Современные иммунологические биомаркеры рака толстой кишки
Автор: Трякин А.А., Хакимова Г.Г., Заботина Т.Н., Борунова А.А., Малихова О.А.
Журнал: Злокачественные опухоли @malignanttumors
Статья в выпуске: 4 т.8, 2018 года.
Бесплатный доступ
В статье кратко описан механизм действия иммунотерапевтических препаратов, подавляющих активность «чекпоинтов» иммунного ответа CTLA-4 и PD-1. Приведены современные данные о клинической эффективности и преимуществах применения ниволумаба, пембролизумаба и атезолизумаба при колоректальном раке. Обобщена существующая доказательная база о потенциальной предиктивной и прогностической роли MSI-статуса и представлена информация о перспективах дальнейшего развития данного метода лекарственного лечения злокачественных опухолей.
Лекарственная терапия, иммунотерапия, чекпоинты, ниволумаб, пембролизумаб, атезолизумаб, биомаркеры, эффективность, сhemotherapy
Короткий адрес: https://sciup.org/140243807
IDR: 140243807 | DOI: 10.18027/2224-5057-2018-8-4-50-58
Текст научной статьи Современные иммунологические биомаркеры рака толстой кишки
Злокачественные новообразования желудочно-кишечного тракта являются наиболее распространенными опухолями во всем мире с увеличением частоты заболеваемости и смертности. Колоректальный рак (КРР) – одно из самых распространенных онкологических заболеваний среди мужчин и женщин. Ежегодно в мире регистрируется около 1 млн новых случаев КРР, таким образом, он занимает третье место по частоте среди онкологических заболеваний [1].
В России в 2016 г. рак ободочной кишки занял 6-е место у мужчин (6,3%) и 5-е у женщин (7,1%), рак прямой кишки – 7-е (5,3%) и 9-е (4,5%) соответственно. За последние 10 лет, с 2006 по 2016 гг., отмечен неуклонный прирост заболеваемости раком ободочной кишки на 28,51%, раком прямой кишки – на 20,61% [2].
Несмотря на тот факт, что с развитием таргетной терапии отмечено увеличение общей выживаемости (ОВ) больных КРР, ожидаемая медианна ОВ до сих пор не превышает 36 мес. для пациентов с мКРР [3]. И в настоящее время приходит понимание того, что многие противоопухолевые ответы даже при классической терапии (цитотоксическая химиотерапия, антитела против EGFR, антиан-гиогенная терапия) реализуются посредством иммунной системы, показанными in vitro и in vivo .
В случаях ускользания от иммунологического надзора в связи с агрессивным фенотипом опухоли классические методы лечения явно терпят неудачу, а иммунотерапевтические подходы являются соблазнительной альтернативой в попытке улучшения прогноза этих пациентов в будущем [4].
Помимо этого, понятие микросателлитной нестабильности (microsatellite instability) прочно вошло в качестве ключевого компонента патогенеза КРР и вполне может быть биомаркером для улучшения прогноза и прогнози- рования эффективности химиотерапии и иммунотерапии [5]. А корреляция ответа на терапию чекпоинт-ингибито-рами и статус MSI является одним из дискутируемых вопросов среди ученых, подтверждая необходимость более детального изучения и дополнительных исследований с расширением арсенала задач при колоректальном раке.
Микросателлитная нестабильность при КРР
У большинства пациентов развитие КРР происходит в результате хромосомной нестабильности, но примерно у 15% из них опухоль возникает из-за аномалий в репарации ошибочно спаренных нуклеотидов (MMR-mismatch repair) ДНК [6].
Основная функция белков MMR заключается в поддержании геномной стабильности посредством коррекции ошибочно связанных нуклеотидов в единичных основаниях, вставок или делеций, которые могут возникать во время репликации ДНК [7].
Соответственно, этот путь включает в себя четыре ключевых процесса: распознавание ошибочных оснований, вставок и делений, удаление этих повреждений, замещение повреждения правильной последовательностью и повторение ДНК (рис. 1).
Микросателлиты же определяются как повторяющиеся последовательности ДНК, состоящие из 2–5 пар оснований, обычно встречающихся 10–60 раз, и рассеянных во всех кодирующих и некодирующих областях генома. MSI относится к фенотипу репликативной ошибки, вызванной мутациями (МТ) при MMR, и подразделяется на две группы: MSI-H или MSS [8].
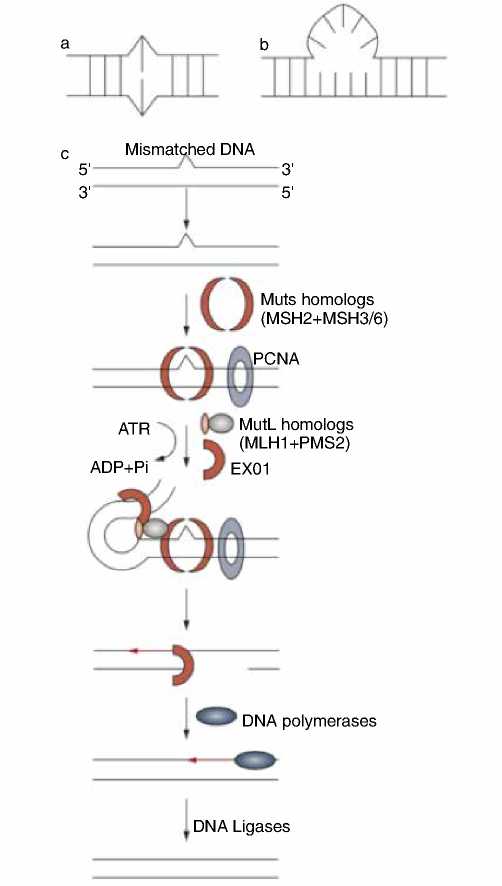
Рисунок 1. Схематическое представление аномалии в репарации и пути MMR. Система MMR распознает ошибочные основания, вставки и деления (а); удаленные вставки цикла (б). Гомологичные мутации связываются с пораженным участком ДНК, который вызывает АТФ-зависимые конформационные изменения и связывание гомологичных мутаций (с). Они, в свою очередь, рекрутируют другие белки, включая PCNA и экзонуклеазы, с последующим удалением поврежденной нити. Взаимодействие связанных белков инициирует скручивание, объединяя две нити ДНК. Образующийся дефект в нити далее заполняется ДНК-полимеразами, и разрыв удаляется ДНК-лигазой.
Опухоли с дефицитом системы MMR несут фенотип MSI-H, а опухоли, обладающие MMR (proficient MMR), – фенотип MSS. Сокращения: eXO1 – экзонуклеаза 1; PCNA – пролиферационно-клеточный антиген
Чаще всего MSI-H развивается спорадически (метилирование промотера MLH1), реже имеет наследственную природу (синдром Линча или наследственный неполипозный рак толстой кишки). Для данных опухолей характерна правосторонняя локализация, более молодой возраст, женский пол, низкодифференцированные аденокарциномы со слизеобразованием, а также высокая плотность TIL (опухоль-инфильтрирующих лимфоцитов) [9].
При этом на ранних стадиях эти опухоли имеют более благоприятный прогноз, чем КРР без фенотипа микроса-теллитной неустойчивости [10].
По данным метаанализа выявлена прямая корреляция между ОВ и MSI КРР независимо от стадии (HR=0,65; 95% ДИ 0,59–0,71) [11].
Помимо этого, опухоли MSI-H характеризуются ранними стадиями на момент постановки диагноза, а адъювантная терапия фторпиримидинами не улучшает выживаемость в этой когорте, частично, вероятно, потому что риск рецидива ниже, чем у MSI-стабильных (MSS) опухолей [12, 13].
К примеру, Popat et al. провели ретроспективную оценку статуса MSI у пациентов, включенных в исследования с адъювантным химиотерапевтическим лечением. Однофакторный анализ, не контролирующий химиотерапию, показал, что пациенты с опухолями MSI-H благоприятнее, чем MSS в целом, и пациенты с опухолями MSI-H при II и III стадиях КРР не получали преимущество от химиотерапии в адъювантом режиме с включением 5-фторураци-ла (5-FU) (HR=1,07, P=0,80). И наоборот, пациенты без наличия дефектов репрации показали лучшие результаты от проведения 5FU-терапии (HR=0,72, P=0,04) [14].
Еще одним примером является исследование Barrat P. L. [15], включавшее пациентов со II и III стадиями MSS, где выявлено значительное преимущество выживаемости (P=0,007) в пользу адъювантного ФУ (HR=0,72; 95% ДИ 0,57–0,92). Напротив, данные по 184 пациентам с MSI показало отсутствие преимуществ с адъювантом ФУ (HR=1,24; 95% ДИ 0,72–2,14).
Тем не менее пациенты с MSI-H КРР III стадии, также как MSS, получают преимущества от адъювантной химиотерапии FOLFOX. Объединенный анализ исследований адъювантной химиотерапии, который оценивал пациентов КРР III стадии, получавших FOLFOX самостоятельно или в сочетании с цетуксимабом, показал, что у пациентов с опухолями MSI-H, а также у пациентов с опухолями MSS (BRAF и KRAS WT) не было принципиальных отличий в трехлетней ВБП [16].
И наконец, еще одним убедительным подтверждением вышесказанного является большое французское ретроспективное исследование AGEO, в котором оценивались 433 пациента КРР MSI-H II или III стадий. Пациенты рандомизировались на исключительно хирургическое лечение (n=263) и в комбинации с химиотерапией (с производными фторпимидинов (n=119) или без оксалиплатина (n=51)). При этом адъювантная химиотерапия для II и III стадий составила 16,7 и 69,0% соответственно. Помимо оценки значимости производных фторпиримидинов, у пациентов КРР MSI-H при многофакторном анализе выявлено значительное улучшение ВБП при добавлении оксалиплатина (HR=0,35; 95% ДИ 0,19–0,65, P<0,001), в отличие от только адъювантного фторпиримидина (HR=0,73; 95% ДИ 0,36–1,49, P=0,38). Также при многофакторном анализе данное статистически значимое превосходство отмечено лишь для КРР III стадии (HR=0,41; 95% ДИ 0,19–0,87, P=0,02), что подтвердило преимущество от химиотерапии FOLFOX в адъювантном режиме [17].
Ингибиторы контрольных иммунных точек при РТК
Несмотря на всю гетерогенность рака толстой кишки и его высокую иммуногенность, dMMR КРР самопроизвольно не уничтожается иммунной системой, указывая на то, что существует множество механизмов ускользания, используемых опухолью. Среди них важное место занимает нарушение регуляции взаимодействия активационных ингибиторных сигналов, модулирующих процесс Т-клеточной активации.
За последнее десятилетие ингибирование PD-1 и CTLА-4 моноклональными антителами изменило ландшафт терапии рака для различных злокачественных новообразований, включая меланому, немелкоклеточный рак легкого, почечно-клеточный рак и лимфому Ходжкина [18].
Американское Общество Клинической Онкологии (American Society of Clinical Oncology, ASCO) признало иммунотерапию главным достижением в области онкологии в 2015 г. [19]. В 2018 г. Дж. Эллисон и Т. Хондзе получили Нобелевские премии за открытие вышеуказанных молекул «контрольных точек» или «чекпоинтов» (от англ. checkpoint) иммунного ответа, способных останавливать развитие иммунной реакции при злокачественных новообразованиях.
К наиболее изученным из подобных контрольных точек регуляции иммунного ответа относятся белок CTLA-4 (от англ. cytotoxic T-lymphocyte associated protein 4 – белок 4 цитотоксических Т-лимфоцитов) и сигнальный путь программируемой клеточной гибели PD-1 (от англ. Programmed cell Death pathway).
PD-1 в основном экспрессируется активированными Т-клетками (CD4+ – CD8+), а также NK, B-клетками и опу-холь-инфильтрирующими лимфоцитами (TIL) и ингибирует активацию Т-клеток путем взаимодействия с PD-L1 (B7-H1) и PD-L2 (B7-DC), которые, в свою очередь, экспрессируются опухолью, стромой и иммунными клетками [20]. Что касается PD-L2, то он также активируется в дендритных клетках, макрофагах и лимфоидной ткани в ответ на стимулы микроокружения опухоли [21]. В отличие от PD-L1, гиперэкспрессия PD-L2 на поверхности опухолевых клеток почти не наблюдается. Поэтому данные о корреляции гиперэкспрессии рецептора PD-L2 и иммунного ответа пока еще не известны, что в очередной раз доказывает многогранную биологию иммуноонкологии.
Из этого следует, что взаимодействие PD-1 с лигандом PD-L1, PD-L2 играет центральную роль в модуляции активности Т-клеток на периферии и в ускользании опухоли от иммунологического надзора [22].
При прогрессировании КРР TIL проявляют повышенную хроническую экспрессию различных антагонистов ICK-ингибиторов, вызывая функциональное истощение и невосприимчивость Т-клеток [23]. Таким образом, когда PD-1 ингибируется, TIL, присутствующие на границе опухоли, активируются и атакуют опухоль. Тем самым изучение экспрессии молекулы PD-L1 на опухолевых клетках и TILs может расширить диапазон знаний в оценке эффективности и продолжительности лечения применяемых в иммунотерапии препаратов, нацеленных на блокирование PD-L1.
В недавнем исследовании Llosa N. J. с соавторами продемонстрировали, что в опухолях MSI-H КРР наблюдается усиление ответа на терапию ингибиторами контрольных точек, такими как PD1, PDL1, CTLA4 [24]. Это открытие позволяет применить терапию ингибиторами ICK в этой специфической когорте пациентов с КРР с целью использования эндогенного иммунного ответа.
В одном из исследований I фазы Brahmer J. R. с коллегами рассмотрели использование анти-PD-1 ниволумаба среди 39 пациентов с распространенными солидными опухолями [25]. Авторы отметили, что среди 14 пациентов с рефрактерным мКРР один достиг полного ответа и опухоль пациента была MSI-H с большим количеством Т-кле-ток и повышенной экспрессией PD-L1. Этот пациент был под наблюдением в течение длительного времени и имел устойчивый частичный ответ (ЧО) более трех лет.
В исследовании Ib фазы Topalian S. L. и др. оценивали 296 пациентов с распространенными солидными опухолями, получавших ниволумаб, и отметили отсутствие объективного ответа (ОЭ) среди 19 пациентов мКРР [26].
В целях дальнейшего изучения использования ингибиторов иммунных контрольных точек среди пациентов с мКРР было начато два исследования среди пациентов с опухолями MSI-H (табл. 1). В исследовании II фазы Keynote-164 Le D. T. с коллегами рассмотрели эффективность пембролизумаба среди пациентов с прогрессирующей метастатической карциномой с или без dMMR [27, 28]. Был сделан ряд интересных наблюдений. Во-первых, секвенирование целых экзонов выявило 1,782 и 73 соматические мутации в опухоли в когорте dMMR и pMMR соответственно (P=0,007). Авторы наблюдали корреляцию между продолжительностью ВБП и высокой соматической мутационной нагрузкой (P=0,02). Также авторы сообщили, что эффективность пембролизумаба ограничивается только группой с MSI-H, при этом частота объективного ответа (ЧОО) – 52 и 0% среди пациентов мКРР с dMMR и pMMR соответственно. Медиана ВБП и ОВ не была достигнута для когорты с дефектом MMR и составила 2,2 и 5,0 мес. соответственно для когорты pMMR. Дальнейшее сравнение двух когорт показало отношение рисков (HR) для прогрессирования или смерти 0,10 (P<0,001), а HR для смерти составлял 0,22 (P=0,05) в пользу группы, у которой были опухоли dMMR. Среди 46 пациентов с неколоректальными опухолями с dMMR ЧОО составила 54%, а медиана ВБП –
Таблица1. Клинические исследования с применением чекпоинт-ингибиторов у пациентов с мКРР
|
Исследование |
Фаза |
Когорта |
Агент |
ЧОО (%) |
СО (%) |
ПО (%) |
ВБП (мес.) |
ОВ (мес.) |
|
Keynote 164 (34) |
II |
MSI-H |
Пембролизумаб |
52 |
30 |
12 |
НД |
НД |
|
CheckMate 142 (25) |
II |
MSI-H |
Ниволумаб |
31,1 |
34 |
3 |
14,3 |
12 (73 %) |
|
CheckMate 142 (35) |
II |
MSI-H |
Ниволумаб + Ипилимумаб |
55 |
31 |
3 |
НД |
НД |
|
Bendell J. (36) |
I |
KRAS-мутация, MSS |
Атезолизумаб + Кобиметиниб |
20 |
20 |
0 |
2,3 |
НД |
|
Tabernero J. (37) |
I |
MSS |
Атезолизумаб + CEA-TCB |
52 |
40 |
0 |
НО |
НО |
ЧОО – частота объективных ответов; СО – стандарт отклонения; ПО – полный ответ; ВБП – выживаемость без прогрессирования; ОВ – общая выживаемость; НД – не достигнута; НО – не оценена
В исследовании CheckMate 142 в популяции больных мКРР с MSI-H изучалось другое анти-PD1 моноклональное антитело ниволумаб [32] (см. табл. 1). При медиане наблюдения 12 мес. ЧОО и контроль заболевания в течение 12 нед. составили 31,1 и 69% соответственно, при этом средняя продолжительность ответа не была достигнута. В недавнем обновлении авторы сообщили, что исход стратифицирован тем, получали ли пациенты три или более линий терапии [33]. Авторы сообщили, что общая медиана ВБП составила 6,6 мес. для всей когорты. В то же время медиана ВБП среди тех пациентов, которые получали больше трех линий терапии, по сравнению с теми, кто не получал меньшее количество линий предшествующей терапии, составила 4,2 мес. Аналогичным образом,
1-годичная ОВ достигла 68 и 81% среди тех, кто получал и не получал больше трех линий терапии. Длительность достигаемых ремиссий вызвала огромный интерес к исследованию ингибиторов иммунной контрольной точки в режиме первой линии терапии пациентов с dMMR мКРР.
В ходе исследования фазы I авторы наблюдали одного пациента КРР, получившего полный ответ на ниволумаб, который оставался прочным в течение 21 мес. Позже исследователи отметили, что опухоль была MSI-H с опухоль-ин-фильтрирующими макрофагами и лимфоцитами, расположенными на PDL-1+. Данный факт подтвердил идею о том, что MSI может быть потенциальным биомаркером для отбора пациентов для иммунотерапевтических подходов в лечении. 31 июля 2017 г. FDA предоставило ускоренное одобрение ниволумаба для пациентов с опухолями мКРР, несущими фенотип MSI-H или dMMR [34]. Тестирование на дефицит MMR опухоли (dMMR) может быть выполнено либо с помощью непосредственного тестирования на экспрессию белков MMR (MLH1, MSH2, MSH6 и PMS2) с использованием иммуногистохимического метода, либо с помощью тестирования MSI, где путем изменения длины 5 микросателлитов в ДНК, оцениваемого с использованием полимеразной цепной реакций (ПЦР), выявляется статус MSI-H, определяемый как нестабильность в двух или более из пяти микросателлитов. Исследования показали, что оба метода конкордантны в 92% случаях [35, 36].
Проведенные клинические исследования указывают на то, что уровень экспрессии молекулы PD-L1 опухолевыми клетками и клетками опухолевого микроокружения является потенциальным биомаркером прогнозирования течения злокачественных новообразований и может быть использован как предиктор ответа на анти-PD1/PDL1 иммунотерапию [37]. Еще одним ингибитором иммунной контрольной точки является анти-CTLA-4. CTLA-4 (также известен как CD152) – мембранный рецептор, который экспрессируется на поверхности Т-лимфоцитов. Активация CTLA-4 приводит к подавлению активности клеток, на поверхности которых он расположен. Точный механизм его подавляющего воздействия на развитие иммунного ответа неизвестен [38].
Ученые, все время стремясь улучшить показатели ответа и сделать опухоли более иммуногенными, изучили комбинированные стратегии (табл. 1). Overman M. J.
с коллегами сообщили о второй группе из 119 пациентов в исследовании CheckMate 142 с опухолями dMMR/MSI-H mCRC, которые получали комбинацию ниволумаба (3 мг/кг) и ингибитора иммунной контрольной точки против CTLА-4 ипилимумаба (1 мг/кг) [39]. В исследование включались пациенты с прогрессированием на предшествующих линиях стандартной терапии. Данная комбинация давалась пациентам каждые 3 нед. в четыре приема, а затем с использованием ниволумаба в монорежиме каждые 2 нед. При медиане наблюдения 13,4 мес. авторы сообщили, что ЧОО и контроль заболевания составили 55 и 80% соответственно. При этом ВБП составила 76% (9 мес.) и 71% (12 мес.), ОВ – 87 и 85% соответственно. И хотя ответ на терапию в обеих исследуемых группах наблюдался независимо от экспрессии PDL-1, статуса BRAF и KRAS и наличия или отсутствия синдрома Линча, комбинация ниволумаба и ипилимумаба продемонстрировала высокие показатели ответа, управляемый спектр токсичности и является перспективно новым вариантом лечения пациентов с dMMR/MSI-H мКРР.
Ингибиторы иммунных контрольных точек также были изучены в сочетании с химиотерапией и бевацизумабом. Известно, что бевацизумаб играет важную роль в отслеживании лимфоцитов и иммунной регуляции. Hochester H. S. с коллегами сообщили о новой комбинации ингибиторов иммунных контрольных точек атезолизумаба и бевациз-умаба [40]. Авторы отметили, что среди 10 пациентов с опухолями MSI-H, 70% из которых ранее получали бе-вацизумаб и 30% имели синдром Линча, ЧОО и контроль заболевания составили 40 и 90% соответственно. Bendell J. C. и др. сообщили о комбинации FOLFOX, бева-цизумаба и атезолизумаба [41]. Среди 23 пациентов, ранее не получавших лечение, авторы наблюдали ЧОО и частичный ответ в 87 и 48% случаях.
Помимо вышеуказанных способов ускользания опухоли от иммунной системы существует еще путь MK-киназы, являющийся одним из наиболее часто дисрегулированных путей рака. Дисрегуляция этого пути приводит к устойчивому ингибированию передачи сигналов RAS, RAF, MEK и ERK, что в конечном итоге приводит к неконтролируемой пролиферации. Ингибиторы MEK могут быть одним из способов остановки данного каскада реакций.
Таким образом, фармакологическое ингибирование MEK потенцирует, а не препятствует противоопухолевым Т-клеткам, нарушая апоптоз, обусловленный Т-клеточным рецептором [42].
Кобиметиниб является высокоселективным, мощным и обратимым ингибитором MEK1 и MEK2. Кобиметиниб был одобрен для лечения меланомы, но показал небольшую активность среди пациентов с мКРР [43]. Доклинические данные показывают, что комбинация атезолизу-маба и кобиметиниба приводит к регрессии опухоли [44]. Bendell J. C. и др. в исследовании Ib фазы по эскалации дозы изучали данную комбинацию в когорте пациентов с местнораспространенными солидными опухолями [45]. Авторы сообщили, что среди 20 пациентов с KRAS-мутированным химиорефрактерным метастатическим раком прямой кишки ЧОО и частичный ответ составили 20% с медианой времени объективного ответа 3,7 мес. Среди пациентов, включенных в исследование, 30% были MSS, 0% имели опухоли MSI-H и у 70% статус MSI был неизвестен. Среди четырех пациентов, у которых был зафиксирован ЧО, трое были с MSI-H опухолями, при этом статус MSI не оценивался. Эти результаты представляют собой преимущество в сравнении с незначительным ЧОО, который обычно наблюдается в этой когорте с ингибиторами иммунных контрольных точек. Позже авторы также сообщили медиану и 6-месячную ВБП, составившие 2,3 мес. и 39% соответственно, а 6-месячная ОВ достигла 77%. Основываясь на этих обнадеживающих ранних результатах, исследование Ib фазы продолжается, а исследование III фазы IMblaze370 (NCT02788279), оценивающее данную комбинацию среди пациентов с химиорефрак-терным мКРР, явилось негативным. В данном исследовании, включавшем 363 пациента мКРР (до 95% MSS) и рандомизированном в соотношении 2:1:1 (атезолизу-маб + кобиметиниб: атезолизумаб: регорафениб), различий в ЧОО, ВБП и ОВ не выявлено. ЧОО составила 2,7% при схеме «атезолизумаб + кобиметиниб» по сравнению с 2,2% в монотерапии атезолизумабом или регорафени-бом. Медиана ОВ составила 8,9 мес. со схемой «атезо-лизумаб + кобиметиниб» (HR=1,00; 95% ДИ 0,73–1,38), 7,1 мес. с атезолизумабом (HR=1,19; 95% ДИ 0,83–1,71) и 8,5 мес. с регорафенибом. Медиана ВБП также значительно не разнилась в терапии со схемой «атезолизумаб + кобиметиниб» (HR 1,25, 95% ДИ, 0,94–1,65), монотерапией атезолизумабом (HR=1,39; 95% ДИ 1,0–1,94) в сравнении с регорафенибом [46].
Еще одним из активно обсуждаемых таргетных препаратов является CEA-TCB. Раковоэмбриональный антиген (РЭА) CD3 T-клеточно биспецифичное (CEA-TCB) антитело представляет собой новое биспецифическое антитело Т-клеток, которое одновременно связывается с опухолью и Т-клетками, участвует и активирует Т-клетки и увеличивает активацию Т-клеток [47]. Более 90% мКРР экспрессируют высокие уровни РЭА, что делает CEA-TCB идеальным средством для исследования в данной когорте пациентов [48]. Доклинические модели продемонстрировали мощную противоопухолевую активность, повышенную ин-траопухолевую Т-клеточную инфильтрацию, активацию и регуляцию PD-1/PDL-1 и усиленную активность в сочетании с атезолизумабом [49]. Tabernero J. и др. недавно сообщили об эффективности и безопасности CEA-TCB как в качестве одного агента, так и в сочетании с атезолиз-умабом в когорте пациентов с мКРР, зарегистрированным в двух текущих исследованиях I фазы [50]. У большинства пациентов был MSS статус. Среди 31 пациента, которые получали CEA-TCB в монорежиме, ЧОО и контроль забо- левания составили 6 и 45% соответственно. Среди 25 пациентов, получавших комбинацию СЕА-ТСВ и атезолиз-умаба, ЧО и контроль заболевания составляли 12 и 52% соответственно.
Таким образом, прогностическое значение экспрессии PD-L1 в качестве потенциального биомаркера ответа опухоли было исследовано в ряде работ, однако результаты оказались спорными в зависимости от исследуемого типа опухоли и типа антител, используемых для тестирования экспрессии PD-L1. Carbognin L. с коллегами провели метаанализ 20 исследований I–III фаз, где рассматривались ингибиторы иммунных контрольных точек при лечении местнораспространенной меланомы, немелкоклеточного рака легких и рака мочеполовой системы [51]. Целью анализа было изучение дифференциальной активности ингибиторов иммунных контрольных точек в солидных новобразованиях на основе экспрессии PDL-1. Авторы сообщили, что объективный ответ был достигнут в 34,1 и 19,9% у PD-L1-положительных и PD-L1-негативных образцов опухоли соответственно. В области КРР новые данные свидетельствуют о том, что экспрессия PDL-1 может не иметь такого же прогностического значения. В исследовании Keynote-028 лишь один из 23 пациентов мКРР с экспрессией PD-L1 продемонстрировал ответ на пем-бролизумаб, и тот, как оказалось, имел опухоль с dMMR [52]. Отсутствие корреляции между экспрессией PD-L1 и эффективностью ниволумаба у пациентов в dMRR было отмечено и в исследовании Checkmate-142 [53]. Таким образом, количество мутаций в опухоли само по себе может выступать в роли биомаркера, предсказывающего эффективность иммунотерапии.
На сегодняшний день ингибиторы иммунных контрольных точек, такие как ипилимумаб, ниволумаб и пембро-лизумаб, нашли свое применение в первой и последующих линиях терапии у пациентов с местнораспространенным и метастатическим раком толстой кишки с дефицитом MMR/MSI-H как в европейских рекомендациях NCCN, так и в отечественных RUSSCO.
Выводы
Основополагающее понимание того, что КРР не является простым заболеванием по своей структуре, а представляет конгломерат ряда важных подтипов, скорее всего, поможет будущему терапевтическому управлению и в конечном итоге улучшит прогностический результат.
Хотя использование ингибиторов иммунных контрольных точек привело к значительным успехам в лечении солидных опухолей, таких как меланома, рак легкого и рак почки, они имеют ограниченный успех при КРР. Их использование и феноменальный успех в подгруппе пациентов с MSI-H мКРР, где ингибиторы иммунных контрольных точек значительно улучшили выживаемость в химиореф-рактерной когорте, которая, как известно, имеет неблагоприятный прогноз, и признание того, что данный успех может быть реплицирован по типам MSI-H солидных опухолей, подчеркивает, что определенные биомаркеры являются агностиками типа опухоли. Будущие исследования в области КРР с иммунологическими терапевтическими подходами теперь, скорее всего, будут сосредоточены на выявлении подходов к улучшению иммуногенности пациентов MSS КРР.
Данные о комбинированных режимах терапии ингибиторами иммунных контрольных точек с другими агентами, безусловно, обнадеживают и активно исследуются учеными всего мира. Эти данные являются особенно впечатляющими в контексте того факта, что последующие открытия в лечении злокачественных опухолей обещают быть прорывами в области иммуноонкологии.
Список литературы Современные иммунологические биомаркеры рака толстой кишки
- Pernot, Simon et al. "Colorectal Cancer and Immunity: What We Know and Perspectives." World Journal of Gastroenterology: WJG. 2014. P. 3738-3750. PMC. Web. 2 July 2018.
- Состояние онкологической помощи населению России в 2016 году/под ред. Каприна А. Д., Старинского В. В., Петровой Г. В. М., 2017. 236 с.
- Pernot S., Terme M., Voron T. et al. Colorectal cancer and immunity: What we know and perspectives. World Journal of Gastroenterology: WJG. 2014. Vol. 20(14). P. 3738-3750 DOI: 10.3748/wjg.v20.i14.3738
- Bilgin B., Sendur M.A., Bulent Akinci M., Sener Dede D., Yalсin B. Targeting PD-1 Pathway: A new hope for Gastrointestinal Cancers. Current Medical Research and Opinion. 2017. Vol. 33(4). P. 749-759 DOI: 10.1080/03007995.2017.1279132
- Hewish M., Lord C.J., Martin S.A. et al. Mismatch repair deficient colorectal cancer in the era of personalized treatment. Nat. Rev. Clin. Oncol. 2010. Vol. 7. P. 197-208.
- Gatalica Z., Vranic S., Xiu J. et al. High microsatellite instability (MSI-H) colorectal carcinoma: a brief review of predictive biomarkers in the era of personalized medicine. Fam Cancer. 2016. Vol. 15. P. 405-412
- DOI: 10.1007/s10689-016-9884-6
- Modrich P. Mechanisms in eukaryotic mismatch repair. J. Biol. Chem. 2006. Vol. 281. P. 30305-9
- DOI: 10.1074/jbc.R600022200
- Jiricny J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006. Vol. 7. P. 335-346.
- Li G.M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008. Vol. 18. P. 85-98.
- Devaud N., Gallinger S. Chemotherapy of MMR-deficient colorectal cancer. Fam Cancer. 2013. Vol. 12. P. 301-306. 10.1007/s10689-013-9633-z
- DOI: :10.1007/s10689-013-9633
- Smyrk T.C., Watson P., Kaul K. et al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001. Vol. 91. P. 2417-2422. 10.1002/1097-0142(20010615)91:123.0.CO;2-U
- DOI: :10.1002/1097-0142
- Benatti P., Gafa R., Barana D., Marino M., Scarselli A., Pedroni M., Maestri I., Guerzoni L. et al. Microsatellite instability and colorectal cancer prognosis. Clin. Cancer Res. 2005. Vol. 11. P. 8332-8340.
- Zaanan A., Shi Q., Taieb J. et al. Analysis of DNA mismatch repair (MMR) and clinical outcome in stage III colon cancers from patients (pts) treated with adjuvant FOLFOX +/-cetuximab in the PETACC8 and NCCTG N0147 adjuvant trials. J. Clin. Oncol. 2015. Vol. 33. P. 3506.
- Popat S., Hubner R., Houlston R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. 2005. J. Clin. Oncol. Vol. 23. P. 609-618.
- Barratt P.L., Seymour M.T., Stenning S.P. et al. Adjuvant x-ray and fluorouracil infusion study: DNA markers predicting benefit from adjuvant fluorouracil in patients with colon cancer -A molecular study. Lancet. 2002. Vol. 360. P. 1381-1391.
- Zaanan A., Shi Q., Taieb J. et al. Analysis of DNA mismatch repair (MMR) and clinical outcome in stage III colon cancers from patients (pts) treated with adjuvant FOLFOX +/-cetuximab in the PETACC8 and NCCTG N0147 adjuvant trials. J. Clin. Oncol. 2015. Vol. 33. P. 3506.
- Tougeron D., Mouillet G., Trouilloud I. et al. Efficacy of adjuvant chemotherapy in colon cancer with microsatellite instability: a large multicenter AGEO study. J. Natl. Cancer Inst. 2016. Vol. 108(7)
- DOI: 10.1093/jnci/djv438
- Mellman I., Coukos G., Dranoff G. Cancer immunotherapy comes of age. Nature. 2011. Vol. 480. P. 480-489.
- Dizon D.S., Krilov L., Cohen E., Gangadhar T., Ganz P.A., Hensing T.A., Hunger S. et al. Clinical Cancer Advances 2016: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J. Clin. Oncol. 2016. Vol. 34. No. 9. P. 987-1011.
- Jacobs J., Smits E., Lardon F., Pauwels P., Deschoolmeester V. Immune Checkpoint Modulation in Colorectal Cancer: What’s New and What to Expect. Journal of immunology research. 2015. Vol. 2015. P. 58038.
- Reiss K.A., Forde P.M., Brahmer J.R. Harnessing the power of the immune system via blockade of PD-1 and PD-L1: a promising new anticancer strategy. Immunotherapy. 2014. Vol. 6(4). P. 459-75.
- Patel S.P., Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015. Vol. 14. P. 847-56.
- Blackburn S.D., Shin H., Haining W.N. et al. Coregulation of CD8. T cell exhaustion by multiple inhibitory receptors during chronic viral infection. NatImmunol. 2009. Vol. 10(1). P. 29-37.
- Llosa N.J., Cruise M., Tam A. et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015. Vol. 5. P. 43-51.
- Brahmer J.R., Tykodi S.S., Chow L.Q. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012. Vol. 366. P. 2455-65.
- Topalian S.L., Hodi F.S., Brahmer J.R. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012. Vol. 366. P. 2443-54.
- Le D.T., Uram J.N., Wang H. et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015. Vol. 372. P. 2509-20.
- Le D.T., Durham J.N., Smith K.N. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017. Vol. 357. P. 409-413.
- Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015. Vol. 372(26). P. 2509-20. 10.1056/NEJMoa1500596
- DOI: :10.1056/NEJMoa1500596
- Diaz L.A., Marabelle A., Delord J.P. et al. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. J. Clin. Oncol. 2017. Vol. 35. P. 3071.
- Lemery S., Keegan P., Pazdur R. First FDA Approval Agnostic of Cancer Site -When a Biomarker De nes the Indication. N. Engl. J. Med. 2017. Vol. 377. P. 1409-12.
- Overman M.J., McDermott R., Leach J.L. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017. Vol. 18. P. 1182-91.
- Overman M.J., Bergamo F., Mcdermott R.S. et al. Nivolumab in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): long-term survival according to prior line of treatment from CheckMate-142. J. Clin. Oncol. 2018. Vol. 36. abstr 554.
- FDA grants nivolumab accelerated approval for MSI-H or dMMR colorectal cancer. Available online: www. fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm569366.htm.
- Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J. Mol. Diagn. 2008. Vol. 10. P. 293-300.
- Shia J., Stadler Z., Weiser M.R. et al. Immunohistochemical staining for DNA mismatch repair proteins in intestinal tract carcinoma: how reliable are biopsy samples? Am. J. Surg. Pathol. 2011. Vol. 35. P. 447-454.
- Chu F., Neelapu S.S. Anti-PD-1 antibodies for the treatment of B-celllymphoma: Importance of PD-1+ T-cell subsets. Oncoimmunology. 2014. Vol. 3(1). P. e28101
- DOI: 10.4161/onci.28101
- Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012. Vol. 12. No. 4. P. 252-264.
- Overman M.J., Lonardi S., Wong K.Y.M. et al. Durable Clinical Benet With Nivolumab Plus Ipilimumab in DNA Mismatch RepairDeficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018. Vol. 36. P. 773-779.
- Hochster H.S., Bendell J.C., Cleary J.M. et al. Efficacy and safety of atezolizumab (atezo) and bevacizumab (bev) in a phase Ib study of microsatellite instability (MSI) -high metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2017. Vol. 35. P. 673.
- Bendell J.C., Powderly J.D., Lieu C.H. et al. Safety and efficacy of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2015. Vol. 33. P. 704.
- Duffy A.G., Makarova-Rusher O.V., Fioravanti S. et al. A pilot study of AMP-224, a PD-L2 Fc fusion protein, in combination with stereotactic body radiation therapy (SBRT) in patients with metastatic colorectal cancer. J. Clin. Oncol. 2016. Vol. 34. P. 560.
- Dhillon A.S., Hagan S., Rath O. et al. MAP kinase signalling pathways in cancer. Oncogene. 2007. Vol. 26. P. 3279-90.
- Ebert P.J.R., Cheung J., Yang Y. et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016. Vol. 44. P. 609-621.
- Bendell J.C., Kim T.W., Goh B.C. et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). J. Clin. Oncol. 2016. Vol. 34. P. 3502.
- Bendell J., Ciardiello F., Tabernero J. et al. Efficacy and safety results from IMblaze370, a randomised Phase III study comparing atezolizumab+cobimetinib and atezolizumab monotherapy vs regorafenib in chemotherapy-refractory metastatic colorectal cancer. Annals of Oncology. 2018. Vol. 29. Issue suppl_5. 10.1093/annonc/mdy208.003
- DOI: :10.1093/annonc/mdy208.003
- Bacac M., Fauti T., Sam J. et al. A Novel Carcinoembryonic Antigen T-Cell Bispeci c Antibody (CEA TCB) for the Treatment of Solid Tumors. Clin. Cancer Res. 2016. Vol. 22. P. 3286-97.
- Tiernan J.P., Perry S.L., Verghese E.T. et al. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br. J. Cancer. 2013. Vol. 108. P. 662-667.
- Lehmann S., Perera R., Grimm H.P. et al. In Vivo Fluorescence Imaging of the Activity of CEA TCB, a Novel T-Cell Bispeci c Antibody, Reveals Highly Speci c Tumor Targeting and Fast Induction of T-Cell-Mediated Tumor Killing. Clin. Cancer Res. 2016. Vol. 22. P. 4417-27.
- Tabernero J., Melero I., Ros W. et al. Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispeci c (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: Preliminary ef cacy and safety in patients with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2017. Vol. 35. P. 3002.
- Carbognin L., Pilotto S., Milella M. et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS One. 2015. Vol. 10. e0130142.
- O’Neil B.H., Wallmark J., Lorente D. et al. Pembrolizumab (MK-3475) for patients (pts) with advanced colorectal carcinoma (CRC): Preliminary results from KEYNOTE-028. Eur. J. Cancer. 2015. Vol. 51. abstr s103.
- Overman M.J., McDermott R., Leach J.L. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017. Vol. 18. P. 1182-91.


