориллонита, так и в составе самостоятельной фазы. С уменьшением размера подфракции увеличивается удельная площадь поверхности.
В пересчете на единицу массы больше ФК сорбируют подфракции III и IV, обладающие большей площадью поверхности. В пересчете на единицу площади поверхности наблюдается обратная зависимость: чем крупнее фракция, тем больше на ней сорбируется ФК. В процессе сорбции фульвокислота фракционируется по амфифильности и химическому составу. Все подфракции ила, выделенного из обоих горизонтов, сорбируют преимущественно гидрофобные компоненты ФК, однако в более тонких подфраци-ях, с минимальным содержанием каолинита, вклад гидрофильных компонентов в общую сорбцию возрастает. В условиях проведенного эксперимента молекулы ФК с молекулярной массой 20 кДа не сорбировались в микропорах, имеющих средний размер ≈ 3.7 нм.
Основным механизмом сорбции ФК на подфракциях ила являются гидрофобные взаимодействия. На основании изменения равновесных значений рН, соотношения C / N в фульвокислоте до и после сорбции на подфракциях, зависимости количества сорбированной ФК от рН и от минерального состава подфракций можно заключить, что гидрофильные компоненты ФК сорбируются посредством электростатических взаимодействий, путем лигандного обмена на боковых сколах глинистых минералов и с образованием мостиковых связей с ионом Ca2+, занимающим обменные позиции в глинах.
В большинстве работ, описывающих механизмы сорбции органического вещества на минеральных поверхностях, исследователи концентрирую свое внимание на отдельных чистых минералах, значительно меньшее количество работ посвящено сорбционным процессам на почвах и почвенных илах. Наша работа, выполненная на самых тонких подфракциях ила, показала особенную важность размеров порового пространства сорбентов, а также продемонстрировала, насколько сильное влияние оказывают амфифильные свойства ФК на сорбционные процессы.
Список литературы Сорбция фульвокислоты на подфракциях ила, выделенных из минеральных горизонтов торфянисто-подзолисто-глееватой почвы
- Айдинян Р.Х. Извлечение ила из почв: краткая инструкция. Методические указания. 1960.
- Марри Р., Греннер Д., Мейес П., Родуэлл В. Биохимия человека. Т. 1. М.: Мир, 1993. 384 с.
- Страйер Л. Биохимия. Т. 1. М.: Мир, 1984. 232 с.
- Ahmat A.M., Thiebault T., Guégan R. Phenolic acids interactions with clay minerals: A spotlight on the adsorption mechanisms of Gallic Acid onto montmorillonite // Applied Clay Science. 2019. Vol. 180. 105188.
- Avena M.J., Vermeer A.W.P., Koopal L.K. Volume and structure of humic acids studied by viscometry pH and electrolyte concentration effects // Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1999. Vol. 151. P. 213-224.
- Avneri‐Katz S., Young R.B., McKenna A.M., Chen H., Corilo Y.E., Polubesova T., Borch T., Chefetz B. Adsorptive fractionation of dissolved organic matter (DOM) by mineral soil: macroscale approach and molecular insight // Org. Geochem. 2017. Vol. 103. P. 113-124. https://doi.org/10.1016/j.orggeochem.2016.11.004.
- Bennett R.H., Hulbert M.H., Curry K.J., Curry A., Douglas J. Organic matter sequestered in potential energy fields predicted by 3-D clay microstructure model // Mar. Geol. 2012. Vol. 315. P. 108-114.
- Blocklehurst K., Baines B.S., Kierstan M.P.J. Papain and other constituents of Cartica papaya L. // Topics on enzyme and fermentation biotechnology / E. Wiseman, E. Horwad (Eds). 1981. Vol. 5. P. 262-335.
- Chen H., Koopal L.K., Xiong J., Avena M., Tan W. Mechanisms of soil humic acid adsorption onto montmorillonite and kaolinite // Journal of Colloid And Interface Science. 2017. Vol. 504. P. 457-467.
- Chen H., Koopal L., Xu J., Wang M., Tan W. Selective adsorption of soil humic acid on binary systems containing kaolinite and goethite: assessment of sorbent interactions // Eur. J. Soil. sci. 2019. Vol. 70. P. 1098-1107.
- El‐sayed M.E.A., Khalaf M.M.R., Gibson D., Rice J.A. Assessment of clay mineral selectivity for adsorption of aliphatic/aromatic humic acid fraction // Chem. Geol. 2019. Vol. 511. P. 21-27. https://doi.org/10.1016/j.chemgeo.2019.02.034.
- Feng X., Simpson A.J., Simpson M.J. Chemical and mineralogical controls on humic acid sorption to clay mineral surfaces // Organic Geochemistry. 2005. Vol. 36. P. 1553-1566.
- Forsyth W.G. Studies on the more soluble complexes of soil organic matter; a method of fractionation // Biochem. J. 1947. Vol. 41. P. 176-181. https://doi.org/10.1042/bj0410176.
- Ghosh S., Wang Z.‐Y., Kang S., Bhowmik P.C., Xing B.S. Sorption and fractionation of a peat derived humic acid by kaolinite, montmorillonite and goethite // Pedosphere. 2009. Vol. 19. P. 21-30. https://doi.org/10.1016/s1002‐0160(08)60080‐6.
- Gonzalez J.M., Laird D.A. Carbon sequestration in clay mineral fractions from C-labeled plant residues // Soil Science Society of America Journal. 2003 Vol. 67. No. 6.
- Hong H., Chen S., Fang Q., Algeo T.J., Zhao L. Adsorption of organic matter on clay minerals in the Dajiuhu peat soil chronosequence, South China // Applied Clay Science. 2019. Vol. 178. Article 105164.
- Isolation of IHSS Samples. URL: https://humic‐substances.org/isolation-of-ihss-samples.
- Kahle M., Kleber M., Jahn R. Retention of dissolved organic matter by phyllosilicate and soil clay fractions in relation to mineral properties // Organic Geochemistry. 2004. Vol. 35. No. 3. P. 269-276.
- Kaiser K., Zech W. Sorption of dissolved organic nitrogen by acid subsoil horizons and individual mineral phases // Eur. J. Soil Sci. 2000. Vol. 51. P. 403-411.
- Kennedy M.J., Löhr S.C., Fraser S.A., Baruch E.T. Direct evidence for organic carbon preservation as clay-organic nanocomposites in a Devonian black shale; from deposition to diagenesis // Earth and Planetary Science Letters. 2014. Vol. 388. P. 59-70.
- Khalaf M., Kohl S.D., Klumpp E., Rice J.A., Tombácz E. Comparison of sorption domains in molecular weight fractions of a soil humic acid using solid‐state 19F NMR // Environ. Sci. Technol. 2003. Vol. 37. P. 2855-2860. https://doi.org/10.1021/es0206386.
- Kleber M., Eusterhues K., Keiluweit M., Mikutta C., Mikutta R., Nico P.S. Mineral‐organic associations: formation, properties, and relevance in soil environments // Adv. Agron. 2015. Vol. 130. P. 1-140. https://doi.org/10.1016/bs.agron.2014.10.005.
- Kleber M., Sollins P., Sutton R. A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonalstructures on mineral surfaces // Biogeochemistry. 2007. Vol. 85. P. 9-24. https://doi.org/10.1007/S10533-007-9103-5.
- Kögel‐Knabner I., Guggenberger G., Kleber M., Kandeler E., Kalbitz K., Scheu S., Eusterhues K., Leinweber P. Organo‐mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry // J. Plant Nutr. Soil Sci. 2008. Vol. 171. P. 61-82.
- Kolchanova K., Tolpeshta I., Izosimova U. Adsorption of fulvic acid and water extractable soil organic matter on kaolinite and muscovite // Agronomy. 2021. Vol. 11. P. 2420. https://doi.org/10.3390/agronomy11122420.
- Kriaa A., Hamdi N., Srasra E. Determination of point of zero charge of tunisian kaolinites by potentiometric and mass titration methods // Chin. Chem. Soc. 2008. Vol. 55. P. 53-61. https://doi.org/10.1002/jccs.200800010.
- Laird D.A., Barak P., Nater E.A., Dowdy R.H. Chemistry of smectitic and illitic phases in interstratified soil smectite // Soil Sci. Soc. Am. J. 1991. Vol. 55. P. 1499-1504.
- Laird D.A., Dowdy R.H. Simultaneous mineralogical quantification and chemical characterization of soil clays // Clays and Clay Minerals. 1994. Vol. 42. No. 6. P. 747-754.
- Liu X., Sprik M., Cheng J., Meijer E.J., Wang R. Acidity of edge surface sites of montmorillonite and kaolinite // Geochim. Cosmochim. 2013. Vol. 117. P. 180-190. https://doi.org/10.1016/j.gca.2013.04.008.
- Liu Y., Alessi D.S., Flynn S.L., Alam M.S., Hao W., Gingras M., Zhao H., Konhauser K.O. Acid‐base properties of kaolinite, montmorillonite and illite at marine ionic strength // Chem. Geol. 2018. Vol. 483. P. 191-200. https://doi.org/10.1016/j.chemgeo.2018.01.018.
- Lützow M.V., Kögel-Knabner A.I., Ekschmitt K. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions - A Review // The European Journal of Soil Science. 2006. Vol. 5. No. 4. P. 426-445.
- Mayer L.M., Schick L.L., Hardy K.R., Wagai R., MCcarthy J. Organic matter in small mesopores in sediments and soils // Geochimica et Cosmochimica Acta. 2004. Vol. 68. No. 19. P. 3863-3872.
- Mayer L.M., Xing B. Organic matter-surface area relationships in acid soils // Soil Sci. Soc. Am. J. 2001. Vol. 65. P. 250-258.
- Miyahara M., Vinu A., Ariga K. Adsorption myoglobin over mesoporous silica molecular sieves: pore size effect and pore-filling model // Mater. Sci. Eng. 2007. Vol. 27. P. 232-236. https://doi.org/10.1016/j.msec.2006.05.012.
- Ndzana G.M., Huang L., Wang J.B., Zhang Z.Y. Characteristics of clay minerals in soil particles from an argillic horizon of Alfisol in central China // Applied Clay Science. 2018. Vol. 151. P. 148-156.
- Ransom B., Bennett R.H., Baerwald R., Shea K. TEM study of in situ organic matter on continental margins: occurrence and the “monolayer” hypothesis // Mar. Geol. 1997. Vol. 138. P. 1-9.
- Ransom B., Kim D., Kastner M., Wainwright S. Organic matter preservation on continental slopes: importance of mineralogy and surface area // Geochim. Cosmochim. 1998. Vol. 62. P. 1329-1345.
- Saidy A.R., Smernik R.J., Baldock J.A., Kaiser K., Sanderman J. The sorption of organic carbon onto differing clay minerals in the presence and absence of hydrous iron oxide // Geoderma. 2013. Vol. 209-210. P. 15-21.
- Singh B., Jones E. Organo‐mineral interactions in contrasting soils under natural vegetation // Front. Environ. Sci. 2014. Vol. 2. No. 2. https://doi.org/10.3389/fenvs.2014.00002.
- Six J., Conant R.T., Paul E.A. Paustian K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils // Plant and Soil. 2002. Vol. 241. P. 155-176.
- Specht C.H., Kumke M.U., Frimmel F.H. Characterization of NOM adsorption to clay minerals by size exclusion chromatography // Water Res. 2000. Vol. 34. P. 4063-4069. https://doi.org/10.1016/S0043‐1354(00)00148‐2.
- Tournassat C., Bourg I.C., Steefel A.I., Bergaya F. Surface Properties of Clay Minerals // Developments in Clay Science. 2015. Vol. 6. P. 5-31.
- Wang K., Xing B. Structural and sorption characteristics of adsorbed humic acid on clay minerals // J. Environ. Qual. 2005. Vol. 34. P. 342-349. https://doi.org/10.2134/jeq2005.0342.
- Wei L., Bu H., Wei Y., Wu H., Wang G., Chen P., Li H. Fractionation of natural algal organic matter and its preservation on the surfaces of clay minerals // Applied Clay Science. 2021. Vol. 213. 106235.
- Yu W.H., Li N., Tong D.S., Zhou C.H., Lin C.H., Xu C.Y. Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review // Applied Clay Science. 2013. Vol. 80-81. P. 443-452.
- Zhang L., Luo L., Zhang S. Integrated investigations on the adsorption mechanisms of fulvic and humic acids on three clay minerals // Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2012. Vol. 406. P. 84-90.
- Zhang Z.Y., Huang L., Liu F., Wang M.K., Fu Q.L., Zhu J. Characteristics of clay minerals in soil particles of two Alfisols in China // Applied Clay Science. 2016. Vol. 120. P. 51-60.


