Современные методы исследования апоптоза с применением проточной цитофлюориметрии
Автор: Власова Виолетта Викторовна, Сайдакова Евгения Владимировна
Журнал: Вестник Пермского университета. Серия: Биология @vestnik-psu-bio
Рубрика: Иммунология
Статья в выпуске: 4, 2018 года.
Бесплатный доступ
В процессе апоптоза - программной гибели - клетка претерпевает ряд консервативных биохимических изменений. Среди них ключевыми событиями являются потеря трансмембранного потенциала митохондрий, активация проапоптотических белков семейства Bcl-2, нарушение липидной асимметрии и повышение проницаемости цитоплазматической мембраны, активация ферментов из семейства каспаз и фрагментация нуклеиновых кислот. Многоцветная проточная цитофлюоримет-рия позволяет детектировать большинство из событий, свидетельствующих об индукции программной гибели клетки, что дает возможность исследования апоптоза на самых ранних его этапах. В настоящей статье описаны основные подходы к детекции апоптоза с применением многоцветной проточной цитофлюориметрии; рассмотрены молекулярные процессы, лежащие в их основе; обозначены преимущества и ограничения в использовании различных методов определения программной гибели клеток в биологии и медицине.
Проточная цитофлюориметрия, апоптоз, митохондрии, проницаемость цитоплазматической мембраны, асимметрия цитоплазматической мембраны, каспазы, фрагментация днк
Короткий адрес: https://sciup.org/147227052
IDR: 147227052 | УДК: 57.083.3 | DOI: 10.17072/1994-9952-2018-4-430-442
Текст научной статьи Современные методы исследования апоптоза с применением проточной цитофлюориметрии
Апоптоз – это генетически обусловленный процесс запрограммированной клеточной смерти, с помощью которого организм избавляется от потерявших свое функциональное значение или опасных для жизнедеятельности элементов. Большое число клеток уничтожается посредством апоптоза еще на стадии эмбриогенеза. Их элиминация необходима для формирования правильных очертаний тела и удаления провизорных органов [Yaron, Steller, 2011]. В сформированном организме апоптоз является важным механизмом регуляции селекции клеток иммунной системы: в процессе со- зревания Т- и В-лимфоцитов уничтожаются клетки, способные приводить к аутоиммунным заболеваниям [Nemazee, 2000]. Также апоптоз направлен на удаление мутантных и зараженных вирусом клеток [Roos, Kaina, 2006; Brazeau et al., 2010].
Нарушения процесса апоптоза ассоциированы с широким спектром патологических состояний. Так, повышенный уровень клеточной гибели играет большую роль в развитии нейродегенеративных заболеваний, таких как болезнь Альцгеймера [Rohn, 2010], болезнь Паркинсона [Samantaray et al., 2007], болезнь Гентингтона [Kim et al., 2001] и боковой амиотрофический склероз [Vucic, Kiernan, 2009]. Сердечно-сосудистые заболевания, среди
которых следует отметить ишемическую болезнь сердца [Whelan, Kaplinskiy, Kitsis, 2010] и сердечную недостаточность [Fujita, Ishikawa, 2011], также характеризуются высоким по сравнению с нормой уровнем апоптоза. Напротив, блокирование сигналов, индуцирующих апоптоз, и, соответственно, снижение числа умирающих клеток характерно для онкологических заболеваний, в том числе рака почек [Gobe et al., 2002], рака легких [Han et al., 2002], рака простаты и прямой кишки [Burz et al., 2009]. Нарушение регуляции апоптоза происходит, в том числе, при аутоиммунных заболеваниях. Так, существует связь между нарушением процесса апоптоза иммунокомпетентных клеток и клеток щитовидной железы при тиреоидите Ха-шимото [Nagata, 2010] и болезни Грейвса [Lin, 2001]. Многие аутоиммунные заболевания, такие как системная красная волчанка [Nagata, 2007] и ревматоидный артрит [Eguchi, 2001], могут быть вызваны недостаточной эффективностью фагоцитов при уничтожении апоптотических клеток [Munoz et al., 2010].
Несмотря на многочисленные исследования, для многих заболеваний, связанных с нарушением апоптоза, не было найдено этиологического лечения. Поэтому изучение процесса программной клеточной смерти несет в себе огромный терапевтический потенциал. Существуют различные методологические подходы к идентификации программной гибели клеток. Так, благодаря тому, что с апопто-тирующими клетками происходят консервативные и хорошо заметные морфологические изменения, впервые этот процесс был описан с использованием светового микроскопа [Kerr, Wyllie, Currie, 1972]. Умирающие клетки становятся более круглыми и уменьшаются в размерах, их хроматин конденсируется и разрушается, а со временем и сама клетка распадается на апоптотические тельца [Anita et al., 2014]. Однако эти события – лишь следствие сложных биохимических процессов, детекция которых позволит приблизиться к пониманию причин и механизмов клеточной гибели. Однако микроскопия не подходит для реализации таких задач. В настоящее время для комплексного изучения апоптоза применяется метод проточной цитофлюориметрии. Он открывает для исследователей возможности мультиплексного анализа, оценки множества клеток за короткий промежуток времени и регистрации индивидуальных характеристик каждой клетки. В то же время большое количество разрозненных литературных данных не позволяет быстро получить представление о существующих подходах к исследованию апоптоза методом проточной цитофлюориметрии. Цель данной статьи – осветить и проанализировать основные цитометрические подходы к исследованию программной гибели клеток.
Нарушение функциональной активности митохондрий
Реализация программной гибели клетки требует больших энергетических затрат, поэтому митохондрии, как главный источник АТФ, играют важную роль в протекании этого процесса. Однако, несмотря на энергетическую зависимость, апоптоз приводит к нарушению функциональной активности митохондрий. Это связано с тем, что во внутреннем пространстве митохондрий содержится ряд проапоптотических факторов, таких, как фактор AIF (Apoptosis inducing factor), способствующий конденсации хроматина и фрагментации ДНК [Kroemer, Reed, 2000]; цитохром С, участвующий в формировании апоптосомы; эндонуклеаза G, которая вносит межнуклеосомальные разрывы в ДНК [Li, Luo, Wang, 2001]; прокаспазы 2, 3 и 9, в форме каспазы участвующие в активации других прокаспаз и расщеплении белков; SMAC (Second mitochondria–derived activator of caspases) или DIABLO (Direct IAP binding protein with low isoelectric point), блокирующий белок IAP (inhibitor of apoptosis protein) – ингибитор апоптоза [Eckelman, Salvesen, Scott, 2006]. Инициация апоптоза связана с выходом митохондриальных факторов в цитозоль. При этом для облегчения процесса транспорта белков мембрана митохондрий может формировать каналы. Возникновение достаточно больших мембранных каналов неизбежно приводит к массовой миграции протонов из межмембранного пространства митохондрий и, следовательно, к потере электрохимического протонного градиента. Для митохондрий его исчезновение означает потерю способности синтезировать молекулы АТФ, а для клетки – потерю ее энергетической функции. Нарушение функции митохондрий – это один из самых ранних маркеров апоптоза, его определение может стать важным инструментом для своевременной диагностики заболеваний, ассоциированных с аномалиями клеточной смерти.
Показателем функционально активной митохондрии является наличие электрохимического протонного градиента, который достигается за счет скопления протонов в межмембранном пространстве митохондрий [Petit et al., 1990]. Разница в концентрации протонов на внешней и внутренней сторонах мембраны стимулирует положительно заряженные молекулы диффундировать во внутреннее пространство митохондрий. Этот эффект является основой метода определения их активности. В клеточных мембранах диффузия молекул ограничена полупроницаемостью, поэтому не каждое вещество способно к движению по градиенту концентрации внутрь матрикса. Этой способностью обладают только молекулы с липофильными свойствами, то есть обладающие сродством к липидам плазматической мембраны. Используя положительно заряженные флюоресцентные молекулы с липофильными свойствами, мы можем визуа- лизировать процесс накопления катионов в матриксе митохондрий живых клеток. В качестве таких маркерных молекул могут выступать липофильные катионные зонды, ярким представителем которых является краситель 3,3'-дигексилоксакарбоцианин йодид (DiOC6(3)).
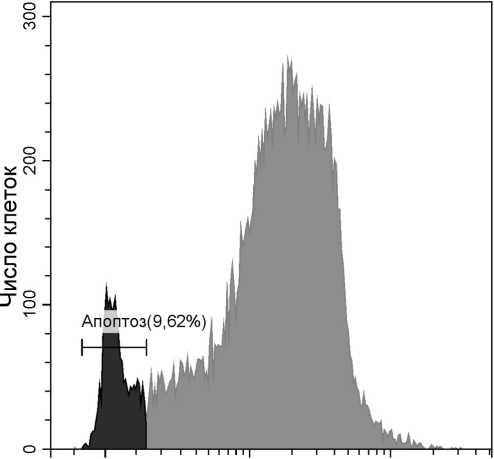
Попадая в живую клетку, DiOC6(3) накапливается в матриксе митохондрий. Проходя через оптическую систему проточного цитофлюориметра, краситель возбуждается синим лазером (488 нм), после чего его флюоресценцию можно зарегистрировать в зеленом спектре (максимум эмиссии 504 нм). В случае, если клетка погибает путем апоптоза, диффузное движение протонов через каналы и разрывы в митохондриальной мембране приводит к уравниванию концентрации заряженных частиц по обе стороны мембраны и накопления DiOC6(3) не происходит. Другими словами, в апоптотирую-щих клетках уровень флюоресценции DiOC6(3) остается низким (рис. 1.). В целях повышения достоверности получаемых результатов анализ функциональной активности митохондрий часто проводят одновременно с исследованием проницаемости цитоплазматической мембраны клетки (метод будет подробно рассмотрен далее в тексте статьи).
DiOC6(3)
0 104 105
DiOC6(3) FITC-A
Рис. 1 . Детекция апоптоза с использованием красителя DiOC6(3)
Мононуклеарные клетки периферической крови человека стимулированы фитогемагглютинином (15 мкг/мл; 24 ч.). На гистограмме представлены апоптотирующие клетки (слева), живые клетки (справа)
Одним из основных преимуществ применения липофильных катионных зондов является возможность детекции ранней стадии апоптоза. Окрашивание DiOC6(3) не требует длительной подготовки образцов и дополнительных реагентов, поэтому во всех отношениях его можно назвать экспресс-методом. Однако у данного метода есть свои ограничения. Во-первых, DiOC6(3) не обладает специфичностью к митохондриям. Его накопление обу- словлено только собственным положительным зарядом. В больших концентрациях этот краситель способен спонтанно связываться с любыми анионами клеточного пространства, создавая риск ложноположительных результатов. Во-вторых, DiOC6(3) токсичен для клеток. Данное свойство не влияет на детекцию апоптоза, но накладывает ограничения на исследователя, изучающего клеточный цикл в целом. В частности, DiOC6(3) препятствует получению достоверных данных об уровне пролиферации клеток. Следует также отметить, что возможность определения ранней стадии апоптоза может быть некорректной. Это связано с тем, что на начальном этапе клетка может сойти с пути апоптоза и сохранить жизнеспособность. О том, насколько последнее ограничение дискредитирует энергетическую функцию клетки как маркер апоптоза, можно судить только исходя из целей исследования.
Активация проапоптотических белков семейства Bcl-2
Изменение проницаемости митохондриальной мембраны происходит под постоянным контролем белков семейства Bcl-2 (B-cell lymphoma-2). Семейство Bcl-2 состоит из проапоптотических и ан-тиапоптотических белков, которые в обычном состоянии блокируют активность друг друга. Нарушения в балансе этой пары приводят либо к инициации, либо к ингибированию апоптоза в клетке [Youle, Strasser, 2008].
Инициация апоптоза связана с активацией про-апоптотических белков. Среди них первыми на сигналы, запускающие программную гибель клетки, реагируют BH3-белки [Happo, Strasser, Cory, 2012]. Так, Bid (BH3 interacting domain death agonist) отвечает на активацию каспазы-8 при запуске апоптоза через рецепторы смерти (Fas, TNF и др.), Bad (Bcl2 antagonist of cell death) – на повышенный уровень факторов роста, Bim (Bcl-2 interacting mediator of cell death) – на нарушение целостности цитоскелета клетки [Mohammad et al, 2005], Noxa и Puma (p53 upregulated modulator of apoptosis) – на повреждения ДНК [Oda et al., 2000; Nakano, Vousden, 2001]. Также отмечается реакция BH3-белков на экспрессию онкогенов и нарушение процессов транскрипции/трансляции [Kaufmann et al., 2007].
Под действием BH3-белков активируются мультидоменные проапоптотические факторы семейства Bcl-2: Bax (Bcl-2-associated X protein) и Bak (Bcl-2 antagonist/killer-1) [Kuwana et al., 2005]. От BH3-белков их отличает наличие дополнительной структуры – митохондриального якоря. Благодаря нему в активном состоянии Bax и Bak образуют олигомеры и участвуют в формировании пор во внешней мембране митохондрий [Autret,
Martin, 2009]. Именно такие поры позволяют протеолитическим белкам мигрировать в цитозоль.
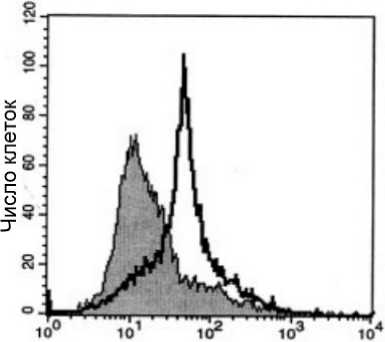
Обнаружить активные формы Bax и Bak в клетке можно с помощью конформационно-специфичных антител [Yethon et al., 2003], таких, например, как 6A7 (#MA5-14003, Thermo Fisher Scientific) и др. Особенность этих антител состоит в том, что они способны связываться только с N-терминальным концом проапоптотических белков, который не доступен для связывания, если белок находится в неактивной форме. Поскольку сами антитела не обладают флуоресцентными свойствами, их молекулы обычно конъюгированы с красителями (например, Alexa Fluor, FITC, PE и др.). Окрашивание таким антителом позволяет с помощью проточной цитофлюориметрии определить клетки, получившие проапоптотический сигнал. Полностью жизнеспособные клетки не будут демонстрировать высоких уровней флуоресценции (рис. 2).
Интенсивность флюоресценции
Рис. 2. Исследование апоптоза с применением моноклональных антител, специфичных к активной форме Bax
Представлены данные, полученные с использованием клеток линии U266 [Panaretakis et al., 2002]. Белый пик – клетки, обработанные доксорубицином (60 нг/мл, 48 ч.). Серый пик – живые, необработанные клетки
Как и в случае с DiOC6(3), использование антител к белкам семейства Bcl-2 позволяет проводить раннюю диагностику апоптоза, что требует некоторой осторожности ввиду обратимости этого этапа программной клеточной смерти. Следует также отметить, что моноклональные антитела, конъюгированные с флуорохромом, значительно дороже химически синтезированных соединений, что объясняет более редкое использование анти-Bcl-2 антител для детекции апоптоза.
Увеличение проницаемости цитоплазматической мембраны
Полупроницаемый характер клеточной мембраны препятствует попаданию в клетку молекул большого размера, однако на ранних этапах апоп- тоза проницаемость мембраны может нарушаться. Определение степени проницаемости клеточной мембраны возможно с применением низкомолекулярных флуоресцентных красителей (менее 900 Да), способных проникать через поры, образованные под влиянием внеклеточного АТФ и активированного им рецептора P2X7 [Virginio et al., 1999; Rassendren et al., 1997]. Одним из часто используемых в проточной цитофлюориметрии красителей, способных проникать в клетки, находящиеся на раннем этапе апоптоза, но не попадающих в живые клетки, является YO-PRO (размер 630 Да; максимум возбуждения/эмиссии 491/509 нм). Следует отметить, что на поздних стадиях апоптоза проницаемость мембраны повышается настолько, что в клетку могут проникать молекулы большего размера, такие как катионные флюоресцентные красители PI (Propidium Iodide; максимум возбуждения/эмиссии 535/617 нм) и 7-AAD (7-Aminoactinomycin D; максимум возбужде-ния/эмиссии 546/647 нм). В обоих случаях красители накапливаются в ядре клетки, связываясь с нуклеиновыми кислотами [Pena et al., 2005].
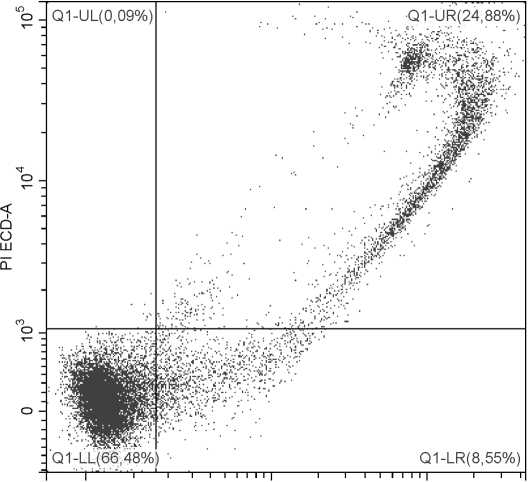
Совместное использование флюоресцентных красителей разных размеров позволяет установить границу между живыми клетками (YO-PRO – /PI – ); клетками на ранней стадии апоптоза (YO-PRO+/PI – ) и клеточными элементами на позднем этапе апоптоза (YO-PRO+/PI+). Зависимость между проницаемостью мембраны и уровнем флуоресценции данных красителей носит нелинейный характер. Интенсивность флуоресценции YO-PRO возрастает до тех пор, пока концентрация PI в клетке остается низкой, что связано со способностью PI подавлять флуоресценцию YO-PRO. На поздней стадии апоптоза происходит не только потеря целостности мембраны, но и повреждение ДНК клетки, что приводит к снижению интенсивности флуоресценции PI. В результате клетки, относящиеся к разным стадиям апоптоза, формируют на графике так называемую «петлю» (рис. 3). Важным преимуществом определения апоптоза с использованием красителей YO-PRO и PI являются малые временные затраты на подготовку образцов.
Нарушение асимметрии клеточной мембраны
Изменения цитоплазматической мембраны в процессе апоптоза не ограничиваются повышенной проницаемостью. Происходит реорганизация всей структуры липидного бислоя. Так, в норме мембраны эукариотических клеток характеризуются липидной асимметрией, главной особенностью которой является расположение анионных фосфолипидов – таких как фосфатидилсерин – только на внутренней (цитоплазматической) стороне. Это связано с тем, что фосфатидилсерин является важным ко-фактором для мембранно-связанных фер- ментов, таких как протеинкиназа С и натрийкалиевая АТФаза [Fadeel, Xue, 2009]. Тем не менее, в ходе различных биологических процессов происходит нарушение липидной асимметрии и появление фосфатидилсерина на поверхности клетки. Его миграция на внешнюю сторону мембраны происходит при свертывании крови [Zwaal, Comfurius, Bevers, 2004], образовании миотрубочек [van den Eijnde et al., 2001], слиянии везикул, активации B-лимфоцитов [Dillon et al., 2000], дегрануляции тучных клеток [Martin et al., 2000] и апоптозе [Fadok et al., 1992]. За распределение фосфолипидов в мембране клетки отвечают ферменты флиппазы. Их действие энергозависимо, поэтому в условиях с пониженным содержанием АТФ стоит ожидать нарушения транспорта фосфа-тидилсерина на внутреннюю сторону мембраны [van Engeland et al., 1998].
YO-PRO : PI
0 104 105
Yo-PRO FITC-A
Рис. 3 . Детекция апоптоза с применением красителей YO-PRO и PI
Выделенные мононуклеарные клетки перифериической крови человека стимулированы фитогемагглютинином (15 мкг/мл; 24 ч.). По оси абсцисс – уровень флуоресценции по каналу FITC (краситель YO-PRO), по оси ординат – уровень флуоресценции по каналу ECD (краситель PI). LL – живые клетки; LR – клетки на ранней стадии апоптоза; UR – клетки на поздней стадии апоптоза
Роль фосфатидилсерина в апоптозе связана с терминальной стадией, на которой происходит фагоцитоз клеток макрофагами – так называемый клиренс. Клиренс играет важную роль в поддержании гомеостаза организма [Fadeel, Xue, Kagan, 2010], так как умирающие клетки, являясь источником аутоантигенов, представляют большую опасность для иммунной системы. Быстрая и эффективная реакция со стороны фагоцитов предотвращает контакт аутоантигенов с антигенпрезентирующими клетками. Нарушения в процессе клиренса могут привести к серьезным заболеваниям, таким как системная красная волчанка [Herrmann, Voll, Kalden, 2000]. Отмечено, что иммунная сис- тема стремится избавиться от умирающих клеток еще на ранних стадиях апоптоза, когда шанс того, что целостность их мембраны будет нарушена, невелик. Поэтому в качестве маркеров для детекции апоптоза можно использовать молекулы, стимулирующие фагоцитоз. Так, фосфатидилсерин выступает в качестве лиганда для макрофагальных рецепторов, передавая фагоцитам от умирающей клетки сигнал «съешь меня». Важная роль фосфа-тидилсерина в процессе апоптоза подтверждается тем фактом, что клетки способны синтезировать данную молекулу de novo, заменяя полярные группы прочих фосфолипидов серином [Kuge, Nishijima, 2003].
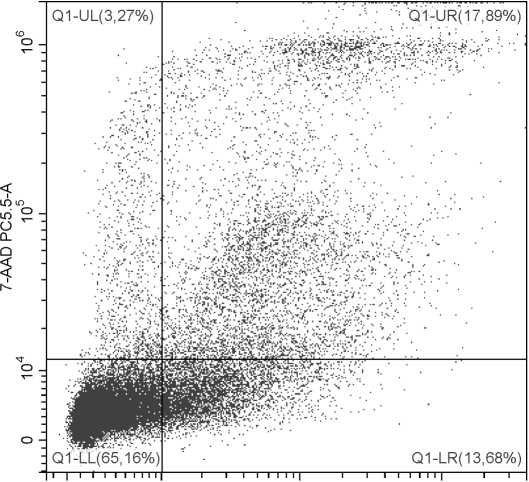
Для определения молекул фосфатидилсерина на поверхности клетки методом проточной цитоф-люориметрии используется белок Annexin V, конъюгированный с флюоресцентной меткой (например, FITC, Alexa Fluor, RPE и др.). Благодаря своему сродству к анионным фосфолипидам, Annexin V (в присутствии кальция) связывается с мембраной апоптотирующей клетки. С живой клеткой это взаимодействие практически невозможно, поскольку данный белок не способен проникать через липидный бислой. На рисунке 4 представлены результаты совместного окрашивания клеток конъюгатом AnnexinV-FITC и красителем 7-AAD, флуоресценция которого говорит о проницаемости цитоплазматической мембраны.
AnnexinV : 7-AAD
0 104 105 106
AnnexinV FITC-A
Рис. 4 . Исследование апоптоза с применением Annexin V и 7-AAD Выделенные мононуклеарные клетки периферической крови человека были стимулированы фитогемагглютинином (15 мкг/мл; 24 часа). По оси абсцисс – уровень флуоресценции по каналу FITC (краситель Annexin V), по оси ординат – уровень флуоресценции по каналу PC5.5 (краситель 7-AAD). LL – живые клетки; LR – клетки на ранней стадии апоптоза; UR, UL – клетки на поздней стадии апоптоза
Тест на определение фосфатидилсерина методом проточной цитофлюориметрии имеет ряд ог- раничений. Во-первых, исследователи [Rysavy et al., 2014] обращают внимание, что связывание Annexin V с молекулами фосфатидилсерина может происходить в условиях, никак не связанных с апоптозом (например, при активации Т- и В-лимфоцитов, тучных клеток и нейтрофилов). Во-вторых, экспрессия фосфатидилсерина на поверхности клетки – это обратимый процесс [Kroemer et al., 2009]. Иными словами, при применении красителя Annexin V в качестве маркера для детекции апоптоза следует учитывать вероятность получения как ложноположительных, так и ложноотрицательных результатов.
Активация протеолитических ферментов
Большинство морфологических (и ряд биохимических) изменений, которые происходят с клеткой во время апоптоза, вызваны действием каспаз-цистеиновых протеаз, специфичных к остаткам аспарагиновой кислоты [Hengartner, 2000]. Субстратом для каспаз могут служить практически все белки клетки, однако активация этих ферментов не приводит к полной деградации всех клеточных структур. Каспазы избирательно расщепляют ограниченный набор целевых белков, внося точечные изменения в последовательность аминокислот (всегда после аспартатного остатка). В большинстве случаев их избирательная «белковая хирургия» приводит не к разрушению целевой молекулы, а к ее инактивации. Каспазы присутствуют в клетке постоянно в виде неактивных форм (прокаспаз) и, как и многие ферменты, реализуют описанные функции только после активации. В результате этого процесса происходит изменение конформации молекулы, ввиду чего ее активный центр получает возможность взаимодействовать с субстратом. Следует отметить, что при детекции апоптоза важно определить только активированные каспазы. Для этих целей в проточной цитофлюоримет-рии применяется меченный флуорохромом ингибитор каспазы FLICA (Fluorochrome-Labeled Inhibitors of Caspases). Особенность FLICA состоит в том, что он с высокой аффинностью связывается с активным центром каспаз [Darzynkiewicz et al., 2004], но не с прокаспазами, постоянно присутствующими в клетке. Таким образом, при детекции методом проточной цитофлюориметрии окрашенные FLICA живые клетки в сравнении с умирающими имеют низкие показатели флуоресценции (рис. 5).
Существует целый ряд каспаз, различных по своим функциям, белковым субстратам и времени активации. Среди этого множества при определении апоптоза методом проточной цитофлюоримет-рии особое внимание стоит уделить каспазе-3, которая относится к эффекторным каспазам; ее функция напрямую связана с появлением характерной для апоптоза морфологии клетки. Появление активной формы этого фермента считается «точкой невозврата» клеточной гибели [Zou et al., 2002]. Можно с уверенностью сказать, что это делает каспазу-3 самым универсальным маркером при определении апоптоза. Распространению этого метода препятствует большая цена реагентов – проблема, присущая, в том числе, и методам, использующим в качестве маркера апоптоза белки семейства Bcl-2.
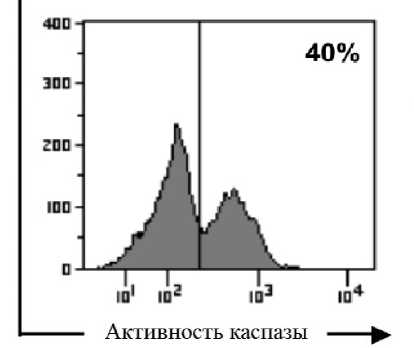
Рис. 5 . Детекция апоптоза методом FLICA В условиях оксидативного стресса, вызванного H 2 O 2 (250 μM), в CD8+ T-лимфоцитах человека происходит активация каспазы-3 и индукция апоптоза [Thorén, Romero, Hellstrand, 2006]. Пик слева – живые клетки с низким уровнем флюоресценции FLICA, пик справа – клетки, в которых присутствует активная форма каспазы-3
Фрагментация ДНК
Один из важных этапов в реализации программной клеточной смерти – фрагментация ДНК. Она является следствием активации каспазы-3, которая приводит к появлению в клетке активных форм фермента под названием CAD (caspase-activated DNase). Под действием CAD ДНК расщепляется на крупные фрагменты размером от 50 до 300 000 пар оснований. За расщеплением, опосредованным ДНКазой, следует фрагментация ДНК на отрезки меньшего размера – интернуклеосо-мальные фрагменты, кратные 180 парам оснований [Nagata et al., 2003]. Роль фрагментации ДНК в процессе апоптоза до конца не ясна, однако существует ряд гипотез, объясняющих необходимость данного процесса. Возможной причиной развития ферментативной системы, деградирующей нуклеиновые кислоты, может являться необходимость противовирусной защиты клеток [Samejima, Earnshaw, 2005]. Простого убийства зараженной клетки может быть недостаточно: если устойчивая молекула вирусного генома после смерти клетки-носителя попадет в цитоплазму здоровой клетки, инфекция возобновится. Риска повторного заражения можно избежать, предварительно разрушив все молекулы ДНК, присутствующие в умирающей клетке. О важной роли фрагментации нуклеиновых кислот говорит не только эволюционная гипотеза, но и эмпирические данные. Во-первых, ДНК не только участвует в процессе апоптоза, но и инициирует его [Nur et al., 2003]. Деградация генетического материала в клетке активирует сигнальную систему, зависимую от повреждений ДНК. Результатом ее активации является стабилизация белка p53. Этот белок выполняет две важных функции: запускает экспрессию проапоптотических генов (Apaf-1, PUMA, Noxa) и инициирует выход цитохрома С из митохондрий [Hedgecock, Sulston, Thomson, 1983]. Как известно, взаимодействие белка Apaf-1, цитохрома С и прокаспаз приводит к запуску митохондриального пути апоптоза [Cain, Bratton, Cohen, 2002]. Во-вторых, одна из функций фрагментации ДНК связана клиренсом обломков апоптотических клеток. Как и молекулы фосфатидилсерина, фрагменты генома могут экспрессироваться на поверхности клеток в качестве сигнала «съешь меня» для фагоцитов [Radic, Marion, Monestier, 2004].
Существуют два базовых цитометрических метода для определения фрагментации ДНК. Первый основан на оценке фракционного содержания ДНК в клетке. Если в процессе апоптоза генетический материал подвергнется деградации, то его фрагменты могут покинуть клетку, пройдя через ее нарушенную мембрану (предварительно обработанную детергентом или этанолом). В живых клетках большой молекулярный вес ДНК не позволит ей покинуть пределы органеллы. Для того чтобы оценить фракционный состав ДНК ядра с помощью проточной цитофолюориметрии, клетки обрабатывают флюоресцентыми ядерными красителями, такими как PI, DAPI, 7-AAD, и др. В результате окрашивания гиподиплоидные клетки (содержащие ДНК в количестве меньшем, чем в диплоидном ядре) в сравнении с живыми (диплоидными) клетками характеризуются пониженным уровнем флюоресценции. Графически это можно отобразить на гистограмме (рис. 6): живые клетки с нормальными ядрами сформируют пик G1/G0 с высоким уровнем флюоресценции; слева от него расположится пик с низкой флюоресценцией, образованный апоптотирующими клетками (Sub-G1); а справа – делящиеся клетки с повышенным содержанием ДНК (G2/M) [Wersto et al., 2001]. Пик Sub-G1 отражает количество клеток с фрагментированным генетическим материалом.
Второй метод определения фрагментированной ДНК в клетке – TUNEL (terminal deoxynucleotided transferase-mediated dUTP-biotin nick-end labelling). Метод основан на появлении в процессе апоптоза множества фрагментов нуклеиновых кислот, а значит и большого числа свободных 3’-концов ДНК. Фермент под названием терминальная дезоксинуклеотидилтрансфераза способен пришивать к свободным 3’-концам фрагментированной ДНК нуклеотид, конъюгированный с флуорохромом (например, dUTP – биотин – FITC) [Darzynkiewicz, Galkowski, Zhao, 2008]. В отличие от живых, апоптотирующие клетки накапливают в себе большое количество красителя (рис. 7). Интенсивность флуоресценции в этом случае прямо пропорциональна числу олигонуклеотидных последовательностей в пробе [Tripathi, Pareek, Sin-gla-Pareek, 2016].
Количество ДНК
Рис. 6 . Определение фракционного содержания ДНК
Апоптоз клеток линии BAF3 индуцирован недостатком IL-3 [Ormerod, 2002]. Гиподиплоидный пик Sub-G1 на гистограмме отражает содержание апоптотирующих клеток
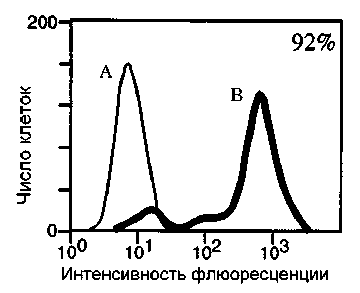
Рис. 7 . Детекция апоптоза с применением метода TUNEL
Программная гибель опухолевых клеток линии Jurkat индуцирована анти-Fas антителами (CH-11, 200 нг/мл) [Gastman et al., 1999]. А – живые клетки, резистентные к Fas-индуцированному апоптозу; B – клетки с фрагментированной ДНК
Стоит отметить, что при определении апоптоза метод, использующий мечение свободных 3’-концов ДНК, в сравнении с определением гиподи-плоидных ядер, является более специфичным. Как и при апоптозе, в процессе некроза, клетка может терять некоторое количество ДНК и приобретать гиподиплоидное ядро. Исключить такие клетки из анализа невозможно. При этом образование множества коротких фрагментов нуклеиновых кислот происходит только в процессе апоптоза. Следует также отметить недостаток работы с PI при определении клеток с гиподиплоидным ядром: необходимость предварительной пермеабилизации клеточной мембраны. Это процедура не позволяет совместить данный маркер апоптоза с другими и получить более достоверный результат. Кроме того, гипердиплоидные или полиплоидные умрающие клетки не попадают в пик Sub-G1. Благодаря высокому содержанию ДНК они могут быть расположены в пространстве между пиками G2 и G1, что, возможно, будет приводить к получению ложноотрицательных результатов.
Изменение формы и размера клеток
При программной клеточной гибели за биохимическими изменениями следует появление характерной морфологии. Основными событиями, имеющими место при апоптотической гибели, являются дегидратация и уменьшение размеров клетки, блеббинг плазматической мембраны и формирование апоптотических телец [Ziegler, Groscurth, 2004]. Проточная цитофлюориметрия, опираясь на показатели светорассеяния, позволяет определять некоторые из этих морфологических изменений [Darzynkiewicz et al., 2004].
Уменьшение клетки, ассоциированное с дегидратацией, наблюдается на ранних стадиях апоптоза и выражается в снижении показателей прямого светорассеяния [Ormerod, Cheetham, Sun, 1995]. Дальнейшее движение клетки по пути апоптоза сопровождается конденсацией цитоплазмы, фрагментацией ядра и хроматина. Цитоскелет клетки также претерпевает значительные изменения. Под действием каспаз разрушаются многие опорные белки, что приводит к появлению динамических образований в виде пузырей на поверхности клетки (блеббинг) [Ndozangue-Touriguine, Hamelin, Breard, 2008]. Описанные изменения увеличивают способность клетки к отражению и преломлению света, что может проявляться в кратковременном увеличении показателей бокового рассеяния [Darzynkiewicz, Bedner, 2000]. На поздних этапах апоптоза клетка значительно уменьшается в размерах, в связи с чем происходит снижение показателей как прямого, так и бокового светорассеяния. Заключительным этапом программируемой клеточной смерти является распад клетки на апопто-тические тельца.
Анализ морфологии клеток на проточном ци-тофлюориметре не зарекомендовал себя как самостоятельный метод детекции апоптоза. Отмечено, что описанные изменения в светорассеянии не являются уникальными и могут встречаться при любых типах клеточной гибели [Lizard, 2001]. Одна- ко информация о том, как изменяется светорассеяние погибающих клеток, может оказаться критической при гейтировании. Поскольку установка границ популяции основана на размере и степени гранулярности элементов, следует принимать во внимание возможные отклонения данных показателей, вызванные апоптотической гибелью клеток.
Заключение
Апоптоз – это энергозависимый процесс клеточной гибели, характеризующийся рядом последовательных биохимических событий. Начало апоптоза ассоциировано с активацией проапопто-тических белков семейства Bcl-2 в ответ на сигнал от рецепторов смерти или внутриклеточный стимул. Эти белки являются одними из главных регуляторов апоптоза, индуцирующих выход апопто-тических факторов (таких, как цитохром С) из митохондрий в цитозоль. Для митохондрий данный процесс заканчивается повышением проницаемости мембраны и потерей способности синтезировать АТФ. Недостаток АТФ приводит к невозможности поддерживать липидную асимметрию и избирательную проницаемость цитоплазматической мембраны клетки. Проапоптотические факторы, высвобожденные при повреждении митохондрий, участвуют в активации цистеиновых протеаз, в частности, эффекторной каспазы-3. Этот фермент – одна из центральных молекул программируемой клеточной гибели и главный инициатор ядерных изменений при апоптозе. Под действием каспазы-3 происходит активация ДНКаз и фрагментация ДНК. Данный этап предвещает завершение апоптоза посредством распада на апоптотические тельца и последующего фагоцитоза клеточного материала.
Проточная цитофлюориметрия позволяет выявлять клетки, погибающие путем апоптоза, на каждом из описанных этапов. Так, активность белков семейства Bcl-2 определяется с использованием конформационно-специфичных антител к Bax и Bak. Нарушение функции митохондрий анализируют путем окрашивания клеток липофильным катионным зондом DiOC6(3). Нарушение асимметрии клеточной мембраны детектируют по экспрессии фосфатидилсерина (реагент Annexin V). Проницаемость цитоплазматической мембраны исследуют при конкурентном окрашивании ядерными красителями YO-PRO и PI. Фрагментация ДНК определяется двумя методами: первый основан на анализе клеточного цикла с использованием ядер-ных красителей, второй – на детекции свободных 3’-концов ДНК, способных связываться с нуклеотидом, меченым флюорохромом.
В данной статье были освещены молекулярные механизмы, лежащие в основе каждого из указанных методов, рассмотрены преимущества и огра- ничения их использования. Кроме того, рассмотрены морфологические изменения, позволяющие установить границы исследуемой на проточном цитофлюориметре клеточной популяции. Полученные данные позволят наиболее эффективно применять проточную цитофлюориметрию для определения апоптоза во многих областях биологии и медицины.
Список литературы Современные методы исследования апоптоза с применением проточной цитофлюориметрии
- Anita et al. Apoptosis (programmed cell death) - A review // World Journal of Pharmaceutical Research. 2014. Vol. 3. P. 1854-1872.
- Autret A., Martin S.J. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis // Mol. Cell. 2009. Vol. 36. P. 355-363.
- Brazeau E. et al. Varicella-zoster virus-induced apoptosis in MeWo cells is accompanied by down-regulation of Bcl-2 expression // J. Neurovi-rol. 2010. Vol. 16. P. 133-140.
- Burz C. et al. Apoptosis in cancer: key molecular signaling pathways and therapy targets // Acta Oncol. 2009. Vol. 48. P. 811-821.
- Cain K., Bratton S.B., Cohen G.M. The Apaf-1 apoptosome: a large caspase-activating complex // Biochimie. 2002. Vol. 84. P. 203-214.
- Darzynkiewicz Z., Bedner E. Analysis of apoptotic cells by flow and laser scanning cytometry // Methods in Enzymology. 2000. Vol. 322. P. 18-39.
- Darzynkiewicz Z., Galkowski D., Zhao H. Analysis of apoptosis by cytometry using TUNEL assay // Methods. 2008. Vol. 44. P. 250-254.
- Darzynkiewicz Z. et al. Cytometric methods to detect apoptosis // Methods in cell biology. 2004. Vol. 75. P. 307-341.
- Dillon S.R. et al. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation // J. Immunol. 2000. Vol. 164. P. 1322-1332.
- Eckelman B.P., Salvesen G.S., Scott F.L. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family // EMBO reports. 2006. Vol. 7. P. 988-994.
- Eguchi K. Apoptosis in autoimmune diseases // Intern. Med. 2001. Vol. 40. P. 275-284.
- Fadeel B., Xue D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease // Critical Reviews in Biochemistry and Molecular Biology. 2009. Vol. 44. P. 264-277.
- Fadeel B., Xue D., Kagan V. Programmed cell clearance: molecular regulation of the elimination of apoptotic cell corpses and its role in the resolution of inflammation // Biochem. Biophys. Res. Commun. 2010. Vol. 396. P. 7-10.
- Fadok V.A. et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages // J. Immunol. 1992. Vol. 148. P. 2207-2216.
- Fujita T., Ishikawa Y. The role of the beta-adrenergic receptor-mediated signaling pathway and p53-mediated signaling pathway in the apoptosis of cardiomyocytes // Circ. J. 2011. Vol. 75. P. 1811-1818.
- Gastman B.R. et al. Caspase-mediated degradation of T-cell receptor {{zeta}}-Chain // Cancer Res. 1999. Vol. 59. P. 1422-1427.
- Gobe G. et al. Apoptosis and expression of Bcl-2, Bcl-XL, and Bax in renal cell carcinomas // Cancer Invest. 2002. Vol. 20. P. 324-332.
- Han H. et al. Prognostic value of immunohistochemi-cal expressions of p53, HER-2/neu, and bcl-2 in stage I non-small-cell lung cancer // Hum. Pathol. 2002. Vol. 33. P. 105-110.
- Happo L., Strasser A., Cory S. BH3-only proteins in apoptosis at a glance // J. Cell Sci. 2012. Vol. 125. P. 1081-1087.
- Hedgecock E.M., Sulston J.E., Thomson J.N. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans // Science. 1983. Vol. 220. P. 1277-1279.
- Hengartner M.O. The biochemistry of apoptosis // Nature. 2000. Vol. 407. P. 770-776.
- Herrmann M., Voll R.E., Kalden J.R. Etiopathogene-sis of systemic lupus erythematosus // Immunol. Today. 2000. Vol. 21. P. 424-426.
- Kaufmann T. et al. The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or Cell-cycle arrest // Cell. 2007. Vol. 129. P. 423-433.
- Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics // Br. J. Cancer. 1972. Vol. 26. P. 239-257.
- Kim Y.J. et al. Caspase 3-cleaved N-terminal fragments of wild-type and mutant huntingtin are present in normal and Huntington's disease brains, associate with membranes, and undergo cal-pain-dependent proteolysis // Proceedings of the National Academy of Sciences of the United States of America. 2001. Vol. 98. P. 12784-12789.
- Kroemer G. et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009 // Cell Death and Differentiation. 2009. Vol. 16. P. 3-11.
- Kroemer G., Reed J.C. Mitochondrial control of cell death // Nature Medicine. 2000. Vol. 6. P. 513-519.
- Kuge O., Nishijima M. Biosynthetic regulation and intracellular transport of phosphatidylserine in mammalian cells // J. Biochem. 2003. Vol. 133. P. 397-403.
- Kuwana et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly // Mol. Cell. 2005. Vol. 17. P. 525-535.
- Li L.Y., Luo X., Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria // Nature. 2001. Vol. 412. P. 95-99.
- Lin J.D. The role of apoptosis in autoimmune thyroid disorders and thyroid cancer // BMJ. 2001. Vol. 322. P. 1525-1527.
- Lizard G. Changes in light scatter properties are a general feature of cell death but are not characteristic of apoptotically dying cells // Cytometry. 2001. Vol. 46. P. 65-65.
- Martin S. et al. Immunologic stimulation of mast cells leads to the reversible exposure of phosphatidylserine in the absence of apoptosis // Int Arch Allergy Immunol. 2000. Vol. 123. P. 249-258.
- Mohammad R.M. et al. Preclinical studies of a non-peptidic small-molecule inhibitor of Bcl-2 and Bcl-X(L) [(-)-gossypol] against diffuse large cell lymphoma // Mol. Cancer Ther. 2005. Vol. 4. P. 13-21.
- Munoz L.E. et al. The role of defective clearance of apoptotic cells in systemic autoimmunity // Nature Reviews Rheumatology. 2010. Vol. 6. P. 280-289.
- Nagata S. Apoptosis and autoimmune diseases // Ann. N Y Acad. Sci. 2010. Vol. 1209. P. 10-16.
- Nagata S. Autoimmune diseases caused by defects in clearing dead cells and nuclei expelled from erythroid precursors // Immunological reviews. 2007. Vol. 220. P. 237-250.
- Nagata S. et al. Degradation of chromosomal DNA during apoptosis // Cell Death and Differentiation. 2003. Vol. 10. P. 108-116.
- Nakano K., Vousden K.H. PUMA, a novel proapoptotic gene, is induced by p53 // Molecular Cell. 2001. Vol. 7. P. 683-694.
- Ndozangue-Touriguine O., Hamelin J, Breard J. Cytoskeleton and apoptosis // Biochemical Pharmacology. 2008. Vol. 76. P. 11-18.
- Nemazee D. Receptor selection in B and T lymphocytes // Annu. Rev. Immunol. 2000. Vol. 18. P. 19-51.
- Nur E.K.A. et al. Single-stranded DNA induces ataxia telangiectasia mutant (ATM)/p53-dependent DNA damage and apoptotic signals // J. Biol. Chem. 2003. Vol. 278. P. 12475-12481.
- Oda E. et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis // Science. 2000. Vol. 288. P. 1053-1058.
- Ormerod M. G. Investigating the relationship between the cell cycle and apoptosis using flow cytometry // J. Immunol. Methods. 2002. Vol. 265. P. 73-80.
- Ormerod M.G., Cheetham F.P.M., Sun X.M. Discrimination of apoptotic thymocytes by forward light scatter // Cytometry. 1995. Vol. 21. P. 300-304.
- Pena F.J. et al. A new and simple method to evaluate early membrane changes in frozen-thawed boar spermatozoa // International Journal of Andrology. 2005. Vol. 28. P. 107-114.
- Petit P.X. et al. Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications // Eur. J. Biochem. 1990. Vol. 194. P. 389-397.
- Radic M., Marion T., Monestier M. Nucleosomes are exposed at the cell surface in apoptosis // J. Immunol. 2004. Vol. 172. P. 6692-6700.
- Rassendren F. et al. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA // J. Biol. Chem. 1997. Vol. 272. P. 5482-5486.
- Rohn T.T. The role of caspases in Alzheimer's disease; potential novel therapeutic opportunities // Apoptosis. 2010. Vol. 15. P. 1403-1409.
- Roos W.P., Kaina, B. DNA damage-induced cell death by apoptosis // Trends Mol. Med. 2006. Vol. 12. P. 440-450.
- Rysavy N.M. et al. Beyond apoptosis: The mechanism and function of phosphatidylserine asymmetry in the membrane of activating mast cells // BioArchitecture. 2014. Vol. 4. P. 127-137.
- Samantaray S. et al. The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats // Neuroscience. 2007. Vol. 146. P. 741-755.
- Samejima K., Earnshaw W.C. Trashing the genome: the role of nucleases during apoptosis // Nat Rev Mol Cell Biol. 2005. Vol. 6. P. 677-88.
- Thoren F.B., Romero A.I., Hellstrand K. Oxygen radicals induce Poly(ADP-ribose) polymerase-dependent cell death in cytotoxic lymphocytes // J. Immunol. 2006. Vol. 176. P. 7301-7307.
- Tripathi A.K., Pareek A., Singla-Pareek S.L. A NAP-family histone chaperone functions in abiotic stress response and adaptation // Plant Physiol. 2016. Vol. 171. P. 2854-2868.
- van den Eijnde S.M. et al. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation // J. Cell Sci. 2001. Vol. 114. P. 3631-3642.
- van Engeland M. et al. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure // Cytometry. 1998. Vol. 31. P. 1-9.
- Virginio C. et al. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor // J. Physiol. 1999. Vol. 519. P. 335-346.
- Vucic S., Kiernan M.C. Pathophysiology of neurodegeneration in familial amyotrophic lateral sclerosis// Curr. Mol. Med. 2009. Vol. 9. P. 255-272.
- Wersto R.P. et al. Doublet discrimination in DNA cell-cycle analysis // Cytometry. 2001. Vol. 46. P. 296-306.
- Whelan R.S., Kaplinskiy V., Kitsis R.N. Cell death in the pathogenesis of heart disease: mechanisms and significance // Annu. Rev. Physiol. 2010. Vol. 72. P. 19-44.
- Yaron F., Steller H. Programmed Cell death in animal development and disease // Cell. 2011. Vol. 147. P. 742-758.
- Yethon J.A. et al. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis // J. Biol. Chem. 2003. Vol. 278. P. 48935-48941.
- Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death // Nature Reviews Molecular Cell Biology. 2008. Vol. 9. P. 47-59.
- Ziegler U., Groscurth P. Morphological features of cell death // News Physiol. Sci. 2004. Vol. 19. P. 124-128.
- Zou H. et al. Regulation of the Apaf-1/Caspase-9 Apoptosome by Caspase-3 and XIAP // The Journal of Biological Chemistry. 2002. Vol. 278. P. 8091-8098.
- Zwaal R.F., Comfurius P., Bevers E.M. Scott syndrome, a bleeding disorder caused by defective scrambling of membrane phospholipids // Biochim Biophys Acta. 2004. Vol. 1636. P. 119-128.


