Современные подходы к специфической профилактике африканской чумы свиней (обзор)
Автор: Чернышев Р.С., Спрыгин А.В., Иголкин А.С., Жбанова Т.В., Перевозчикова Н.А., Роменская Д.В., Груздев К.Н., Мазлум А.
Журнал: Сельскохозяйственная биология @agrobiology
Рубрика: Обзоры, проблемы
Статья в выпуске: 4 т.57, 2022 года.
Бесплатный доступ
Африканская чума свиней (АЧС), впервые охарактеризованная R.E. Montgomery еще в начале ХХ века (R.E. Montgomery, 1921), уже более 100 лет остается одной из острых проблем в мировом свиноводстве. Поиски эффективных и универсальных средств специфической профилактики во всем мире ведутся с 1933 года (J. Walker, 1933). В представленном обзоре суммированы данные литературы из открытых источников по наиболее важным и успешным событиям в истории разработки вакцин против АЧС, обсуждены перспективы и результаты использования аттенуированных (C. Muñoz-Pérez с соавт., 2021), инактивированных (E. Cadenas-Fernández с соавт., 2021), субъединичных (J.G. Neilan с соавт., 2004) и векторных (J.K. Jancovich с соавт., 2018) вакцин против АЧС. При широком использовании природно ослабленных негемадсорбирующих изолятов в качестве вакцин во второй половине XX века в странах Европы наблюдалось хроническое течение АЧС у значительного числа свиней (J. Manso Ribeiro с соавт., 1963). Несмотря на генетические изменения в геноме вируса, последовательные пассажи полевых изолятов вируса АЧС на различных культурах клеток не показали должного результата в ослаблении вирулентных свойств возбудителя (I. Titov с соавт., 2017). Использование технологий получения делетированных мутантов ASFV-G-ΔI177L создало перспективу для разработки эффективных вакцин-кандидатов (M.V. Borca с соавт., 2020). Показано, что инактивированные, а также субъединичные вакцины на основе рекомбинантных белков, вызывали образование специфических иммуноглобулинов в высоких титрах, однако не обладали протективными свойствами (G. Burmakina с соавт., 2016). Новой вехой в борьбе с многими инфекционными болезнями животных, в частности с АЧС, стали векторные вакцины. Исследования последних лет указывают на перспективу создания эффективных и сертифицированных ДНК-вакцин, среди векторов для разработки которых хорошо себя зарекомендовали аденовирус человека 5 (rAd) и модифицированный вирус осповакцины Анкара (MVA) (L.C. Goatley с соавт., 2020). Аттенуированные вакцины на основе генетически модифицированных вирусов с делецией генов I226R и 18-7GD (Y. Zhang с соавт., 2021; W. Chen с соавт., 2020) нуждаются в экспертизе с привлечением международных организаций для дальнейшей регистрации и внедрения в ветеринарную практику.
Африканская чума свиней, ачс, вакцина, инактивированная вакцина, аттенуированная вакцина, днк-вакцина, рекомбинантная вакцина, crispr/cas9
Короткий адрес: https://sciup.org/142236345
IDR: 142236345 | УДК: 636.4:619:578:577.2:571.27 | DOI: 10.15389/agrobiology.2022.4.609rus
Текст обзорной статьи Современные подходы к специфической профилактике африканской чумы свиней (обзор)
Африканская чума свиней (АЧС) — контагиозная, природно-очаговая, трансграничная болезнь домашних свиней и диких кабанов, которая может протекать сверхостро, остро, подостро, хронически и бессимптомно. Ее этиологический агент — содержащий двуцепочечную ДНК (dsDNA) оболочечный вирус (African swine fever virus — ASFV, род Asfivirus , семейство Asfarviridae ) (1) (рис. 1).
Геном вируса состоит из 170-193 т.п.н. и содержит 151-167 открытых рамок считывания (ОРС) (2). Возбудитель имеет 9 сероиммунотипов, идентифицированных в реакции задержки гемадсорбции (РЗГАд) и в иммунопробе на восприимчивых животных, и 24 генотипа на основе вариабельности гена B646L , кодирующего капсидный белок vр72 (2-4). В связи с консервативностью последнего также проводится внутригенотиповая дифференциация изолятов вируса АЧС на основе анализа трех высоковариабельных генов — гена В602L (CVR), кодирующего неструктурный шаперон, ко-
∗ Работа поддержана грантом Министерства науки и высшего образования Российской Федерации на реализацию отдельных мероприятий Федеральной научно-технической программы развития генетических технологий на 2019-2027 годы (Соглашение ¹ 075-15-2021-1054).
торый участвует в сборке капсида, а также генов E183L и CP204L , кодирующих соответственно структурные белки vp54 и vp30 (5, 6).
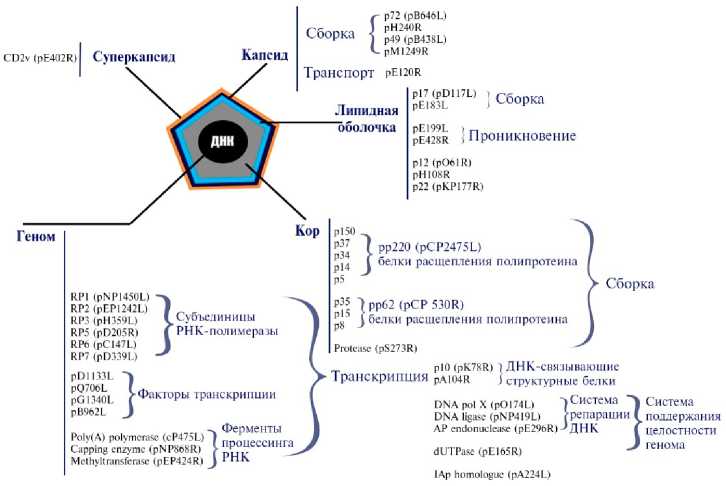
Сборка
Капсид
Транспорт pEI20R
Геном
Сборка
АР endonuclease (pE296R)Э ДНК dUTPasc (pEI65R)
IAp homologue (pA224L)
Система "
■репарации
RP1 (pNP1450L)
RP2 (pEP1242L)
RP3 (pH359L)
RP5 (pD2O5R)
RP6 (pC147L)
RP7 (pD339L)
Липидная "оболочка
Poly(A) polymerase (cP475L)
Capping enzyme (pNP868R)
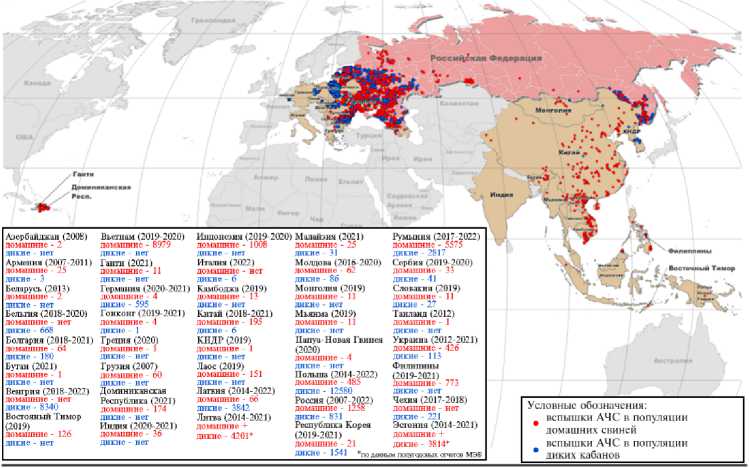
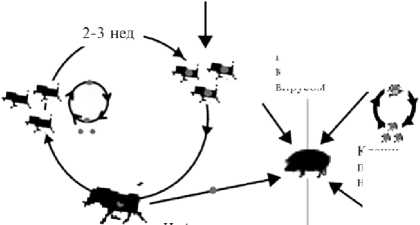
Methyltransferase pl 50 p37 p!4 p5 p72 (pB646L) pH240R p49 (pB438L) pMI249R DNA pol X (pOl74L) DNA ligase (pNP419L) pl2 (pO61R) pHIOSR p22 (pKPI77R) Система поддержания целостности генома рр62 (рСР 530R) белки расщепления полипротеина рр220 (pCP2475L) белки расщепления полипротеина Ферменты процессинга РНК PEI99L , п PE428R i Проникновение Субъединицы РНК-полимеразы №?117Ц ; Сборка рОПЗЗЬ pG^UOL /Факторы транскрипции PB962L J Protease (pS273R) рЮ (pK78R) ) ДНК-связываюшие Транскрипция pAI04R j структурные белки CD2v (pE402R)| Суперкапсид Рис. 1. Морфология вириона вируса африканской чумы свиней (1). Эпизоотическая ситуация по АЧС в РФ, странах Европы, Азии и Америки, 2007-2022 годы (по данным срочных сообщений МЭБ на 17.01.2022) Рис. 2. Эпизоотическая ситуация по африканской чуме свиней (АЧС) в мире, 2007-2022 годы (11). В XXI веке за относительно короткий период времени АЧС стала глобальной мировой проблемой. За последние 8 лет стремительное распространение инфекции в странах Европы (95 % очагов в популяции дикого кабана) и Юго-Восточной Азии (97 % очагов в популяции домашних свиней), ранее свободных от этой болезни, приняло катастрофический характер. В современной панзоотии АЧС (2007-2022) официально первые очаги инфекции возникли на территории Грузии в начале 2007 года, когда контаминированная вирусом АЧС мясная продукция, доставленная в порт г. Поти на морских судах из стран Юго-Восточной Африки, была реализована среди местного населения. После этого в течение весенне-летнего сезона вокруг г. Поти регистрировали массовый падеж свиней, в том числе находившихся на свободном выгуле (7). В ноябре 2007 года была зарегистрирована первая вспышка АЧС на территории Российской Федерации в Чеченской Республике среди диких кабанов (8). В 2012 году очаги АЧС нотифицированы в Украине, в 2013 году — в Беларуси, в 2014 году — в Польше и Эстонии, в 2017 году — в Чехии, в 2018 году — в Венгрии, Бельгии и в Китае, в 2020 году — в Германии, в 2021 — в Малайзии, Республике Гаити и Доминиканской Республике (9, 10). По информации OIE, по данным на 2021 год АЧС зарегистрирована в 13 странах Европы и в 11 странах Азии (рис. 2) (11). В период с января 2020 года по ноябрь 2021 года общие потери от АЧС в Европе и Азии составили соответственно 1168354 и 373693 гол. домашних свиней (12). Три цикла передачи вируса АЧС Сильватический цикл Цикл передачи у домашних свиней Цикл передачи у дикого кабана Пищевые отходы И нфи цированные клещи отпадают с покровов бородавочника Прямой контакт с живыми свиньями, их трупами и продуктами жизнедеятельности, с контаминированными Клещи, паразитирующие на коже свиней Прямой контакт с трупами/продуктами жизнедеятельности/ предметами, контаминированными вирусом Прямой контакт с живыми свиньями, их трупами, продуктами жизнедеятельности и предметами, >. контаминированными вирусом Прямой контакт с живыми кабанами, их трупами и предметами, контаминированными вирусом, включая транспорт объектами окружающей сЬеды Рис. 3. Механизмы передачи вируса АЧС (13). Механизм передачи вируса АЧС осуществляется по трем основным путям: через мягких клещей (сильватический цикл: страны Южной и ЮгоВосточной Африки, о. Сардиния), контактная трансмиссия и алиментарное заражение, причем последние два включают участие как домашних свиней, так и диких кабанов (рис. 3) (13). В большинстве регионов Африки мутуалистические отношения между Ornithodoros moubata (клещ семейства Arga-sidae) и бородавочниками Phacochoerus africanus формируют устойчивый сильватический цикл, поддерживая тем самым циркуляцию вируса в природе (14). В условиях северного полушария дикие кабаны могут осуществлять передачу вируса домашним свиньям контактным путем (15). Дополнительно выделяют еще один механизм — антропогенный, связанный с активной деятельностью человека, в том числе с транспортировкой продукции свиноводства и охотничьего промысла, контаминированной вирусом АЧС, ее попаданием в корм здоровых восприимчивых животных (16). Отличительная особенность инфекции — образование большого количества антител, однако полной элиминации вируса из организма больного животного не происходит (17). Мало того, из-за наличия FCγ-рецеп-тора у макрофага может наблюдаться антителозависимое усиление инфекции, приводящее к чрезмерному синтезу IL-10, и, как следствие, к активации Th2-клеток (16, 18-20). Экспериментально установлено, что роль антител заключается в снижении титров первичной виремии и, как следствие, в задержке проявления клинических признаков (21). В связи со сложностью изучения механизма иммунных реакций в организме свиней в ответ на заражение вирусом АЧС, поиск подходов к разработке эффективных и безопасных средств специфической профилактики продолжается до настоящего времени (22). В последние годы в условиях беспрецедентного распространения АЧС в мире достигнут, к сожалению, незначительный прогресс в создании эффективной вакцины. В связи с этим в своем обзоре мы рассмотрим вопросы, касающиеся современного положения дел в разработке аттенуированных, инактивированных и рекомбинантных вакцин против АЧС. Роль клеточного и гуморального иммунитета в защите против АЧС. Большая часть имеющихся данных указывает на важнейшее значение NK-клеток в развитии иммунного ответа против АЧС. В исследовании in vitro установлено, что вирулентные изоляты вируса АЧС ингибируют активность NK-клеток (23). Высокий уровень цитотоксических CD8+ Т-лимфоцитов, которые уничтожают инфицированные макрофаги в организме, играет не менее значимую роль в иммунном ответе у свиней (24). Однако гипергаммаглобулинемия, а также повышенное содержание плазматических клеток и цитотоксических Т-лимфоцитов могут вызывать как специфическую иммуносупрессивную, так и опосредованную реакцию организма через IL-4 и IL-10 (25, 26). На сегодня наиболее эффективными показали себя аттенуированные вакцины, полученные при делеции определенных генов, и рекомбинантные вакцины, индуцирующие сильный клеточный иммунитет, который обусловлен ранней активностью NK-клеток и цитотоксических CD8+ Т-лимфоцитов. Основная сложность, связанная с использованием ДНК-вакцин, заключается именно в том, чтобы обеспечить формирование клеточного иммунитета, связанного с ранней активностью NK-клеток и цитотоксических CD8+ Т-лимфоцитов, что, безусловно, играет большую роль, чем короткая сероконверсия к белкам вируса АЧС, которые экспрессируются в клетках хозяина (27). В настоящее время известны работы, доказывающие существование вируснейтрализующих антител (17). Сыворотки реконвалесцентов, полученные после иммунизации свиней аттенуированным на культуре клеток CV-1 вариантом E75CV1-4, защищали животных от заражения исходным изолятом E75, а также изолятами E70, Lisbon 60, Malawi Lil 20/1 вируса АЧС в 86-97 % случаях (28). У свиней, пассивно иммунизированных очищенными иммуноглобулинами к вирусу АЧС, наблюдалась задержка первичной виремии в течение 3 сут по сравнению с контрольной группой животных (29). В исследовании вируснейтрализующей активности антител на культурах клеток Vero и альвеолярных макрофагов свиней показана 80 % нейтрализация меченного радиоактивным изотопом вируса АЧС, при этом его интернализация в клетки продолжалась (30). Антитела к цитоплазматическому динеин-домену DLC8 белка vp54 играли ведущую роль в нейтрализации вируса АЧС в культуре клеток Vero (31, 32). Несмотря на противоречивость данных, полученных относительно как уровня, так и самого факта формирования гуморального иммунитета против вируса АЧС, большинство исследователей сходятся во мнении, что специфические антитела важны в реакции задержки гемадсорбции при повторном заражении вирусом АЧС и в задержке первичной виремии, однако они не играют протективной роли (17). Аттенуированные вакцины. Аттенуированными называют вакцинные препараты на основе природно ослабленных (или аттенуированных) вирусов, полученных в лабораторных условиях посредством последовательных пассажей на чувствительных культурах клеток, а также при генетической модификации вирулентного вируса в результате делеции определенных генов. Естественно аттенуированные вакцины. Первые данные об использовании аттенуированных вакцин были опубликованы в 1933 году J. Walker (33). Процент выживших свиней при иммунизации такими вакцинами был невысок, а введение интактным свиньям сывороток реконвалесцентов не защищало от заражения вирулентным вирусом. Автор тогда предположил, что низкая иммунная защита свиней от АЧС может быть связана с высокой антигенной изменчивостью изолятов из-за большого числа штаммов возбудителя по сравнению с вирусом классической чумы свиней (33). Впоследствии было установлено, что иммунизация аттенуированными вакцинами может защищать от заражения только изолятом гомологичного генотипа, тогда как при заражении гетерологичным изолятом вируса развивается клиническая картина АЧС (34, 35). В опыте F. Boinas с соавт. (36) свиньям вводили природно аттенуированный изолят вируса АЧС OURT88/3 (генотип I), выделенный из клещей Ornithodoros erraticus семейства Argasidae и не проявляющий гемадсор-бирующих свойств, с последующим заражением вирулентным гемадсорби-рующим вирусом OURT88/1 (генотип I) в качестве штамма-пробойника. При этом ни виремии, ни клинических признаков АЧС у животных не отмечали. Однако после заражения таких свиней изолятом Lisbon 57 (генотип I) гибель всего поголовья произошла через 10-14 сут (36). При заражении свиней негемадсорбирующим изолятом NH/P68 (генотип I), выделенным в 1968 году в Португалии от свиней с хронической формой АЧС, наблюдалось бессимптомное течение болезни, при этом регистрировали раннюю активность NK-клеток (с 7-х сут), позднюю виремию (после 14 сут) и высокий уровень специфических IgM, IgG1, IgG2 и IgA и цитотоксических Т-лимфоцитов, выявленных на 7-18-е сут после заражения. Свиньи, у которых наблюдали инаппарантнаю инфекцию, раннюю активность и высокое содержание NK-клеток, были устойчивы к заражению вирулентным изолятом АЧС L60 (генотип I). Однако у животных с хронической формой АЧС уровень NK-клеток был низким и приближался к таковому у свиней из контрольной группы (37, 38). K. King с соавт. (39) иммунизировали свиней авирулентным изоля-том OURT88/3, а затем исходным природно ослабленным низковирулентным штаммом OURT88/1 вируса АЧС. При заражении таких свиней вирулентными изолятами Benin 97/1 (генотип I) и гетерологичным Uganda 1965 (генотип X) уровень иммунной защиты составил соответственно 85,7 и 100 %. Более того, у 78 % иммунизированных свиней, которых заражали изолятом Benin 97/1, и у 50 % свиней, зараженных изолятом Uganda 1965, не наблюдали виремии и клинических признаков болезни (39). При иммунизации свиней негемадсорбирующим естественно аттенуированным изолятом вируса АЧС Lv/17/WB/Rie1 (генотип II), выделенного от дикого кабана на территории Латвии в 2017 году, с последующим контактом со свиньями, зараженными вирулентным вирусом Arm07 (генотип II), у 50 % животных опытной группы отмечали клинические признаки и виремию, а 50 % оставались клинически здоровыми, однако виремия у них сохранялась. При заражении свиней контрольной группы родственным гемадсорбирующим вирулентным изолятом Lv17/WB/Zieme3 гибель наступала на 12-е сут (40). Пероральная вакцинация диких кабанов штаммом Lv/17/WB/Rie1 показала 92 % иммунную защиту против контрольного заражения Arm07 (41). В настоящее время проходит оценка безопасности вакцины-кандидата на основе Lv/17/WB/Rie1 в популяции дикого кабана. Отмечен разный процент выживаемости животных при вакцинации и ревакцинации в дозах 103 ТЦД50 и 104 ТЦД50, что показывает важность дальнейших исследований этого прототипа вакцины (42). Таким образом, естественно аттенуированные вакцинные варианты вируса АЧС демонстрируют широкую вариабельность в отношении протек-тивных свойств даже против заражения изолятами гомологичного генотипа (17). Эта особенность препятствует разработке унифицированного препарата с широким спектром протективной активности. Лабораторно аттенуированные вакцины. Культуральные вакцины. При аттенуации вирулентного изолята BA71 вируса АЧС (генотип I) провели 36 пассажей на макрофагах свиней с последующими 23 пассажами на перевиваемой культуре клеток Vero. В результате был получен вариант BA71V, который зарекомендовал себя в качестве стандарта для титрования вируса АЧС по числу бляшкообразующих единиц (БОЕ), однако этот вариант не снижал летальность свиней при заражении (43). Проведя 50 последовательных пассажей вирулентного изолята вируса АЧС К49 (генотип I) на перевиваемой культуре клеток эмбриональной почки свиньи (SPEV) и 262 пассажа на первичной культуре клеток костного мозга свиней, получили авирулентный штамм КК262. После двух введений КК262 свиньям опытной группы (в 1-е и 21-е сут) у 33 % животных наблюдалась виремия на 28-е сут, но при заражении исходным вирулентным изолятом К49 на 42-е сут с начала испытания все свиньи оставались живыми (44). При изучении биологических свойств изолята Odintsovo 02/14 (генотип II), выделенного от дикого кабана, выполнили три последовательных пассажа вируса на культуре клеток костного мозга свиньи (КМС). Важно отметить, что исходный полевой изолят, выделенный из селезенки павшего дикого кабана, вызывал 87,5 % летальность у домашних свиней после введения изолята пяти животным внутримышечно в дозе 10 ГАдЕ50 и пяти — интраназально в дозе 50 ГАдЕ50 (27, 45). Адаптация изолята Odintsovo 02/14 на перевиваемой культуре клеток CV-1 в течение 30 последовательных пассажей (вирус назван АЧС/ВНИИЗЖ/CV-1/30) привела к снижению гемад-сорбции с 40-50 до 20-30 эритроцитов, прикрепленных к инфицированной клетке. Заражение свиней таким вариантом показало снижение летальности до 16,7 % и устойчивость выживших животных к контрольному заражению вирулентным изолятом Arm 07 (46-48). При адаптации изолята Georgia 2007/1 на культуре клеток Vero провели 110 пассажей. На 80-м пассаже вариант ASFV-G/VP80 показал сниженную в 10 раз способность реплицироваться в первично трипсинирован-ной культуре клеток макрофагов свиней, а на 110-м пассаже у варианта ASFV-G/VP110 репликация снижалась в 105-106 раз. При иммунизации свиней вариантом ASFV-G/VP110 не отмечали клинических признаков болезни в течение 21 сут наблюдения. Однако контрольное заражение изоля-том Georgia 2007/1 не показало эффективности подобной иммунизации, так как у всех свиней проявились клинические признаки АЧС и регистрировалась гибель на 9-е сут (49). Результаты проведенных исследований по лабораторной аттенуации полевых изолятов позволяют заключить, что, как правило, не происходит такого ослабления вирулентных свойств вируса АЧС, чтобы в дальнейшем применять его для безопасной иммунизации. Однако при разработке вакцин может оказаться успешной целевая модификация генов аттенуированных вариантов вируса АЧС с помощью методов молекулярной биологии. Вакцины на основе генетически модифицированных вирусов. Один из новых перспективных подходов при получении безопасных аттенуированных вакцин предполагает делетирование специфических генов вируса АЧС, кодирующих белки, которые служат факторами вирулентности (22, 50). Однако в этом случае значительные трудности связаны с тем, что функции многих белков не изучены. Например, делеция гена KI69R, кодирующего тимидинкиназу, приводила к потере вирулентности у изолята Malawi Lil-20/1 (генотип VIII), но при этом сохраняла вирулентность у изо-лята Georgia 2007/1 (генотип II), что, по-видимому, объясняется компенсаторными мутациями в других участках генома (51, 52). При удалении шести генов мультигенного семейства MGF360 и MGF505 у Georgia 2007/1 (MGF505-1R, MGF360-12L, MGF360-13L, MGF360-14L, MGF505-2R, MGF505-3R) происходит полное ослабление вирулентных свойств вируса. Иммунизация животных таким делетированным вариантом в дозах 102 и 104 ГАдЕ50 не вызывала развития клинических признаков АЧС, а после контрольного заражения вирулентным штаммом Georgia 2007/1 не отмечалось признаков хронического течения болезни, однако на протяжении примерно 7,5 сут детектировалась умеренная виремия (53). При иммунизации вариантом Georgia 2007/1 с делецией гена 9GL, кодирующего фосфорилазу, более высокий уровень защиты наблюдали при сочетании с де-лецией гена UK (DP96R) (50, 54). Однако при одновременной делеции генов MGF360 и MGF505, кодирующих ингибиторы интерферона I типа, и 9GL защитный эффект при иммунизации не наступал (55). Свиньи, которых иммунизировали вариантом BA71ΔCD2 с деле-цией гена EP402R, полученным при гомологичной рекомбинации, оказались устойчивы к заражению исходным вирулентным изолятом BA71 (генотип I), а также вирулентными изолятами Е75 (генотип I), Georgia 2007/1, RSA/11/2017 (генотип XIX), Ken06.Bus (генотип IX) (56, 57). Делеция гена DP148R у изолята вируса АЧС Benin 97/1 (генотип I) приводила к полной потере вирулентности. У свиней, иммунизированных BeninΔDP148R, при внутримышечном заражении исходным изолятом отмечали 100 % защиты, тогда как при интраназальном этот показатель составил 83,3 % (58). При иммунизации свиней вариантами NH/P68DA238L-COS7 с делецией гена A238L, NH/P68DA224L-COS7 с делецией гена A224L, NH/P68DEP153R-COS7 с делецией гена EP153R в дозе 106 ТЦД50 и NH/P68DA276R-PAM в дозе 102 ТЦД50 с последующим контрольным заражением 10 ГАдЕ50 штаммом Arm07 ослабление вирулентных свойств было достигнуто только в случае удаления гена A224L. Иммунная защита при этом достигала 100 % (59). В исследованиях по делетированию генов MGF505-1R, MGF505-2R, MGF505-3R, MGF360-12L, MGF360-13L, MGF360-14L, EP402R, 9GL, DP148R, кодирующих семь различных белков, китайские ученые (60) получили модифицированный вариант вируса АЧС HLJ/18-7GD, после внутримышечной инокуляции которого свиньи оставались клинически здоровыми в течение 3 нед наблюдения. При заражении свиней вирулентным изолятом HLJ/18 (генотип II) в дозе 200 ЛД50 у животных, иммунизированных 103 ТЦД50 HLJ/18-7GD, наблюдалась лихорадка в течение 3-9 сут с максимальным подъемом температуры до 42 °С, однако у свиней, иммунизированных 105 ТЦД50 HLJ/18-7GD, незначительный подъем температуры до 40,7 °С регистрировался только в течение первых суток (60). В 2020 году группа ученых из США опубликовала данные о 100 % защите поголовья свиней, иммунизированных аттенуированным вариантом ASFV-G-ΔI177L вируса АЧС, полученным из исходного вирулентного изо-лята ASFV-G (Georgia 2007/1) в результате делеции гена I177L, который до этого исследования не был типичен для таких экспериментов. В опытной группе свиньям вводили ASFV-G-ΔI177L в дозе 102 ГАдЕ50, после чего регистрировали незначительные титры при виремии (101,8-105 ГАдЕ50/см3 на 4-е сут, пик при 104-107,5 ГАдЕ50/см3 на 11-е сут и последующее снижение до 102,3-104 ГАдЕ50/см3 вплоть до 28-х сут) и отсутствие каких-либо клинических признаков АЧС в течение 28 сут наблюдения. Впоследствии таких свиней заражали 102 ГАдЕ50 исходного изолята ASFV-G. В течение 21 сут у животных не было клинических признаков АЧС, виремия развивалась, титры не превышали таковые в первом периоде наблюдения, а в крови вирулентный вирус не детектировался методом ПЦР-РВ (qPCR) (61). Работа M.V. Borca с соавт. (61) — первое сообщение о формирования стерильного иммунитета против АЧС в истории изучения этой инфекции. При адаптации ASFV-G-ΔI177L на культуре клеток PIPEC (Plum Island porcine epithelial cells) был получен стабильный изолят ASFV-G-ΔI177L/ΔLVR с делецией генов MGF и X69R, защищающий 100 % свиней от заражения вирулентным ASFV-G, что может быть использовано при производстве универсальной вакцины против АЧС (62). В дальнейшем авторы показали эффективность экспериментальной вакцины на основе ASFV-G-ΔI177L при ороназальном введении, что имеет важнейшее значение с точки зрения перспектив иммунизации диких свиней (в особенности европейского кабана Sus scrofa). При одинаковой дозе с внутримышечной инокуляцией титры при виремии в случае ороназального введения были значительно меньше, тогда как титры IgG1, IgG2 и IgM сохранялись на том же уровне (63). Недавнее исследование показало, что экспериментальная вакцина на основе ASFV-G-ΔI177L успешно индуцировала иммунную защиту поголовья вьетнамских свиней от полевых изолятов вируса АЧС (генотип II) во Вьетнаме (64). Аналогичные исследования проводились в КНР в 2021 году, где в качестве вакцины-кандидата использовался изолят SY18ΔI226R с делецией не описанного до этого функционального гена I226R, кодирующего консервативный белок pI226R, локализующегося в виросоме цитоплазмы клеток («вирусных фабриках»). После введения SY18ΔI226R одной группе свиней в дозе 104 ТЦД50, другой — 107 ТЦД50 у животных не отмечали повышения температуры тела выше 40,1 °С и каких-либо клинических признаков болезни. При заражении первой группы свиней исходным изолятом SY18 (генотип II) в дозе 102,5 ТЦД50 регистрировали 2-суточную лихорадку с максимальным повышением температуры до 41,4 °С. Во второй группе при заражении 104 ТЦД50 SY18 за период наблюдения не отмечали лихорадки и иных клинических признаков АЧС. Выживаемость свиней в обеих группах составила 100 % (65). Опубликованные данные по вакцинным препаратам на основе генетически модифицированного вируса АЧС, в том числе с указанием генов для редактирования и процента выживаемости после контрольного заражения, представлены в таблице 1. Здесь обобщены сведения о проведенных за последнее десятилетие исследованиях по генетической модификации вируса АЧС с использованием наиболее охарактеризованных и изученных вирулентных изолятов (в частности, Georgia 2007, Benin, E75). 1. Генетически модифицированные варианты вируса африканской чумы свиней, использованные в качестве перспективных вакцин Экспериментальный вирус АЧС Удаленные гены Контрольный изолят (генотип) Инфицировано/вы-жило (протекция, %) Ссылка BA71ΔCD2 в дозе 106 ГАдЕ50 BA71ΔCD2 EP402R (CD2v) BA71 (I) 6/6 (100 %) (56) в дозе 103 ГАдЕ50 BA71ΔCD2 в дозе 3,3½104 EP402R (CD2v) BA71 (I) 6/2 (33 %) (56) или 106 ГАдЕ50 BA71ΔCD2 EP402R (CD2v) Е75 (I) 12/12 (100 %) (56) в дозе 103 ГАдЕ50 BA71ΔCD2 в дозе 3,3½104 EP402R (CD2v) Е75 (I) 6/1 (17 %) (56) или 106 ГАдЕ50 BA71ΔCD2 в дозе 3,3½104 EP402R (CD2v) Georgia 2007/1 (II) 18/18 (100 %) (56) или 106 ГАдЕ50 BA71ΔCD2 в дозе 3,3½104 и EP402R (CD2v) RSA/11/2017 (XIX) 6/5 (83,3 %) (57) 106 ГАдЕ50 EP402R (CD2v) Ken06.Bus (IX) 8/4 (50 %) (57) HLJ/18-7GD MGF505-1R, MGF505-2R, MGF505-3R, MGF360-12L, MGF360-13L, MGF360-14L, EP402R, 9GL, DP148R HLJ/18 (II) 4/4 (100 %) (60) BeninΔDP148R DP148R Benin (I), внутримышечное заражение 11/11 (100 %) (58) BeninΔDP148R DP148R Benin (I), интра-назальное заражение 6/5 (83,3 %) (58) ASFV-G-ΔI177L в дозе 102 ГАдЕ50 ASFV-G-ΔI177L I177L Georgia 2007/1 (II) 10/10 (100 %) (61) в дозе 104 ГАдЕ50 I177L Georgia 2007/1 (II) 5/5 (100 %) (61) ASFV-G-ΔI177L в дозе 106 ГАдЕ50 ASFV-G-ΔI177L (ороназаль- I177L Georgia 2007/1 (II) 5/5 (100 %) (61) ная иммунизация) I177L Georgia 2007/1 (II) 10/10 (100 %) (63) SY18ΔI226R I226R SY18 (II) 10/10 (100 %) (65) Georgia 2007/1 9GL (B119L) и UK (DP96R) Georgia 2007/1 (II) 5/5 (100 %) (50) Pr4Δ9GL 9GL (B119L) Pr4 (XX) 4/4 (100 %) (76) NH/P68DA238L-COS7 в дозе 106 ТЦД50 A238L Arm07 (II) 4/0 (0 %) (59) NH/P68DA224L-COS7 в дозе 106 ТЦД50 A224L Arm07 (II) 4/4 (100 %) (59) NH/P68DEP153R-COS7 в дозе 106 ТЦД50 EP153R Arm07 (II) 4/0 (0 %) (59) NH/P68DA276R-PAM в дозе 102 ТЦД50 A276R Arm07 (II) 5/0 (0 %) (59) ASFV-G-ΔMGF MGF505-1R, Georgia 2007/1 (II) 10/10 (100 %) (55) в дозе 102 ГАдЕ50 MGF360-12L, MGF360-13L, MGF360-14L, MGF505-2R, MGF505-3R ASFV-G-ΔMGF MGF505-1R, Georgia 2007/1 (II) 10/10 (100 %) (55) в дозе 104 ГАдЕ50 MGF360-12L, MGF360-13L, MGF360-14L, MGF505-2R, MGF505-3R Видно (см. табл. 1), что благодаря биоинформатическому анализу генома вируса АЧС выбор генов-мишеней для делетирования с целью получения кандидатной вакцины расширился. При иммунизации свиней экспериментальными вирусами дозы варьировались от 10 до 106 ГАдЕ50. Полу- ченные к настоящему времени результаты свидетельствуют о том, что деле-ции по разным генам (например, I177L, 9GL и I226R) снижают вирулентность вируса и обеспечивают достаточную защиту от повторного заражения. Протективность препарата может различаться в зависимости от генотипа исходного изолята, дозы иммунизации и даже способа введения вируса. Более того, делеция ряда генов может неодинаково влиять на вирулентность вируса и степень защиты от повторного заражения, зависящую исключительно от способа иммунизации. Такие результаты демонстрируют сложность определения наилучшего способа вакцинации при борьбе с АЧС. Инактивированные вакцины. Инактивированными вакцинами называют биологические препараты, в которых под действием какого-либо химического вещества подавлена репликативная активность вируса. Попытки разработать эффективную инактивированную вакцину против АЧС по аналогии с другими инфекционными болезнями животных предпринимались одновременно с созданием аттенуированных вариантов вируса. Однако уже в 1967 году при исследовании иммунного ответа на инактивированную вакцину выяснилось, что у иммунизированных свиней не развивается устойчивость к заражению вирулентным изолятом (66). При иммунизации культуральной вакциной, инактивированной глицеральальдегидом, у некоторых животных отмечали устойчивость к повреждению селезенки, однако защитный иммунитет при заражении вирулентным изолятом вируса АЧС не формировался (67). Несмотря на первые неудачи, исследования продолжались. Так, при использовании инактивированного вируса АЧС, выделенного из экстракта селезенки больных свиней, и N-октилглюкозида в качестве адъюванта иммунизированные свиньи демонстрировали устойчивость к заражению гомологичным изолятом, но были чувствительны к гетерологичному изоляту (68). В 2021 году при инактивации вируса бинарным этиленимином при низких температурах использовались новые и чрезвычайно активные адъюванты (Silicaoil, mGNE и другие). Однако при заражении иммунизированных свиней вирулентным изолятом вируса АЧС протективный иммунитет не развивался (69). В связи с однозначными результатами о нецелесообразности применения такого типа вакцин против АЧС проводятся контрольные эксперименты для доказательства того, что инактивированные вакцины против АЧС не имеют перспектив (70). В итоге следует отметить, все подходы, использованные для разработки инактивированной вакцины, успехом не увенчались. Это, в свою очередь, поднимает ряд важных вопросов в отношении генетической и антигенной изменчивости вируса, требующей детального изучения. Субъединичные вакцины. В конце 1990-х годов внимание ученых было сосредоточено на рекомбинантных белках, полученных в бакуло-вирусной системе экспрессии для иммунизации свиней против АЧС. В эксперименте синтезированный таким способом белок CD2v вводили свиньям в разных дозах. После заражения свиней интактным вирусом наблюдалась временная задержка гемадсорбции и временная дозозависимая задержка развития инфекции, однако защитный иммунитет не формировался (71). При введении рекомбинантных белков vp12, vp30 и vp54 у свиней вырабатывались специфические иммуноглобулины, задерживающие проникновение вируса в хозяйские клетки-мишени, однако высокие титры антител не приводили к устойчивости свиней при заражении вирулентным вирусом АЧС (72, 73). Использование химерного белка vp54/30, который получили при экспресии гена CP204L, интегрированного в сайт рестрикции гена E183L, в бакуловирусной системе, в культуре клеток макрофагов свиней приводило к 50 % нейтрализации вируса АЧС специфическими антителами. Биопроба с иммунизацией химерным белком vp54/30 и последующим заражением изолятом Е75 показала 100 % выживаемость свиней при хроническом течении АЧС (74). При использовании химерных белков CD2v и лектина типа С, кодируемого геном EP153R, была доказана роль специфических антител, защищающих от заражения гомологичным изолятом вируса АЧС (75). В 2004 году проводился опыт на чувствительных животных для сравнения субъединичных вакцин и вакцин на основе генетически модифицированного вируса АЧС, при этом использовали две группы свиней: первую иммунизировали изолятом Pr4Δ9GL с делецией гена B119GL, второй вводили рекомбинантные белки vp30, vp54, vp72 и vp22, экспрессированные в бакуловирусной системе. После заражения исходным изолятом Pr4 (генотип ХХ) у животных из первой группы проявлялись клинические признаки АЧС и виремия с незначительным титром (2,9±0,6 ТЦД50/мл), однако гибели не происходило, тогда как во второй группе виремия развивалась с задержкой на 2 сут, после чего титр вируса составил 9,1±0,3 ТЦД50/мл, и на 8,5±0,5 сут после заражения наступала гибель животных (76). Такие данные поставили под сомнение существование вируснейтрализующих антител, участвующих в формировании иммунной защиты против АЧС. 2. Использованные прототипы субъединичных вакцин против африканской чумы свиней (АЧС) Белки вируса АЧС Система экспрессии Степень защиты Ссылка vp12 Бакуловирус Нет защиты (73) CD2v Бакуловирус Нет защиты (71) vp54 и vp30 Бакуловирус Нет защиты (73) Химерный белок vp54/30 Бакуловирус Выживаемость 100 % (2/2), хроническое течение АЧС (74) vp30, vp54, vp72 и vp22 Бакуловирус Нет защиты (76) CD2v и лектин типа С Бакуловирус Частичная защита (75) Как показывает таблица 2, в качестве субъединичных вакцин-кандидатов для иммунизации свиней могут использоваться различные рекомбинантные белки, но почти при всех комбинациях белков добиться защиты иммунизированных животных от повторного заражения не удается, и все полученные к настоящему времени результаты малоперспективны. Единственным исключением оказался химерный белок vp54/30, иммунизация которым обеспечивала выживание свиней при контрольном заражении вирулентным изолятом, однако при этом АЧС принимала хроническое течение и вирус все еще выделялся из организма свиней. Подобные работы, связанные с использованием субъединичных вакцин в качестве кандидат-ных, пока также нельзя считать перспективными. Во многом это связано с малой изученностью многих антигенов, способных защитить свиней от заражения вирусом АЧС. Рекомбинантные (векторные) вакцины. В связи с развитием генной инженерии и молекулярной биологии стала методически доступна разработка рекомбинантных вакцин, в том числе против АЧС, в которых в качестве вектора могут использоваться плазмиды и гетерологичные вирусы (77-79). Например, при использовании плазмидной конструкции pCMV-UbsHAPQ, кодирующей химерные белки vp54, vp30 и CD2v, соединенные с убиквитином для увеличения возможности экспрессии совместно с молекулами MHC I класса на цитоплазматической мембране клеток-мишеней свиней, как и ожидалось, отмечался высокий уровень цитотоксических CD8+ Т-лимфоцитов с пиком на 3-и сут после иммунизации. После заражения вирулентным изолятом E75 наблюдалась частичная защита свиней от АЧС с летальностью 66,0 % (78). Плазмида BacMam-sHAPQ может применяться в качестве перспективного вектора для экспрессии белков vp54, vp30 и sHA, сочетание которых при иммунизации свиней показало высокий уровень цитокинов у четырех из шести животных (78). При конструировании модифицированных аденовирусов для экспрессии белков вируса АЧС в аденовирусный геном икорпорировали гены A151R, B119L, B602L, EP402RΔPRR, B438L, K205R и A104R изолята Georgia 2007/1. В культуре клеток костного мозга свиней (КМС) такая рекомбинантная конструкция индуцировала высокий уровень γ-интерферона на 7-е сут после инокуляции, но из-за отсутствия испытаний на восприимчивых животных нельзя сделать вывод о наличии иммунной защиты против вирулентных изолятов АЧС (80). Иммунизация диких кабанов 35 антигенами вируса АЧС, экспрессированными с использованием в качестве вектора аденовируса человека 5, не показала иммунной защиты при контрольном заражении вирулентным изолятом Arm 07 (78). Клонирование 47 антигенов вируса АЧС с использованием плазмид и вируса осповакцины в качестве векторов не дало значительных результатов в поиске рекомбинантных вакцин, так как при заражении иммунизированных рекомбинантным вариантом свиней вирулентным изолятом Georgia 2007/1 в дозе 104 ГАдЕ50 у всех животных опытной группы наблюдалось острое течение болезни, несмотря на высокий уровень продукции γ-интерферона цитотоксическими CD8+ Т-лимфоцитами in vitro (6½105 клеток) (81). В аналогичном исследовании вектором служила плазмида Escherichia coli pGEX 4T-1, в которой клонировали гены вируса АЧС в двух вариантах: в одном — кодирующие белки CD2v, vp32, vp72 и vp17, в другом — белки vp15, vp35, vp54 и vp17. Уровень γ-интерферона, синтезированного в сенсибилизированных рекомбинантным вариантом мононуклеарных клетках крови, едва превышал таковой в контрольной пробе, а при заражении иммунизированных свиней вирулентным изолятом Arm 07 вируса АЧС среди животных наблюдалась 100 % летальность (82). Весьма обнадеживающие результаты показало исследование, в котором восемь генов вируса АЧС изолята OURT88/3 (B602L, B646L, E183L, E199L, CP204L, F317L, EP153R, MGF505-5R) инкорпорировали в векторы человеческого аденовируса 5 (rAd) и модифицированного вируса осповак-цины Анкара (MVA). При введении свиньям рекомбинантного вируса rAd в дозе 1,5½1010 МЕ и рекомбинантного вируса MVA в дозе 2½108 БОЕ и повторного заражения вирулентным изолятом АЧС OURT88/1 наблюдалась виремия в течение 6 сут, при этом ни оно животное не погибло (83). Важным этапом в развитии молекулярной биологии стала технология CRISPR/Cas9, которая уже экспериментально показала результаты в экспрессии белка vp30, кодируемого геном CP204L вируса АЧС (84). В системе CRISPR/Cas9 был синтезирован рекомбинантный вариант высоковирулентного изолята ASFV-Kenya-IX-1033 (генотип IX) с делецией гена A238L (85). Этот метод рассматривают как самый перспективным для редактирования генома вирусов, в том числе возбудителя АЧС. Доступная обобщенная информация о генно-инженерных вакцинах против АЧС представлена в таблице 3. Из данных этой таблицы видно, что 620 использование рекомбинации различных генов, экспрессируемых с помощью плазмидных и вирусных векторов, — один из наиболее интересных и перспективных подходов при разработке вакцины против АЧС. Различные гены можно объединять в пулы для достижения наилучшего результата, который варьируется от полного отсутствия защиты до 100 % протекции (см. табл. 3). Успех таких результатов зависит как от комбинации используемых генов, так и от их влияния друг на друга. 3. Данные об использовании рекомбинантных конструкций в качестве перспективных вакцин против африканской чумы свиней Белки вируса АЧС ^ Система экспрессии, вектор 1 Степень защиты ] Ссылка Убиквитин-CD2v, vp54 и vp30 Плазмида pCMV-UbsHAPQ Частичная защита (33 %) (78) vp54, vp30, sHA Плазмида BacMam-sHAPQ Частичная защита (77) 7 антигенов: vpA151R, vpB119L, vpB602L, vpEP402RΔPRR, vpB438L, vpK205R и vpA104R Аденовирус человека 5 Биопроба не проводилась (80) 47 антигенов Плазмида pCMVi-LS и модифициро-Нет защиты ванный вирус осповакцины (81) CD2v, vp32, vp72, vp17 Плазмида Escherichia coli pGEX 4T-1 Нет защиты (82) vp15, vp35, vp54 и vp17 Плазмида Escherichia coli pGEX 4T-1 Нет защиты (82) 35 антигенов Аденовирус человека 5 Нет защиты (79) 8 антигенов: vpB602L, vpB646L, vpE183L, vpE199L, vpCP204L, vpF317L, vpEP153R, vpMGF505-5R Аденовирус человека 5 Полная защита (100 %) (83) 8 антигенов: vpB602L, vpB646L, vpE183L, vpE199L, vpCP204L, vpF317L, vpEP153R, vpMGF505-5R Модифицированный вирус осповак- Полная защита (100 %) цины Анкара (MVA) (83) Таким образом, можно заключить, что, несмотря на значительный накопленный опыт изучения генома вируса АЧС, а также функциональных и иммунологических характеристик вирусных белков, на сегодняшний день в мире не существует ни одной сертифицированной вакцины против АЧС. После относительно длительного затишья новая эпизоотия АЧС в 20072021 годах привела к многомиллиардным убыткам во всех секторах свиноводства и охоты. В КНР потери от АЧС в 2018-2019 году составили 0,78 % ВВП страны (86). В связи с этим вновь остро стал вопрос о необходимости эффективной защиты хозяйств от АЧС не только методами общей профилактики, но и с помощью вакцинации. Для независимой оценки препаратов специфической профилактики АЧС требуются дальнейшие исследования возможных вакцин-кандидатов в товарном свиноводстве разных стран, чтобы избежать хронического течения АЧС, персистирующей в конкретно взятом государстве (87). Для осуществления стратегии по дифференциации инфицированных животных от вакцинированных (DIVA) предлагается применять вакцинный вариант вируса АЧС с индуцированной делецией консервативного гена E184L иммуногенного белка, что позволяет четко определить отсутствие антител к этому белку у вакцинированных особей (88). Итак, разработка эффективных и безопасных вакцин против африканской чумы свиней (АЧС) продолжается почти 90 лет. Широкое применение в середине XX века аттенуированных вакцин против гомологичных изолятов вируса АЧС во многих странах Европы (Испании, Португалии) привело к масштабной циркуляции патогенного вируса в популяции домашних и диких свиней и к росту численности поголовья с хроническим течением болезни. Попытка ослабления вирулентных свойств вируса методом последовательных пассажей на многих первичных и перевиваемых линиях культур клеток с целью создания вакцины не увенчалась успехом. Инактивированные, а также субъединичные вакцины на основе рекомбинантных белков обладали высокой иммуногенностью, однако протективных свойств не имели независимо от того, какие адъюванты и инактиванты использовались. Применение технологии гомологичной рекомбинации для получения клонов вируса АЧС с делецией определенных генов привело к созданию успешных вакцин-кандидатов. Человеческий аденовирус 5 (rAd) и модифицированный вирус осповакцины Анкара (MVA) зарекомендовали себя в качестве векторных конструкций для переноса ДНК вируса АЧС при разработке эффективных рекомбинантных (векторных) вакцин. Самыми перспективными, с нашей точки зрения, представляются экспериментальные вакцины на основе генетически модифицированных вирусов ASFV-G-ΔI177L и SY18ΔI226R, демонстрирующие 100 % защиты свиней, как минимум, от гомологичных изолятов генотипа II вируса АЧС. Подобные ожидания порождает и кандидатная вакцина на основе HLJ/18-7GD.


Список литературы Современные подходы к специфической профилактике африканской чумы свиней (обзор)
- Urbano A.C., Forth J.H., Olesen A., Dixon L., Rasmussen T., Cackett G., Werner F., Karger A., Andrés G., Wang X., Pérez-Núcez D., Galindo I., Malogolovkin A., Revilla Y., Alonso C., Gallardo C., Blome S., Arabyan E., Zakaryan H., Ferreira F. African swine fever virus: cellular and molecular aspects. In: Understanding and combatting African swine fever. A European perspective /L. Iacolina, M.-L. Penrith, S. Bellini, E. Chenais, F. Jori, M. Montoya, K. Stehl, D. Gavier-Widén (eds.). Wageningen Academic Publishers, 2021: 25-61 (doi: 10.3920/978-90-8686-910-7_2).
- Dixon L.K., Chapman D.A.G., Netherton C.L., Upton C. African swine fever virus replication and genomics. Virus Research, 2013, 173(1): 3-14 (doi: 10.1016/j.virusres.2012.10.020).
- Балышев В.М., Болгова М.В., Балышева В.И., Болгова М.В., Князева М.В. Получение типовых задерживающих гемадсорбцию референс-сывороток к вирусу африканской чумы свиней. Вопросы нормативно-правового регулирования в ветеринарии, 2015, 2: 23-25.
- Quembo C.J., Jori F., Vosloo W., Heath L. Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transboundary and Emerging Diseases, 2018, 665(2): 420-431 (doi: 10.1111/tbed.12700).
- Nix R.J., Gallardo C., Hutchings G., Blanco E., Dixon L.K. Molecular epidemiology of African swine fever virus studied by analysis of four variable genome regions. Archives of Virology, 2006, 151(12): 2475-2494 (doi: 10.1007/s00705-006-0794-z).
- Mazloum A., Igolkin A.S., Vlasova N.N. African swine fever virus: use genetic markers in analysis of its routes of spread. Veterinary Science Today, 2019, 30: 9-14 (doi: 10.29326/2304-196X-2019-3-30-3-8).
- Beltrán-Alcrudo D., Lubroth J., Depner K., Rocque S. African swine fever in the Caucasus. EMPRES Watch, 2008, 1: 1-8 (doi: 10.13140/RG.2.1.3579.1200).
- Россельхознадзор. Федеральная служба по ветеринарному и фитосанитарному надзору. Эпизоотическая ситуация по АЧС на территории Российской Федерации в 2007 г. Режим доступа: https://fsvps.gov.ru/fsvps-docs/ru/iac/asf/archive/asf_2007.pdf. Дата обращения: 16.01.2022.
- Россельхознадзор. Федеральная служба по ветеринарному и фитосанитарному надзору. Эпизоотическая ситуация по АЧС на территории Российской Федерации и стран Восточной Европы. Данные МЭБ с 2007 по 2020 гг. Режим доступа: https://fsvps.gov.ru/fsvps-docs/ru/iac/asf/2020/09-14/05.pdf. Дата обращения: 16.01.2022.
- Россельхознадзор. Федеральная служба по ветеринарному и фитосанитарному надзору. Эпизоотическая ситуация по АЧС на территории Российской Федерации и странах Европы и Азии. Данные МЭБ с 2007 по 2021 гг. Режим доступа: https://fsvps.gov.ru/fsvps-docs/ru/iac/asf/2022/01- 18/04.pdf. Дата обращения: 18.01.2022.
- OIE — World Organization for Animal Health. Analytics: Quantitative data. Режим доступа: https://wahis.oie.int/#/dashboards/qd-dashboard. Дата обращения: 18.12.2021.
- OIE — World Organization for Animal Health. African swine fever (ASF) — Situation report. Report Date: 03.12.2021. Режим доступа: https://www.oie.int/app/uploads/2021/12/report-65-current-situation-of-asf.pdf. Дата обращения: 18.12.2021.
- Beltrán-Alcrudo D., Arias M., Gallardo C., Kramer S., Penrith M.L. African swine fever: detection and diagnosis — а manual for veterinarians. FAO, 2017.
- Wilkinson P.J., Pegram R.G., Perry B.D., Lemche J., Schels H.F. The distribution of African swine fever virus isolated from Ornithodoros moubata in Zambia. Epidemiology and Infection, 1988, 101(3): 547-564 (doi: 10.1017/s0950268800029423).
- Макаров В.В., Сухарев О.И., Цветнова И.В. Эпизоотологическая характеристика вируса африканской чумы свиней. Ветеринарная практика, 2013, 1: 6-16.
- Макаров В.В. Африканская чума свиней. М., 2011.
- Escribano J.M., Galindo I., Alonso C. Antibody-mediated neutralization of African swine fever virus: myths and facts. Virus Research, 2013, 173(1): 101-109 (doi: 10.1016/j.virusres.2012.10.012).
- Gaudreault N., Richt J. Subunit vaccine approaches for African swine fever virus. Vaccines, 2019, 7(2): 56 (doi: 10.3390/vaccines7020056).
- Halstead S.B., Mahalingam S., Marovich M.A., Ubol S., Mosser D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. The Lancet Infectious Diseases, 2010, 10(10): 712-722 (doi: 10.1016/S1473-3099(10)70166-3).
- Suhrbier A., La Linn M. Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection. Trends in Immunology, 2003, 24(4): 165-168 (doi: 10.1016/s1471-4906(03)00065-6).
- Netherton C.L., Goatley L.C., Reis A.L., Portugal R., Nash R.H., Morgan S.B., Gault L., Nieto R., Norlin V., Gallardo C., Ho C.S., Sánchez-Cordón P.J., Taylor G., Dixon L.K. Identification and immunogenicity of african swine fever virus antigens. Front. Immunol., 2019, 10: 1318 (doi: 10.3389/fimmu.2019.01318).
- Mucoz-Pérez C., Jurado C., Sánchez-Vizcaíno J. African swine fever vaccine: turning a dream into reality. Transboundary and Emerging Diseases, 2021. 68(5): 2657-2668 (doi: 10.1111/tbed.14191).
- Mendonza C., Videgain S.P., Alonso F. Inhibition of natural killer activity in porcine mononuclear cells by African swine fever virus. Research in Veterinary Science, 1991, 51(3): 317-321 (doi: 10.1016/0034-5288(91)90084-2).
- Martins C., Lawman M., Schol T., Mebus C., Lunney J. African swine fever virus specific porcine cytotoxic T cell activity. Archives of Virology, 1993, 129(1-4): 211-225 (doi: 10.1007/BF01316896).
- Ribeiro A.S., Arala-Chaves M.P., Vilanova M., Porto M.T., Coutinho A. Role of B and T lymphocytes in the specific immunosuppression induced by a protein released by porcine monocytes infected with African swine fever virus. International Immunology, 1991, 3(2): 165-74 (doi: 10.1093/intimm/3.2.165).
- Vilanova M., Ferreira P., Ribeiro A., Arala-Chaves M. The biological effects induced in mice by p36, a proteinaceous factor of virulence produced by African swine fever virus, are mediated by interleukin-4 and also to a lesser extent by interleukin-10. Immunology, 1999, 96(3): 389-395 (doi: 10.1046/j.1365-2567.1999.00629.x).
- Pershin A., Shevchenko I., Igolkin A., Zhukov I., Mazloum A., Aronova E., Vlasova N., Shev-tsov A. A long-term study of the biological properties of ASF virus isolates originating from various regions of the Russian Federation in 2013-2018. Veterinary Sciences, 2019, 6(4): 99 (doi: 10.3390/vetsci6040099).
- Zsak L., Onisk D.V., Afonso C.L., Rock D.L. Virulent African swine fever virus isolates are neutralized by swine immune serum and by monoclonal antibodies recognizing a 72-kDa viral protein. Virology, 1993, 196(2): 596-602 (doi: 10.1006/viro.1993.1515).
- Onisk D.V., Borca M.V., Kutish G., Kramer E., Irusta P., Rock D.L. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology, 1994, 198(1): 350-354 (doi: 10.1006/viro.1994.1040).
- Gómez-Puertas P., Rodríguez F., Oviedo J.M., Ramiro-Ibáñez F., Ruiz-Gonzalvo F., Alonso C., Escribano J.M. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. Journal of Virology, 1996, 70(8): 5689-5694 (doi: 10.1128/JVI.70.8.5689-5694.1996).
- Alonso C., Miskin J., Hernáez B., Fernandez-Zapatero P., Soto L., Cantó C., Rodríguez-Crespo I., Dixon L., Escribano J.M. African swine fever virus protein p54 interacts with the micro-tubular motor complex through direct binding to light-chain dynein. Journal of Virology, 2001, 75(20): 9819-9827 (doi: 10.1128/JVI.75.20.9819-9827.2001).
- Hernáez B., Tarragó T., Giralt E., Escribano J.M., Alonso C. Small peptide inhibitors disrupt a high-affinity interaction between cytoplasmic dynein and a viral cargo protein. Journal of Virology, 2010, 84(20): 10792-10801 (doi: 10.1128/JVI.01168-10).
- Walker J. East African swine fever. University of Zürich, 1933.
- Stone S.S., DeLay P.D., Sharman E.C. The antibody response in pigs inoculated with attenuated African swine fever virus. Can. J. Comp. Med., 1968, 32(3): 455-460.
- Hamdy F.M., Dardiri A.H. Clinical and immunologic responses of pigs to African swine fever virus isolated from the Western Hemisphere. American Journal of Veterinary Research, 1984, 45(4): 711-804.
- Boinas F., Hutchings G., Dixon L., Wilkinson P. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol., 2004, 85: 2177-2187 (doi: 10.1099/vir.0.80058-0).
- Leitäo A., Cartaxeiro C., Coelho R., Cruz B., Parkhouse R., Portugal F.C., Vigário J.D., Martins C.L.V. The non-haemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti-virus immune response. J. Gen. Virol., 2001, 82(3): 513523 (doi: 10.1099/0022-1317-82-3-513).
- Vigário J.D., Terrinha A.M., Moura Nunes J.F. Antigenic relationships among strains of African swine fever virus. Archiv für die gesamte Virusforschung, 1974, 45(3): 272-277 (doi: 10.1007/BF01249690).
- King K., Chapman D., Argilaguet J.M., Fishbourne E., Hutet E., Cariolet R., Hutchings G., Oura C.A., Netherton C.L., Moffat K., Taylor G., Le Potier M.F., Dixon L.K., Takamatsu H.H. Protection of European domestic pigs from virulent African isolates of African swine fever virus by experimental immunisation. Vaccine, 2011, 29(28): 4593-4600 (doi: 10.1016/j.vaccine.2011.04.052).
- Gallardo C., Soler A., Rodze I., Nieto R., Cano-Gómez C., Fernandez-Pinero J., Arias M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transboundary and Emerging Diseases, 2019, 66(3): 1399-1404 (doi: 10.1111/tbed.13132).
- Barasona J.A., Gallardo C., Cadenas-Fernández E., Jurado C., Rivera B., Rodríguez-Bertos A., Arias M., Sánchez-Vizcaíno J.M. First oral vaccination of Eurasian wild boar against African swine fever virus genotype II. Frontiers in Veterinary Science, 2019, 6: 137 (doi: 10.3389/fvets.2019.00137).
- Barasona J.A., Cadenas-Fernández E., Kosowska A., Barroso-Arévalo S., Rivera B., Sánchez R., Porras N., Gallardo C., Sánchez-Vizcaíno J.M. Safety of African swine fever vaccine candidate Lv17/WB/Rie1 in wild boar: overdose and repeated doses. Frontiers in Immunology, 2021, 12: 761753 (doi: 10.3389/fimmu.2021.761753).
- Enjuanes L., Carrascosa A.L., Moreno M.A., Vicuela E. Titration of African swine fever (ASF) virus. J. Gen. Virol, 1976, 32(3): 471-477 (doi: 10.1099/0022-1317-32-3-471).
- Titov I., Burmakina G., Morgunov Y., Morgunov S., Koltsov A., Malogolovkin A., Kolbasov D. Virulent strain of African swine fever virus eclipses its attenuated derivative after challenge. Archives of Virology, 2017, 162(10): 3081-3088 (doi: 10.1007/s00705-017-3471-5).
- Elsukova A.A., Shevchenko I.V., Varentsova A., Puzankova O.S., Zhukov I.Y., Pershin A.S., Remyga S.G., Zinyakov N.G., Mazloum A., Vlasov I.N., Igolkin A.S., Lozovoy D.A., Gruzdev K.N., Vlasova N.N. Biological properties of African swine fever virus Odintsovo 02/14 isolate and its genome analysis. International Journal of Environmental and Agriculture Research, 2017, 3: 26-37 (doi: 10.25125/agriculture-journal-IJ0EAR-0CT-2017-15).
- Mazloum A., Zinyakov N.G., Pershin A.S., Shevchenko I.V., Zhukov I.Y., Fedoseyeva D.N., Sharypova D.V., Igolkin A.S., Vlasova N.N. Analysis of changes in African swine fever virus genetic structure and biological properties during adaptation to continuous cell culture. Veterinary Science Today, 2018, 27: 21-25 (doi: 10.29326/2304-196X-2018-4-27-21-25).
- Dixon L.K., Chapman D.A., Netherton C.L., Upton C. African swine fever virus replication and genomics. Virus Research, 2013, 173(1): 3-14 (doi: 10.1016/j.virusres.2012.10.020).
- Mazloum A., Igolkin A.S., Zinyakov N.G., Van Schalkwyk A., Vlasova N.N. Changes in the genome of African swine fever virus (Asfarviridae: Asfivirus: African swine fever virus) associated with adaptation to reproduction in continuous cell culture. Вопросы вирусологии, 2021, 66(3): 211-216 (doi: 10.36233/0507-4088-50).
- Krug P.W., Holinka L.G., O'Donnell V., Reese B., Sanford B., Fernandez-Sainz I., Gladue D.P., Arzt J., Rodriguez L., Risatti G.R., Borca M.V. The progressive adaptation of a Georgian isolate of African swine fever virus to Vero cells leads to a gradual attenuation of virulence in swine corresponding to major modifications of the viral genome. Journal of Virology, 2015, 89(4): 23242332 (doi: 10.1128/JVI.03250-14).
- O'Donnell V., Risatti G.R., Holinka L.G., Krug P.W., Carlson J., Velazquez-Salinas L., Azzi-naro P.A., Gladue D.P., Borca M.V. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. Journal of Virology, 2016, 91(1): e01760-16 (doi: 10.1128/JVI.01760-16).
- Moore D.M., Zsak L., Neilan J.G., Lu Z., Rock D.L. The African swine fever virus thymidine kinase gene is required for efficient replication in swine macrophages and for virulence in swine. Journal of Virology, 1998, 72(12): 10310-10315 (doi: 10.1128/JVI.72.12.10310-10315.1998).
- Sanford B., Holinka L., O'Donnell V., Krug P., Carlson J., Alfano M., Carrillo C., Wu P., Lowe A., Risatti G., Gladue D., Borca M. Deletion of the thymidine kinase gene induces complete attenuation of the Georgia isolate of African swine fever virus. Virus Research, 2016, 213: 165-171 (doi: 10.1016/j.virusres.2015.12.002).
- O'Donnell V., Holinka L.G., Gladue D.P., Sanford B., Krug P.W., Lu X., Arzt J., Reese B., Carrillo C., Risatti G.R., Borca M.V. African swine fever virus Georgia isolate harboring deletions of MGF360 and MGF505 genes is attenuated in swine and confers protection against challenge with virulent parental virus. Journal of Virology, 2015, 89(11): 6048-6056 (doi: 10.1128/JVI.00554-15).
- Lewis T., Zsak L., Burrage T.G., Lu Z., Kutish G.F., Neilan J.G., Rock D.L. An African swine fever virus ERV1-ALR homologue, 9GL, affects virion maturation and viral growth in macrophages and viral virulence in swine. Journal of Virology, 2000, 74(3): 1275-1285 (doi: 10.1128/jvi.74.3.1275-1285.2000).
- Wu L., Yang B., Yuan X., Hong J., Peng M., Chen J.L., Song Z. Regulation and evasion of host immune response by African swine fever virus. Frontiers in Microbiology, 2021, 12: 698001 (doi: 10.3389/fmicb.2021.698001).
- Monteagudo P.L., Lacasta A., López E., Bosch L., Collado J., Pina-Pedrero S., Correa-Fiz F., Accensi F., Navas M.J., Vidal E., Bustos M.J., Rodríguez J.M., Gallei A., Nikolin V., Salas M.L., Rodríguez F. BA71ACD2: a new recombinant live attenuated African swine fever virus with cross-protective capabilities. Journal of Virology, 2017, 91(21): e01058-17 (doi: 10.1128/JVI.01058-17).
- Lopez E., van Heerden J., Bosch-Camys L., Accensi F., Navas M.J., López-Monteagudo P., Argilaguet J., Gallardo C., Pina-Pedrero S., Salas M.L., Salt J., Rodriguez F. Live attenuated African swine fever viruses as ideal tools to dissect the mechanisms involved in cross-protection. Viruses, 2020, 12(12): 1474 (doi: 10.3390/v12121474).
- Reis A.L., Goatley L.C., Jabbar T., Sanchez-Cordon P.J., Netherton C.L., Chapman D.A.G., Dixon L.K. Deletion of the African swine fever virus gene DP148R does not reduce virus replication in culture but reduces virus virulence in pigs and induces high levels of protection against challenge. Journal of Virology, 2017, 91(24): e01428-17 (doi: 10.1128/JVI.01428-17).
- Gallardo C., Sánchez E.G., Pérez-Núcez D., Nogal M., de León P., Carrascosa Á.L., Nieto R., Soler A., Arias M.L., Revilla Y. African swine fever virus (ASFV) protection mediated by NH/P68 and NH/P68 recombinant live-attenuated viruses. Vaccine, 2018, 36(19): 2694-2704 (doi: 10.1016/j.vaccine.2018.03.040).
- Chen W., Zhao D., He X., Liu R., Wang Z., Zhang X., Li F., Shan D., Chen H., Zhang J., Wang L., Wen Z., Wang X., Guan Y., Liu J., Bu Z. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci,, 2020, 63(5): 623-634 (doi: 10.1007/s11427-020-1657-9).
- Borca M.V., Ramirez-Medina E., Silva E., Vuono E., Rai A., Pruitt S., Holinka L.G., Velazquez-Salinas L., Zhu J., Gladue D.P. Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. Journal of Virology, 2020, 94: e02017-19 (doi: 10.1128/JVI.02017-19).
- Borca M.V., Rai A., Ramirez-Medina E., Silva E., Velazquez-Salinas L., Vuono E., Pruitt S., Espinoza N., Gladue D.P. A cell culture-adapted vaccine virus against the current African swine fever virus pandemic strain. Journal of Virology, 2021, 95(14): e0012321 (doi: 10.1128/JVI.00123-21).
- Borca M.V., Ramirez-Medina E., Silva E., Vuono E., Rai A., Pruitt S., Espinoza N., Velazquez-Salinas L., Gay C.G., Gladue D.P. ASFV-G-AI177L as an effective oral nasal vaccine against the Eurasia strain of Africa swine fever. Viruses, 2021, 13(5): 765 (doi: 10.3390/v13050765).
- Tran X.H., Le T.T.P., Nguyen Q.H., Do T.T., Nguyen V.D., Gay C.G., Borca M.V., Gladue D.P. African swine fever virus vaccine candidate ASFV-G-AI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transboundary and Emerging Diseases, 2021, 69: e497-e504 (doi: 10.1111/tbed.14329).
- Zhang Y., Ke J., Zhang J., Yang J., Yue H., Zhou X., Qi Y., Zhu R., Miao F., Li Q., Zhang F., Wang Y., Han X., Mi L., Yang J., Zhang S., Chen T., Hu R. African swine fever virus bearing an I226R gene deletion elicits robust immunity in pigs to African swine fever. Journal of Virology, 2021, 95(23): e0119921 (doi: 10.1128/JVI.01199-21).
- Stone S.S., Hess W.R. Antibody response to inactivated preparations of African swine fever virus in pigs. American Journal of Veterinary Research, 1967, 28(123): 475-481.
- Forman A.J., Wardley R.C., Wilkinson P.J. The immunological response of pigs and Guinea pigs to antigens of African swine fever virus. Archives of Virology, 1982, 74(2-3): 91-100 (doi: 10.1007/BF01314703).
- Bommeli W., Kihm U., Ehrensperger F. Preliminary study on immunization of pigs against African swine fever In: Proceedings of a CEC/FAO Research Seminar, Sassari, Sardinia, Italy, 23-25 September 1981. Sassari, 1981: 217-223.
- Cadenas-Fernández E., Sánchez-Vizcaíno J.M., van den Born E., Kosowska A., van Kilsdonk E., Fernández-Pacheco P., Gallardo C., Arias M., Barasona J.A. High doses of inactivated African Swine fever virus are safe, but do not confer protection against a virulent challenge. Vaccines, 2021, 9(3): 242 (doi: 10.3390/vaccines9030242).
- Blome S., Gabriel C., Beer M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine, 2014, 32(31): 3879-3882 (doi: 10.1016/j.vaccine.2014.05.051).
- Ruiz-Gonzalvo F., Rodríguez F., Escribano J.M. Functional and immunological properties of the baculovirus-expressed hemagglutinin of African swine fever virus. Virology, 1996, 218(1): 285-289 (doi: 10.1006/viro.1996.0193).
- Carrascosa A.L., Sastre I., Vicuela E. Production and purification of recombinant African swine fever virus attachment protein p12. Journal of Biotechnology, 1995, 40(2): 73-86 (doi: 10.1016/0168-1656(95)00035-o).
- Gómez-Puertas P., Rodríguez F., Oviedo J.M., Brun A., Alonso C., Escribano J.M. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology, 1998, 243(2): 461-471 (doi: 10.1006/viro.1998.9068).
- Barderas M.G., Rodríguez F., Gómez-Puertas P., Avilés M., Beitia F., Alonso C., Escribano J.M. Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Archives of Virology, 2001, 146(9): 1681-1691 (doi: 10.1007/s007050170056).
- Burmakina G., Malogolovkin A., Tulman E.R., Zsak L., Delhon G., Diel D.G., Shobogo-rov N.M., Morgunov Y.P., Morgunov S.Y., Kutish G.F., Kolbasov D., Rock D.L. African swine fever virus serotype-specific proteins are significant protective antigens for African swine fever. J. Gen. Virol., 2016, 97(7): 1670-1675 (doi: 10.1099/jgv.0.000490).
- Neilan J.G., Zsak L., Lu Z., Burrage T.G., Kutish G.F., Rock D.L. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology, 2004, 319(2): 337-342 (doi: 10.1016/j.virol.2003.11.011).
- Argilaguet J.M., Pérez-Martín E., López S., Goethe M., Escribano J.M., Giesow K., Keil G.M., Rodríguez F. BacMam immunization partially protects pigs against sublethal challenge with African swine fever virus. Antiviral Res., 2013, 98(1): 61-65 (doi: 10.1016/j.antiviral.2013.02.005).
- Argilaguet J.M., Pérez-Martín E., Nofrarías M., Gallardo C., Accensi F., Lacasta A., Mora M., Ballester M., Galindo-Cardiel I., López-Soria S., Escribano J.M., Reche P.A., Rodríguez F. DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PLoS ONE, 2012, 7(9): e40942 (doi: 10.1371/journal.pone.0040942).
- Cadenas-Fernández E., Sánchez-Vizcaíno J.M., Kosowska A., Rivera B., Mayoral-Alegre F., Rodríguez-Bertos A., Yao J., Bray J., Lokhandwala S., Mwangi W., Barasona J.A. Adenovirus-vectored African swine fever virus antigens cocktail is not protective against virulent arm07 isolate in Eurasian wild boar. Pathogens, 2020, 9(3): 171 (doi: 10.3390/pathogens9030171).
- Lokhandwala S., Waghela S.D., Bray J., Sangewar N., Charendoff C., Martin C.L., Hassan W.S., Koynarski T., Gabbert L., Burrage T.G., Brake D., Neilan J., Mwangi W. Adenovirus-vectored novel African swine fever virus antigens elicit robust immune responses in swine. PLoS ONE, 2017, 12(5): e0177007 (doi: 10.1371/journal.pone.0177007).
- Jancovich J.K., Chapman D., Hansen D.T., Robida M.D., Loskutov A., Craciunescu F., Bo-rovkov A., Kibler K., Goatley L., King K., Netherton C.L., Taylor G., Jacobs B., Sykes K., Dixon L.K. Immunization of pigs by DNA Prime and recombinant vaccinia virus boost to identify and rank African swine fever virus immunogenic and protective proteins. Journal of Virology, 2018, 92(8): e02219-17 (doi: 10.1128/JVI.02219-17).
- Sunwoo S.Y., Pérez-Núñez D., Morozov I., Sánchez E.G., Gaudreault N.N., Trujillo J.D., Mur L., Nogal M., Madden D., Urbaniak K., Kim I.J., Ma W., Revilla Y., Richt J.A. DNAprotein vaccination strategy does not protect from challenge with African swine fever virus Armenia 2007 strain. Vaccines, 2019, 7(1): 12 (doi: 10.3390/vaccines7010012).
- Goatley L.C., Reis A.L., Portugal R., Goldswain H., Shimmon G.L., Hargreaves Z., Ho C.S., Montoya M., Sánchez-Cordón P.J., Taylor G., Dixon L.K., Netherton C.L. A pool of eight virally vectored African swine fever antigens protect pigs against fatal disease. Vaccines, 2020, 8(2): 234 (doi: 10.3390/vaccines8020234).
- Hübner A., Petersen B., Keil G.M., Niemann H., Mettenleiter T.C., Fuchs W. Efficient inhibition of African swine fever virus replication by CRISPR/Cas9 targeting of the viral p30 gene (CP204L). Sci. Rep., 2018, 8(1): 1449 (doi: 10.1038/s41598-018-19626-1).
- Abkallo H.M., Svitek N., Oduor B., Awino E., Henson S.P., Oyola S.O., Mwalimu S., Assad-Garcia N., Fuchs W., Vashee S., Steinaa L. Rapid CRISPR/Cas9 editing of genotype IX African swine fever virus circulating in Eastern and Central Africa. Frontiers in Genetics, 2021, 12: 733674 (doi: 10.3389/fgene.2021.733674).
- You S., Liu T., Zhang M., Zhao X., Dong Y., Wu B., Wang Y., Li J., Wei X., Shi B. African swine fever outbreaks in China led to gross domestic product and economic losses. Nature Food, 2021, 2: 802-808 (doi: 10.1038/s43016-021-00362-1).
- Manso Ribeiro J., Nines Petisca J.L., Lopes Frizao F., Sobral M. Vaccination contre la peste porcine africaine. Bulletin de l'Office International des Epizooties, 1963, 80: 921-937.
- Ramirez-Medina E., Vuono E., Rai A., Pruitt S., Espinoza N., Velazquez-Salinas L., Pina-Pedrero S., Zhu J., Rodriguez F., Borca M.V., Gladue D.P. Deletion of E184L, a putative DIVA target from the pandemic strain of African Swine fever virus, produces a reduction in virulence and protection against virulent challenge. Journal of Virology, 2022, 96(1): e0141921 (doi: 10.1128/JVI.01419-21).


