Совершенствование автоматизированных систем управления технологическим процессом промывки нитробензола
Автор: О. М. Ионов, Н. О. Кулигина, А. А. Попов, Н. И. Кечкина, Т. Н. Павлычева
Журнал: Современные инновации, системы и технологии.
Рубрика: Прикладные вопросы и задачи применения систем и технологий
Статья в выпуске: 5 (2), 2025 года.
Бесплатный доступ
В статье рассматриваются вопросы модернизации автоматизированных систем управления технологическим процессом промывки нитробензола на химическом производстве. Основное внимание уделено повышению эффективности и безопасности процесса, а также обеспечению соответствия современным требованиям к качеству продукции, экологической безопасности и защите труда. Предложено внедрение современных микропроцессорных средств автоматизации, распределённых систем управления (DCS) и программируемых логических контроллеров (PLC), что позволяет повысить точность регулирования ключевых параметров процесса (температуры, pH, дозировки компонентов), снизить риск аварийных ситуаций и улучшить условия труда персонала. Результаты внедрения подтверждают повышение стабильности технологического процесса, снижение количества дефектов продукции и соответствие строгим нормативным требованиям.
Автоматизированные системы управления, технологический процесс, промывка нитробензола, микропроцессорная автоматизация, безопасность производства, качество продукции, экологическая безопасность
Короткий адрес: https://sciup.org/14133034
IDR: 14133034 | DOI: 10.47813/2782-2818-2025-5-2-5021-5029
Текст статьи Совершенствование автоматизированных систем управления технологическим процессом промывки нитробензола
DOI:
With increasingly stringent requirements for safety and product quality in chemical manufacturing, the modernization of automated process control systems is of paramount importance. This article focuses on the nitrobenzene washing process—a critical stage in the production chain—where compliance with technical regulations and environmental standards is essential. Nitrobenzene serves as a key raw material in the production of aniline and various aromatic nitrogen-containing compounds such as benzidine, quinoline, and azobenzene. It is also widely used as a solvent for cellulose ethers, a component in metal polishing compounds, and a mild oxidizer. Furthermore, nitrobenzene derivatives find applications as explosives, rocket fuel components, and as fragrant or odor-fixing substances in perfumery (including artificial musks). Historically, nitrobenzene itself was produced under the name "bitter-almond" or "mirban" oil. Some derivatives are also used in varnishes, paints, and medicine [1-3].
The primary objective of this research is to develop and implement contemporary automation solutions aimed at enhancing both the efficiency and safety of the nitrobenzene washing process. At present, improving the competitiveness of products manufactured by chemical enterprises is highly relevant. This goal can be achieved by increasing product quality and reducing defects. The implementation of this project addresses the challenges of improving process safety, product quality, and working conditions. The development of automated process control systems based on modern microprocessor-based automation tools makes it possible to significantly increase the speed and fault tolerance of operations, as well as to ensure the safety and accuracy of the ongoing process.
In the chemical industry, particular attention must be paid to process automation due to the complexity and high speed of technological processes, their sensitivity to deviations from optimal conditions, and the explosion and fire hazards associated with the substances involved [4-5]. Automation of technological processes is a decisive factor in increasing labor productivity, ensuring production safety, and improving product quality. The effectiveness of any automated control system depends on its design, implementation, and operation. Among these factors, design is especially important, as it lays the foundation for the long-term, trouble-free operation of the automation system [6].
Modernization of automation systems as a whole reduces production costs while maintaining or even improving product quality, thereby increasing profitability. In the chemical industry, ensuring process safety and environmental friendliness is of great importance. This article includes a dedicated section focused on developing measures to minimize the risk of industrial injuries, occupational diseases, poisoning, and the occurrence of explosive, fire-hazardous, and emergency situations.
-
• Use of more reliable microelectronic computer technology.
-
• Greater flexibility in upgrading hardware and software, with the ability to increase the computing power of the automated process control system.
-
• Maximum compliance with technological regulations.
-
• High accuracy in dosing washing components, which is essential for obtaining a high-quality product.
-
• Objective control and forecasting of technological process development.
These improvements are expected to set new standards for safety, efficiency, and reliability in nitrobenzene production.
MATERIALS AND METHODS
The modernization of automated process control systems for the nitrobenzene washing stage was carried out at a chemical production facility, with the aim of improving both the efficiency and safety of this critical process. The nitrobenzene production process is continuous and consists of several stages, including acid mixture preparation, benzene nitration, nitrobenzene acid washing, oil washing, water washing, drying, packaging, labeling, and transportation. This study specifically addresses the washing stage, which is essential for removing impurities and ensuring the quality of the final product [7,8].
The main equipment involved in the washing stage comprises continuous washing devices with a working volume of 4.3 m³, each equipped with a mixing (basket) zone and a separation zone. These apparatuses are fitted with coils for temperature control and agitators to ensure thorough mixing. The process utilizes nitrobenzene as the primary raw material, along with water for acid washing and aqueous ammonia for alkaline washing. Nitrobenzene is a toxic, flammable liquid with a characteristic odor, widely used as an intermediate in the production of aniline and various aromatic nitrogen-containing compounds, as well as in pharmaceuticals, perfumery, and as a solvent for cellulose ethers. Aqueous ammonia, a clear, colorless liquid with a characteristic odor, is used for neutralization in the alkaline washing stage; while it is non-flammable, its vapors can be hazardous if allowed to accumulate.
The acid washing process involves supplying crude nitrobenzene and water to the mixing zone of the washing apparatus, where mineral acids and watersoluble impurities are removed. The mixture then flows into the separation zone, where nitrobenzene is separated from the acidic water. The temperature in the apparatus is automatically maintained within the range of 35–50°C by regulating the flow of hot or cooled water through the coil. The separated nitrobenzene is then directed by gravity to the alkaline washing apparatus [9].
In the alkaline washing stage, nitrobenzene from the acid washing is mixed with an ammonia solution for neutralization. The temperature is similarly controlled, and the product is separated and sent to the next stage. The process is designed to ensure maximum compliance with technological regulations, high accuracy in the dosing of wash components, and thorough mixing for deep purification.
The existing automated process control system, implemented in 1994, was analyzed for technical limitations and compliance with current safety, quality, and environmental standards. The modernization project involved upgrading to advanced microprocessor-based automation tools, including distributed control systems (DCS) and modern programmable logic controllers (PLCs), to increase reliability, flexibility, and computing power. Real-time monitoring, data acquisition, and advanced process control (APC) software were integrated to optimize process parameters and detect deviations. Enhanced safety measures, such as interlocks and emergency shutdown procedures, were introduced to minimize the risk of industrial injuries, poisoning, and hazardous incidents.
The automation system was designed to maintain strict control over key process variables, including temperature, pressure, flow rates, and dosing accuracy. The main control actions included regulating the flow of coolant into the coils and the supply of ammonia solution for pH control. The system also monitored the rotation speed of the agitator, ensuring the required mixing intensity. Any deviation from set parameters, such as a drop in agitator speed or an increase in temperature, triggered appropriate safety responses. Process data were collected automatically and used to evaluate improvements in process stability, product quality, and compliance with safety and environmental regulations. The results were compared with historical data from the previous system to assess the effectiveness of the modernization.
Through these technical upgrades, the new automation system is expected to set new standards for safety, efficiency, and reliability in nitrobenzene production, while also addressing current regulatory and environmental challenges. The modernization ensures the long-term, trouble-free operation of the production facility and contributes to improved product quality and reduced defect rates.
RESULTS AND DISCUSSION
Description of the Technological Process
General information on the production process of nitrobenzene
The production of nitrobenzene is a continuous process involving several key stages: acid mixture preparation, benzene nitration, and subsequent washing steps—acid, alkaline, and water washing— followed by drying, packaging, and transportation. The washing stage is critical for improving the quality of nitrobenzene by removing residual acids and impurities. Equipment involved includes multiple washing apparatuses, separators, receivers, and pumps, each with specific operational parameters such as volume, temperature, and flow rate (Table 1).
T able 1 - D evices involved in the process .
|
1. Flushing apparatus pos. 1/1 |
Vp = 5.5 m3, Vpw = 4.25 m3, F = 5.3 m2, H = 4750 mm, L = 1800 mm |
|
2. Flushing apparatus pos. 1/2 |
Vp = 4.5 m3, Vpw = 4.0 m3, F = 5.3 m2, H = 5020 mm, L = 1700 mm |
|
3. Flushing device |
Vp = 5.5 m3, Vpw = 4.25 m3, F = 5.3 m2, H = 4750 mm, L = 1800 mm |
|
4. Nitrobenzene receiver (raw product separator) |
V=10m3 |
|
5. Mother Cell Receiver |
V=4m3 |
|
6.Condensate receiver |
Vp=8m3, Vpw=6.4m3, L=2880mm, L=2000mm |
|
7. Vertical centrifugal pump |
Q=5-10m3/h, electric engine. 4 AIR 112 M U2 |
|
8. Vertical centrifugal pump |
Q=5-10m3/h, electric engine. AIR 112 M U2 |
|
9. Queen cell separator |
Vp=0.68m3 |
|
10. Mixer |
Vp = 16 L |
Vp is the total volume, Vpw is the working volume, F is the section of the basket, H is the height, D is the diameter, V is the volume, L is the length, Q is the flow rate of the liquid.
A notable challenge in the washing stage is the need for precise temperature control (35-50°C) and pH regulation, both of which are essential for product quality and safety. The process is highly sensitive to disturbances such as fluctuations in nitrobenzene flow or coolant temperature. Automation is necessary to maintain stable conditions and prevent the formation of persistent emulsions or excessive impurities.
Water-based technical ammonia
Empirical formula NH 4 OH
A clear, colurless liquid with a characteristic odor. It has the properties of bases and it forms salts with acids. Aqueous ammonia is not flammable or explosive. However, during degassing, ammonia vapors can create dangerous concentrations in the room that can lead to an explosion. Gaseous ammonia is a gas with a pungent odor, toxic, explosive, flammable.
The concentration limits of ignition are 15-28%. The auto-ignition temperature is 6500 ° C. Aqueous ammonia must meet the requirements and standards of GOST 9-92.
Technical nitrobenzene
The empirical formula is C 6 H 5 NO 2 . Nitrobenzene is a toxic transparent liquid from light yellow to light brown color with a smell of bitter almonds, flammable.
Nitrobenzene is easily soluble in ethanol, ether, soluble in benzene, hardly soluble in water (0.19%). With water, it forms azeotrope (boiling point 98.6 ° C, 88% water).
In terms of the degree of harmful effect on the human body, it belongs to the second class of hazard (highly hazardous substance) according to GOST 12.1.007-76. Nitrobenzene is used as an intermediate product in the aniline-color, pharmaceutical, and perfume industries. Based on nitrobenzene, aniline, benzidine, t-nitroaniline, t-phenylenediamine, p-aminosalipilic acid (PASA) are obtained. Nitrobenzene dissolves many organic compounds, including cellulose nitrate, forming a gelatinous mass with it. In the petroleum industry, nitrobenzene is used as a solvent for the purification of lubricating oils [10].
Acid washing of nitrobenzene
To remove mineral acids and water-soluble impurities, acid nitrobenzene is subjected to water washing in a washing apparatus (pos. 1/1), which has a mixing (basket) and separation zones, equipped with a coil and an agitator.
Acidic separated nitrobenzene is supplied to the mixing zone of the apparatus (pos. 1/1), where fire water (PVC) is simultaneously supplied from the network through a filter.
From the mixing zone, nitrobenzene with acidic water enters the separation zone of the apparatus through a cut-out in the side wall, where the product is separated from the acidic water.
The separated product enters the product pipe, which does not reach 5 cm to the bottom of the apparatus, from where it continuously flows by gravity into the alkaline washing apparatus (pos. 1/2).
Acidic water from the apparatus (pos. 1/1) flows continuously, by gravity, into the diluent.
The temperature in the basket of the apparatus (pos. 1/1) is regulated automatically or remotely, within the range of 35-50 ° C by supplying hot or circulating (cooled) water to the coil of the apparatus.
The washing apparatus pos.1/1 of the acid washing of nitrobenzene is connected to the system of absorption of nitrogen oxides, vapors from the equipment (devices, separator) of nitration units, dilution of nitrobenzene. Nitrogen oxides and vapours enter the absorber [10].
Alkaline washing of nitrobenzene
Alkaline flushing (neutralization) of nitrobenzene is carried out in a flushing apparatus (item 1/2), which has a mixing (basket) and separation zones, equipped with a coil and an agitator.
Nitrobenzene is continuously supplied to the washing basket of the apparatus (pos. 1/2) from the washing apparatus (pos. 1/1) through the overflow, as well as an ammonia solution pre-mixed in the mixer.
The temperature in the basket of the apparatus is maintained automatically or remotely within the range of 35-50 ° C, by supplying hot or circulating (cooled) water to the coil of the apparatus.
Nitrobenzene from the bottom of the separation zone of the alkaline apparatus after neutralization by gravity through the overflow, the queen cell continuously enters the separator of the mother cell from the upper part of the separation zone of the device. Nitrobenzene from the separator is discharged by gravity to the water washing device, and the separated mother cell is transferred to the mother cell receiver, from where it is transferred by a centrifugal vertical pump, after which it is sent for incineration.
The finished product of this production is nitrobenzene, which meets the requirements of TU 636-0204208-107-89.
Characteristics of the process in terms of
EXPLOSION AND FIRE HAZARD
The technological process in the building is explosive. The explosive zone classification is B-1a [11]. According to ONTP 105-95, the building is classified as fire hazard category A. According to SNiP 2.09.04-87, nitrobenzene production is classified as an industry with harmful working conditions (group ZB of production processes). In accordance with RD 34.21.122-87, the lightning protection category for the building is I.I.
Regulatory restrictions on process
INDICATORS AND TECHNOLOGICAL PARAMETERS
Regulatory restrictions on process indicators and technological parameters are specified in the technological regulations in production.
Exceeding the permissible values of technological parameters can lead to emergencies and various violations of the technological process, as well as affect the quality of the nitrobenzene produced.
Consequences of violation of regulatory restrictions
Production of high-quality nitrobenzene in a continuous process is possible only if the technological regime is strictly observed.
Failure to comply with the raw nitrobenzene washing regime causes the formation of persistent emulsions and an increase in the amount of nitrophenols impurities in the finished product.
The rinsing process takes place with thorough stirring for deeper purification of the nitrobenzene obtained in the nitration stage.
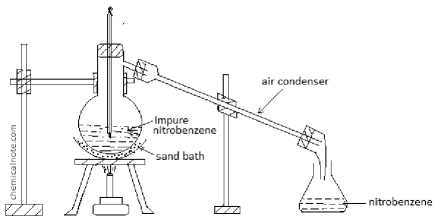
Figure 1. Distillation setup for nitrobenzene purification.
Figure 1 illustrates the laboratory apparatus used for the purification of nitrobenzene by distillation. The setup consists of a round-bottom flask containing impure nitrobenzene, which is heated in a sand bath to initiate the distillation process. The vapors produced pass through an inclined air condenser, where they cool and condense back into liquid form. The purified nitrobenzene is then collected in a receiving flask at the end of the condenser. This configuration enables the effective separation of nitrobenzene from non-volatile impurities, ensuring a higher purity of the final product. The apparatus is supported by stands and clamps to maintain stability and safety during the distillation process.
The physicochemical parameters of the washed nitrobenzene are influenced by the following factors: increase or decrease in temperature in washing devices pos. 1/1-2 (due to the cessation of cooling or heating with water), a change in the pH of the medium or the separation mode.
If, as a result of violation of regulatory restrictions, nitrobenzene is spilled, then its maximum permissible concentration in the air of the working area will increase, which will lead to gas contamination with nitrobenzene vapors. This is an emergency situation and the emergency exhaust ventilation unit must be turned on.
ANALYSIS OF THE TECHNOLOGICAL PROCESS AS AN OBJECT OF CONTROL
Any technological process, as an object of control, is characterized by the following main groups of variables:
These variables in the process of regulation must be maintained at a given level or changed according to a given law, by changing which the regulatory system can affect the object for the purpose of control -control (regulatory) actions, the changes of which are not related to the impact of the regulatory system. These changes reflect the influence of external conditions on the object of control, changes in the characteristics of the object itself, etc.
The production of nitrobenzene at the washing stage is a rather complex control object in which various parameters are monitored. The technological process includes several devices, each of which has input and output parameters.
The process is explosive or fire hazardous, the class of the explosive zone was defined as B-1a, in terms of the degree of fire hazard it belongs to category A, the production of nitrobenzene belongs to industries with harmful working conditions (group 3B of production processes), the category of lightning protection is I.I.
Solving the problems of analysis involves a complete description of the existing actions in the system for the subsequent obtaining of transformations – regularities according to which input actions are transformed into reactions [12].
Description of the system coordinates: X – control action; Z – perturbing effect; f – uncontrolled disturbance; q – controlled disturbance; S – transformations; Y – Steerable coordinates.
The control action X is set by a person or is generated by a control device. Control actions include those values, the values of which can be varied in the process of management.
Perturbations Z are independent of the control system. They can occur both outside the system and within it.
The object of control in the production of nitrobenzene is considered to be continuous washing devices with a capacity of 4.3 m3, equipped with mixers and coils.
The temperature in the washing machines must be maintained in accordance with the regulations. Exceeding the established limits will lead to a violation of the washing regime, as a result of which the resulting nitrobenzene will not meet the requirements due to the excess of the content of impurities, the presence of which in nitrobenzene is unacceptable. The temperature in the devices is maintained by regulating hot and cold water entering the coil. The main disturbing effects are changes in the flow rate of nitrobenzene entering the apparatus and changes in the temperature of hot and cold water. The output variable is the temperature in the apparatus.
The regulating effect is the flow of the coolant into the coils of the apparatus. The constancy of the flow rate of flushing substances into the flushing apparatus is achieved by control valves at the inlet (stabilization of the flow rate of flushing liquids). The pH of the medium in the washing unit is regulated by the supply of ammonia water. When the pH increases above the regulated value, the degree of valve opening on the ammonia solution line increases, and the pH normalizes over time.
Control of the rotation of the agitator in the apparatus ensures the required amount of product. When the agitator is stopped or its rotation speed drops below the permissible value, the dosages of flushing liquids into the apparatus must be cut-off [12].
Analysis of the existing system
The current system employs manual or semiautomatic control to manage essential parameters such as temperature and pH, mainly by adjusting coolant flow and the addition of ammonia solution. Despite these measures, this approach presents significant challenges. Manual adjustments are not sufficiently responsive to sudden changes in input flow or temperature, which can result in deviations from the desired process conditions and compromise the stability of the operation. The reliance on human intervention also heightens the risk of operator errors, potentially leading to process upsets or safety incidents. Furthermore, fluctuations in process parameters can cause inconsistencies in product quality, resulting in the presence of unacceptable impurities in the final nitrobenzene. Additionally, the process operates under hazardous conditions -classified as explosive and fire hazardous (zone B-1a, category A) with harmful working environments (group ZB) - making robust automation essential for ensuring both safety and compliance with regulatory requirements
Proposed solutions
To address the identified issues, a comprehensive modernization of the automation system is proposed, focusing on both technical and software upgrades. The implementation of advanced process control (APC) introduces automated control loops for temperature and pH regulation, leveraging real-time feedback from sensors to dynamically adjust coolant flow and ammonia solution dosing. This ensures that process parameters remain stable within the required ranges, minimizing the risk of deviations and improving overall product quality. Enhanced safety measures include the integration of emergency shutdown mechanisms and automatic activation of ventilation systems in response to parameter deviations or accidental spills, thereby ensuring strict compliance with safety standards and reducing the likelihood of hazardous incidents.
Additionally, user-friendly human-machine interface (HMI) systems are developed to enable operators to monitor process conditions efficiently and intervene manually when necessary, further supporting operational flexibility and safety. The incorporation of data logging and analysis tools allows for continuous tracking of process performance, facilitating the identification of trends or anomalies and supporting proactive maintenance and optimization efforts.
Collectively, these modernization initiatives are designed to bolster process stability, reduce operator workload, and elevate both product quality and workplace safety.
Implementation and results
Following implementation, the proposed automation system was rigorously evaluated for its effectiveness in managing the nitrobenzene washing stage. Automated control loops played a crucial role in maintaining temperature and pH within the strictly defined operational ranges, which resulted in a marked reduction in process upsets and increased overall process stability.
The consistency of process conditions directly contributed to a decrease in impurities present in the final product, thereby enhancing its quality and ensuring full compliance with the established technical specifications, specifically TU 6-36
0204208-107-89.
Additionally, the integration of advanced emergency systems and automated responses to parameter deviations significantly reduced the risk of accidents, leading to a safer working environment for operators and staff (Table 2).
T able 2. K ey parameters and automation features of the nitrobenzene washing process .
|
Parameter |
Description |
|
Temperature Range |
35-50°C |
|
pH Regulation |
Automatic dosing of ammonia solution |
|
Applied Technologies |
Microprocessor automation, DCS, PLC, APC, HMI |
|
Modernization Results |
Increased process stability, reduced defects, improved safety |
|
Explosion Hazard Class |
B-1a |
|
Fire Hazard Category |
Category A |
When compared to the previous manual or semiautomatic control methods, the modernized system demonstrated superior performance across several key metrics: reliability, product quality, and safety were all notably improved. The capability for continuous monitoring and data analysis further enriched the operational perspective, providing actionable insights that support ongoing process optimization. In conclusion, the modernization of the nitrobenzene washing stage automation system delivered substantial enhancements in process control, product quality, and safety, fully aligning with regulatory requirements and industry best practices.
CONCLUSION
The modernization of automated process control systems for the nitrobenzene washing stage represents a decisive step forward in meeting the increasingly stringent requirements for safety, product quality, and environmental compliance in chemical manufacturing. By replacing outdated manual and semi-automatic controls with advanced microprocessor-based automation tools - including distributed control systems (DCS), programmable logic controllers (PLCs), and real-time monitoring -this study has successfully addressed key challenges associated with the explosive, fire-hazardous, and environmentally sensitive nature of nitrobenzene production.
The new system ensures precise regulation of critical process parameters such as temperature and pH, minimizes operator errors, and significantly reduces the risk of process upsets and hazardous incidents. Enhanced safety features, including automated emergency shutdowns and ventilation activation, provide robust protection for both personnel and equipment, while continuous data logging and analysis support proactive process optimization and maintenance.
Overall, the implementation of modern automation has resulted in improved process stability, higher product quality, and greater compliance with regulatory standards. These advancements not only bolster the competitiveness of chemical enterprises but also set new benchmarks for operational safety and reliability in the production of nitrobenzene.


