Современные представления об эпидемиологии, клинико-патогенезу, иммунопатологии, дополнительных факторах поддержания воспаления, диагностике, лечению COVID-19 в условиях высокогорья (обзор литературы)
Автор: Алымкулов А.Т., Узаков О.Ж., Атыканов А.О.
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Медицинские науки
Статья в выпуске: 2 т.10, 2024 года.
Бесплатный доступ
Выполнен анализ данных по актуальному вопросу - пандемии COVID-19. Интенсивный показатель по инфицированности населения составил 285,5 человек на 10000 населения КР. Доля смертности населения за весь период пандемии в КР составила 0,42 на 1000 человек. Таким образом, аспект влияния климатического региона на исследуемые показатели, является актуальной ввиду высокой вероятности появления новых типов коронавирусов человека.
Ковид-19, климат, высокогорье, среднегорье
Короткий адрес: https://sciup.org/14129854
IDR: 14129854 | УДК: 616-035.57.084.1 | DOI: 10.33619/2414-2948/99/31
Текст обзорной статьи Современные представления об эпидемиологии, клинико-патогенезу, иммунопатологии, дополнительных факторах поддержания воспаления, диагностике, лечению COVID-19 в условиях высокогорья (обзор литературы)
Бюллетень науки и практики / Bulletin of Science and Practice
УДК 616-035.57.084.1
Коронавирусная болезнь 2019 (COVID-19) — острое инфекционное заболевание обладающая высокой контагиозностью среди человеческой популяции , .
С момента первого документального подтверждения COVID-19 в Ухане (декабрь 2019
года), до момента приобретения статуса пандемии (30 января 2020 года) не прошло и двух полных месяца [1–6].
За кратчайший срок COVID-19 приобрела титул «самой глобальной проблемой в области здравоохранения со времен Второй мировой войны» [4].
Таксонометрии можно выделить что Sars-CoV-2 относится к роду Betacoronaviris, семейству Orthocoronavirinae, отряду Coronaviridae Nidoviriles [11-14].
В настоящее время известны четыре основных типа коронавирусов: альфа (α), бета (β), гамма (γ) и дельта (δ). Начиная от впервые выявленных коронавирусов, способных поражать человека, наиболее опасными для человека считаются α- и β-коронавирусы, ввиду идентифицированных случаев патологий вызванных серотипами 229E, NL63 (α) и OC43, NKU1 (β) [11].
Sars-CoV-2 как и его предшественники, стимулировал коэволюцию коронавирусов, став короновирусом человека (HCoV) [15].
За период пандемии COVID-19 было заражено более 648 млн. человек, в том числе зарегистрированных 6.6 млн. смертей .
Уровень смертности варьировал от 0 до 20% в зависимости от страны [33].
На территории Кыргызской Республики, общее число зараженных составило 200993 случаев, при населении более 6 млн. человек. Умерли 2991 человек, согласно официальной статистике уровень летальности составил 1.48 .
Ввиду разнородности климатических регионов оценка локальных показателей в областях КР наиболее целесообразной в данной диссертационной работе. Таким образом, Бишкек, как самый густонаселенный регион, выявил у себя, 95281 зараженного, 1670 смертей с коэффициентом смертности 1.75, тогда как в менее населенном регионе, Нарынской области (высокогорье), выявленно 4231 случаев и смертельным исходом у 87 человек, коэффициент смертности 2.05. Регион среднегорья (Иссык-Кульская область) — 14183 случая, умерло 219 человек, коэффициент летальности 1.5. В целях ранжирования — южный регион: г.Ош — 9882 инфицированных, умерло 86, коэффициент летальности 0.87 .
Цель данной работы: изучить современные данные по клинико-патогенетическим, иммунологическим, терапевтическим особенностям COVID-19 в зависимости от климатического региона.
Основными базами данных, использованными для поиска важной медицинской литературы, представленной в этом обзоре, были PubMed, ScienceDirect и Google Scholar. Ключевые слова, используемые как по отдельности, так и в сочетании, включали: «передача», COVID-19, «коронавирус», SARS-CoV-2, «регион», «высокогорье», «климат», «прогностический», «прогностический» и «маркеры». Были включены только статьи на английском языке. Найдено 33 500 статей по ключевым словам, из которых были отсеяны статьи не связанные с тематикой. Итого, было отобрано 220 статей из журналов с высоким коэффициентом доверия и цитируемости.
Общая характеристика Sars-CoV-2 и видов коронавирусов преодолевших видовой барьер.
Коронавирусы — большое семейство вирусов, которые распространились среди людей и др. видов животных. Ранее было отмечено только зоонозные пути передачи с последующей мутаций коронавирусов что привело к первой эпидемии вызванной Sars-CoV и последующей Mers-CoV [5–7]. Таким образом, до настоящего времени считалось что человек может заразиться Sars-CoV-2 только при прямом контакте с промежуточным хозяином [3].
После преодоления видового барьера Sars-CoV-2 приобрел число репродукции (R 0 – 2.2) с постоянным ее ростом в корреляции с выявленными случаями во всем мире [8-10].
Плеоморфная вирусная частица содержит одноцепочечную РНК (ssRNA+) в качестве своего генома [15, 16].
Структурные белковые образования на поверхности мембраны состоят из основных 4 типов: шипов (S), оболочки (E), мембраны (М) и нуклеокапсида (N) [16, 17].
Наиболее значимую роль в вопросах проникновения в клетку человека имеет белок S [18], белок N — в репликации [19].
Между S и N белками расположен интегральный гликопротеин называемый матриксный белок, который контактируя с N-белком координирует функционал жизненного цикла Sars-CoV-2 [19, 20].
Относительно внедрения в клетку, главным входным рецептором, является ACE2, под действием протеазы хозяина S – белок претерпевает ряд изменений что позволяет Sars-CoV-2 в 10–20 раз превосходить Sars-CoV в способности к проникновению в клетку [21-23].
Ряд авторов отмечают роль TMPRSS-2, TMPRSS-4 и HAT в проникновении, но все же ведущую роль играет рецептор ACE2 [24, 25].
Таким образом в настоящее время известно что Sars-CoV-2 использует рецептор ACE2 дыхательных путей как основной путь проникновения [26], но также возможно проникновение через экспрессию вышесказанного рецептора на энтотелиальных клетках тонкого кишечника [27].
В момент проникновения вируса в организм человека стартует каскадный процесс по внедрению вируса в клетку альвеоцитов, презентующих на своей поверхности рецептор ACE2 [18], так же отмечено что Sars-CoV-2 и предшественники, могут использовать аналогичный рецептор на мембранах энтероцитов вызывая клиническую симптоматику острого энтерита. S-пептид Sars-CoV-2 контактирует с ACE2 инициируя процесс выделения протеаз со стороны клетки, таким образом контактная часть рецептора и вируса под воздействием протеазы активирует процесс эндоцитоза, поглощая вирусную единицу с последующей ее транспортировкой в клетку [28–30].
Проникнув в цитоплазму, РНК Sars-CoV-2 внедряется в структуру рибосом активируя процесс формирования структурных компонентов вирусного матрикса. РНК с формированием РНК-полимеразы на ее старт конце, внедряется в структуру ядра и последующим созданием матричной РНК вирусного агента, которая в зарубежной литературе обозначается как Small-RNK. Small-RNK возвращаясь в пространство цитоплазмы, проникает в систему гранулярной эндоплазматической сети, где составные компоненты вирусной капсулы синтезируются в больших масштабах. Элементы капсулы и РНК вирусного агента собираются в цельный конгломерат внутри вакуоль аппарата Гольджи. После чего вырываются в межклеточное пространство посредством экзоцитоза или разрыва цитоплазматической мембраны клетки [31, 32].
Эпидемиология COVID-19 в мире и Кыргызской Республике
Интенсивный показатель заболеваемости COVID-19 в КР, составил 28.71 на 1000 населения, в г. Бишкеке — 86,73, в г. Нарын — 14,38, на Иссык-Куле — 28,04 и в г. Ош — 29,6. Любопытно заметить, что Нарынская область при самом высоком коэффициенте летальности, имеет наименьший относительно указанных регионов интенсивный показатель по заболеваемости COVID-19. Эпидемиологические показатели по некоторым странам, приведено в Таблице.
Таблица
ЭПИДЕМИОЛОГИЧЕСКИЕ ПОКАЗАТЕЛИ COVID-19
В СТРАНАХ БЛИЖНЕГО И ДАЛЬНЕГО ЗАРУБЕЖЬЯ (01 декабря 2022 года)
Страны и территории о и о 50 Й о б л S о о ж -0 Е* * S S § * США 100 743 392 98 191 251 1 106 378 0,011 301 Индия 44 673 984 44 137 617 530 624 1,188 32 Франция 37 916 052 36 935 415 159 026 0,419 578 Германия 36 530 020 35 853 000 158 109 0,433 435 Бразилия 35 336 482 34 257 388 689 998 1,953 164 Республика Корея 27 208 800 26 187 426 30 621 0,113 530 Япония 24 911 367 20 713 863 49 826 0,2 198 Италия 24 260 660 23 587 105 181 098 0,746 402 Великобритания 24 000 101 23 744 855 196 821 0,82 350 Россия 21 597 613 21 003 575 39 206 0,182 148 Турция 17 005 537 16 904 137 101 400 0,596 198
Из Таблицы 1, мы видим, что общий коэффициент летальности варьирует от 0,11 до 1,9, и КР по своим средним показателям соответствует мировому тренду, но регионы КР по отдельности, имеют большой люфт от 0,87 (г. Ош) до 2,05 (г. Нарын).
Касаясь вопроса первичного механизма передачи коронавирусов, стоит отметить что, основным путем остается зоонозный тип передачи. Первые данные по механизму передачу получены Риу и др. в их исследовании отражено что передача Sars-CoV и Sars-CoV-2 идентичны с показателями R 0 — 2,24–3,58 с посылом о возможной быстрой глобализации устойчивой передачи от человека к человеку [34–36].
Первым доказательством гипотезы Риу и др. стала работа Chan et all в экспериментальной модели [37, 38].
С момента выявления Sars-CoV-2, установлено что средняя продолжительность инкубационного периода составляет от 1 до 14 дней. Инкубационный период характеризуется постоянным нарастанием вирусной нагрузки с проявлением пика в продром заболевания. Интересным моментом является отсутствие зависимости интенсивности вирусной нагрузки от тяжести клинического состояния в продромальном периоде [39].
Основным путем передачи является воздушно-капельный [40], тогда как фекальнооральный путь имеет низкий уровень доказательности [41–43], что можно отметить и в вертикальном механизме передачи [44–48].
Механизм патогенеза COVID-19 вызванной Sars-CoV-2
Sars-CoV-2, как и его предшественники, имеет высокую таргетность относительно дыхательной системы человека. В настоящее время известно, что передача происходит за счет контактные капли и фомиты от инфицированных лиц с последующим бессимптомным или симптоматическим течением в период разгара COVID-19 [49].
S-гликопротеин коронавируса взаимодействуя с клеточной мембраной альвеоцитов II типа, активируют протеазы (TMPRSS2 и фурин) клеток хозяина, протеазы разрезают S- гликопротеин по установленным участкам, тем самым обнажая участки S-гликопротеина сливающихся с ACE2 [49, 50].
Слияние S-гликопротеина и ACE2 активирует механизм эндоцитоза c погружением в цитоплазму как вириона, так и рецептора ACE2. В цитоплазме, вирион освобождается от оболочки, и геном вируса располагается непосредственно в цитоплазме клетки хозяин [31].
РНК-геном Sars-CoV-2 могут непосредственно синтезировать свои белки на рибосомах эндоплазматического ретикулума. Рибосомы хозяина трансформируют вирусную РНК в белок РНК-полимеразу, последняя, снова считывает положительную цепь (ssRNA+) для будущего синтеза одноцепочечной цепи РНК с отрицательным смыслом (ssRNA-). Цепи ssRNA используются РНК-полимеразой в качестве матрицы для синтеза дополнительных цепей ssRNA+. РНК новых вирусов и их структурные компоненты мигрируют в аппарат Гольджи для упаковки в нуклеокапсид, в целях создания новых вирионов. Упакованные вирусные частицы, удаляются из клетки хозяина путем экзоцитоза посредством секреторных везикул [32].
Данный механизм проникновения в клетку и выход и нее аналогичен с Sars-CoV [51].
Выход реплицированных вирусных единиц в межклеточное пространство, а ровно и высокая вирусная нагрузка в момент контакта с источником инфекции, происходит активация миграции тканевых макрофагов. Макрофаги фагоцитируя вирусные частицы выделяют интерфероны и цитокины [32], на это указывают высокие титры ИЛ-6, γ-интерферона и IFN-γ у инфицированных одной из человеческих коронавирусов [52].
Интерфероны инициируют активацию каскадной реакции специфического и неспецифического антивирусного иммунитета [53].
В случае с Sars-CoV-2 данная реакция иммунитета, проявляет себя как звено в развитии цитокинового шторма [32].
Цитокины производят дополнительный эффект в миграции а зону пораженной ткани макрофагов и нейтрофилов, в сочетании с прямым провоспалительным эффектом, усугубляя процесс воспаления в легочной ткани [54].
Активированная и самоподдерживающая воспалительная реакция легочной ткани приходит к раздражению нервных волокон, чем и объясняется ранний сухой кашель у больных COVID-19. В последующем к рефлекторному кашлю от раздражения нервных волокон периферического отдела легких, присоединяется продуктивный кашель выведения патологических продуктов скопившихся в бронхиальном дереве [54].
ИЛ-8 увеличивает миграцию нейтрофилов в очаг воспаления тем самым, провоцируя фагоцитоз с выделением дополнительных токсичных продуктов таких как: арахидоновая кислота, линоленовая кислота и др. которые в свою очередь выступают дополнительными бронхоконстрикторами что напрямую влияет на тяжесть гипоксического состояния и провоцируют неконтролируемую лихорадку [55].
Лихорадка инициированная организмом как защитный механизм под действием продуктов распада нейтрофилов становится патологического формата, именно данное явление становится дополнительным фактором оценки тяжести состояния пациентов. ИЛ-1 и ФНО-α действуют на гладкую мускулатуру сосудистого русла, расслабляя ее, что увеличивает объем жидкости в них с последующей ее выходом, в межклеточное пространство. А также увеличивая проницаемость сосудов, создают дополнительную инициацию миграции макрофагов и нейтрофилов в очаг попутно усугубляя гипоксических компонент в клиническом проявлении COVID-19 [56].
В комплексе вышесказанных факторов и сочетание поражение альвеоцитов II типа и понижению сурфактанта в паренхиме легких — это приводит к спаданию респираторных альвеол, и как итог, респираторному дистресс синдрому [57]. Патогенетический механизм показан на Рисунке 1 [58].
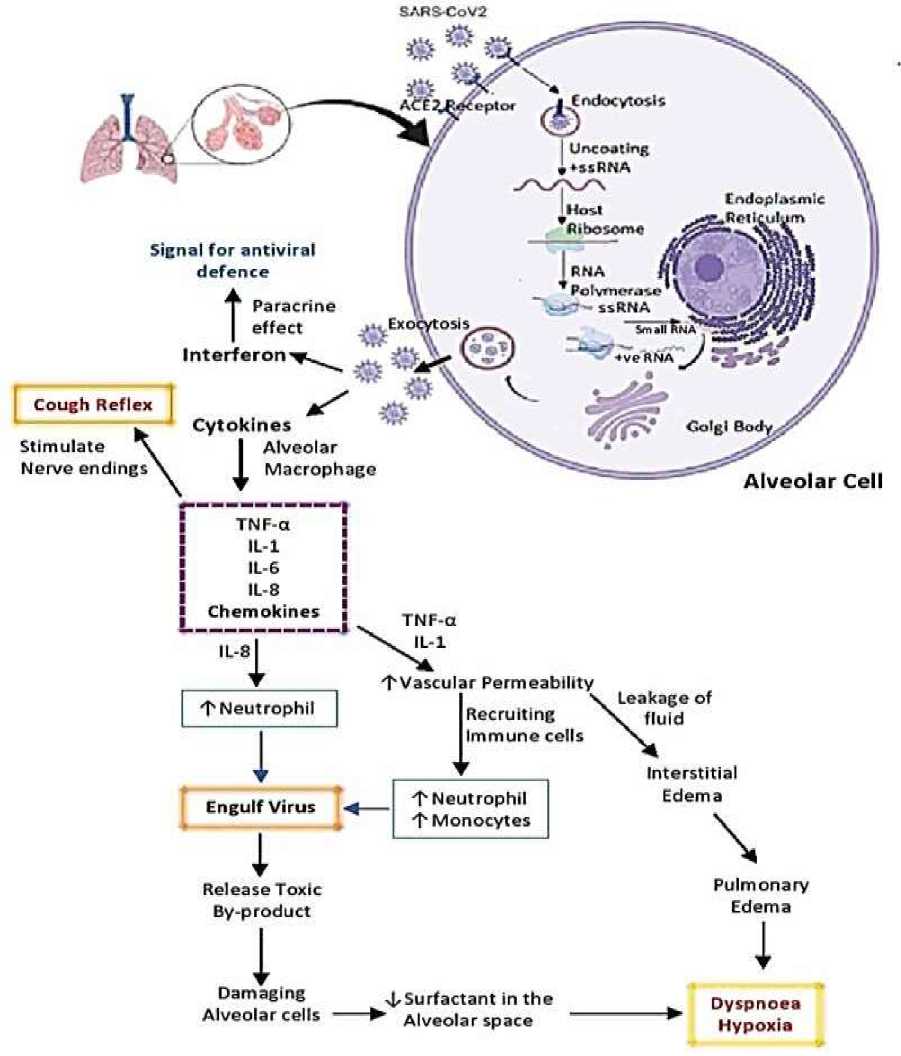
Рисунок 1. Патогенетический механизм, развивающийся при COVID-19
Классификация, клиника, диагностика, лечение, реабилитация и профилактика COVID-19
Классификация COVID-19
Современная классификация строится на определении степени тяжести состояния пациента , , , , :
-
а) легкая степень (включает в себя симптомы: лихорадка, кашель, усталость, потеря
аппетита, потеря обоняния/вкуса; другие неспецифические симптомы: боль в горле, заложенность носа, головная боль, диарея, тошнота (рвота), головокружение, возбуждение, слабость, усталость, снижение внимания, мобильности).
-
б) средней тяжести (включает в себя: клинические признаки пневмонии: лихорадка, кашель, одышка, учащенное дыхание, но без признаков тяжелой пневмонии, SpO 2 ≥90%).
-
в) тяжелая степень (включает в себя: клинические признаки пневмонии: лихорадка, кашель, одышка, быстрое дыхание, плюс одно из приведенного: ЧДД>30 движений/мин; Тяжелый респираторный дистресс синдром; SpO2<90% при дыхании комнатным воздухом или подтверждённая рентгенологически или КТ).
-
г) крайне тяжелая степень (включает в себя: наличие острого респираторного дистресс-синдрома (ОРДС), сепсиса, септического шока, острого тромбоза (ТЭЛА, ОКС, инсульт).
По степени риска , согласно приложению 2, клинического руководства по диагностике и лечению коронавирусной инфекции (COVID-19) (Версия 5) для всех уровней здравоохранения, утвержденного Приказом №424 МЗ КР от 16.04.2021: а) низкий риск; б) средний риск; в) высокий риск.
По осложнениям : а) осложненный; б) не осложненный.
Клинические проявления COVID-19
Основные симптомы при COVID-19 проявляются в виде: высокой температуры, кашля, одышки (вновь возникшая или ухудшение имеющийся одышки), аносмии или других нарушений в восприятии запахов, агевзии (нарушение вкуса) или другие нарушения вкусового восприятия, фарингита/ларингита, миалгии, озноба/дрожи головной боли, ринореи, тошноты/рвоты, диареи, болью или чувством тяжести в груди , , , , .
Выделяют так же жизнеугрожающие признаки обозначенными «Красными флагами» клинического состояния пациентов: Сильная одышка или затрудненное дыхание; Кровохарканье; Боль или давление в груди; Синие губы или лицо (диффузный цианоз); Холодный и липкий пот с пятнистой кожей; Изменение сознания; Становится трудно разбудить; Значительно сниженный диурез.
Значительную информацию о клиническом состоянии, дают лабораторные показатели. Так, в общем анализе крови не проявляется специфичных изменений, помимо лейкоцитоза в легкой и средней тяжестях и лимфопенией в тяжелом или крайне тяжелом состоянии. Анализ мочи с биохимическим анализом крови малоинформативны относительно дифференциальной диагностики, но как индикаторы общего состояния организма и выбора симптоматической терапии, незаменимы. Значимым показателем в оценке состояния воспалительных процессов при COVID-19 является С-реативный белок. При COVID-19, уровень СРБ имеет прямую корреляционную связь с тяжестью состояния, помимо сказанного, определяет период начала глюкокортикостероидной терапии. Ферритин и прокальцитонин, оценивают сочетанную бактериальную инфекцию при коронавирусе, и их значения определяют начало антибактериальной терапии. Наиболее важными в прогностическом значении являются показатели коагулограммы. При COVID-19, коагулограмма претерпевает отклонения в сторону большей свертываемости , , , , .
Диагностика COVID-19
Согласно стандартам разработанным в национальных руководствах, диагностика проводилась выявления антигенной структуры Sars-CoV-2 посредством полимеразной цепной реакции (ПЦР). Методика позволила быстро и точно определять зараженных и эффективно и быстро реагировать во всем мире. Эпидемиологическое расследование, несмотря на сложности в реализации, принесли значительный результат по выявлению потенциально зараженных и бессимптомных носителей [59]. Сочетание двух методик по организации, диагностических мероприятий, позволили США эффективно управлять эпидемиологической ситуацией без полной поставки экономической деятельности страны .
Минусами в диагностике ПЦР явились то что результат применим только в реальном времени, без возможности выявления иных возбудителей [59] и отсутствие результатов у реконвалисцентов [60].
Помимо вышесказанных минусов, в условиях КР, технически и процедурно тяжелая методика ПЦР, вероятно, приводила к ошибкам в заключении. Серологические методы диагностики, важны как метод оценки иммунного ответа на внедрения вируса. Так по состоянию IgM — мы можем определить острую фазу выработки антител, тогда как IgG – говорит о пройденном иммунологическом процессе с формированием полного специфического иммунного ответа против Sars-CoV-2. Минусом метода является невозможность исключить перекрестную реакцию на антитела, генерируемыми разными коронавирусами [61, 62].
В настоящее время, в целях экспресс диагностика, используют, тесты системы с адсорбированными и зафиксированными антителами к Sars-CoV-2 или антигенами Sars-CoV-2 на планшетах. Данная методика позволяет, разворачивать пункт по контролю в любом месте без оборудования лаборатории [62].
Лечение COVID-19
В настоящее время в терапии COVID-19 используются стандартные принципы фармакотерапии: а) этиотропная терапия; б) патогенетическая терапия; в) симптоматическая терапия. Начав обзор этиотропных принципов терапии, важно отметить что противовирусные лекарственные средства и все что было заявлено в качестве этиотропной терапии, не имеет достаточного уровня доказательности по вопросам эффективности и безопасности. Было проведено множество клинических исследований с применением трех основных групп лекарственных средств в роли этиотропной терапии. Так, группа противомалярийных препаратов, а именно хлорохин и гидроксихлорохин в сочетании с азитромицином или без таковой, оказались сомнительной эффективности и безопасности при проведении, рандомизированного исследования.
Вторая обширная группа — противовирусные средства (лопиновир, ремдесевир, интерферон-β-1а и т. п.) так же не проиллюстрировал эффективность относительно COVID-19. То же касается терапии реконвалисцентной плазмы как один из вариантов этиотропной терапии которые применялись и в КР.
Патогенетическая терапия, вышла на первый план в стратегии лечения COVID-19. Данный подход в терапии, включает себя следующие направления:
-
а) Антикоагулянтная терапия — в целях разрыва патогенетических цепей формирования тромбов в сосудистом русле пациентов, применяют в качестве препарата выбора низкомолекулярный гепарин (НМГ) — эноксапарин, в случае отсутствия последнего
используется нефракционированный гепарин (НФГ).
-
б) Заместительная инфузионная терапия — применяется как способ восполнения компонентов гомеостаза в организме пациента. В качестве препарата выбора применяют свежезамороженную плазму, дозу и объем СЗП подбирают в зависимости от тяжести состояния.
-
в) Гастропротективная терапия — применяется на фоне проводимой антикоагулянтной и антиагрегатной терапии у пациентов с сопутствующей патологией желудочно-кишечного тракта [68].
-
г) Противовоспалительная терапия глюкокортикостероидами, терапия направленная на общую мобилизацию организма, сильное противодействие воспалению и приостановке иммунологических реакций у пациентов с COVID-19. Важно отметить что применение ГКС регламентировано: применять рекомендуются только в случаях тяжелой и крайне тяжелой формах клинического течения COVID-19. Применение ГКС в легкой и средней степени тяжести приводит у увеличению времени виремии и усугубляет риск утяжеления клинического состояния пациентов. Препаратами выбора являются преднизолон, дексаметазон, метилпреднизолон и другие синтетические ГКС системного действия [63-67].
-
д) Оксигенотерапия — поражение легочной ткани и сопутствующие патологические механизмы при COVID-19, нарушают перфузионно-вентиляционный градиент, что приводит к гипоксии. Оксигенотерапия необходима как поддерживающая гомеостаз терапия. Так на сегодняшний день используют инвазивные и неинвазивные методы оксигенации крови [63].
Симптоматическое лечение — применяется не зависимости от степени тяжести и в случаях легкой и средней степени тяжести являются рекомендуемым подходом в терапии COVID-19. Так, данная терапия направленна на снижения или удаления клинического проявления COVID-19. Например; применение парацетамола/ибупрофена в случаях лихорадки, энтеральная или парентеральная дегидратация в случаях дефицита жидкости в период болезни и т д. , , , , .
Реабилитационные мероприятия для пациентов с COVID-19
В настоящее время реабилитационные мероприятия начинаются с первого дня госпитализации/амбулаторного наблюдения и продолжается после выписки пациента. В период госпитализации/наблюдения реабилитация сводится к предотвращению ухудшения состояния и носят профилактический характер относительно ослабленных функций органов и их систем , ,
Во втором этапе, в первые 30 дней после выписки подключаются различные физиотерапевтические, трудотерапевтические и психотерапевтические мероприятия в целях восстановления функционального состояния органов, систем и организма в целях укрепления в период реконвалесценции [64–66].
Профилактические мероприятия COVID-19
Профилактические мероприятия включают в себя: отслеживание случаев, изоляцию, социальную дистанцию, личную гигиену и вакцинацию [67–72].
В период начала и разгара пандемии COVID-19, а также отсутствия вакцины от COVID-19, комплекс мероприятий направленный на изоляцию и пропаганду личной гигиены, показал наилучшие результаты по сдерживанию волнообразного наплыва инфицированных в больницы Сингапура [72].
Эффективность своевременного и этапного внедрения карантинных мер снизил количество госпитализаций в реанимационные отделения и палаты интенсивной терапии в Италии и США [35, 73, 74].
Ношение масок, соблюдение социальной дистанции, введение карантинных мер, в 6 раз снизил передачу вируса R 0 от 2,6 до 1,1 и тем самым признаны наиболее эффективными методами по сдерживанию инфекционных заболеваний и смертностью от них [8, 75, 76].
В настоящее время, продолжаются профилактические мероприятия специфического характера — вакцинация от COVID-19 [70, 71].
Иммунопатологические процессы при COVID-19
Первостепенным, в иммунной системы человека при COVID-19, является отсутствие защитного иммунного механизма [77], что нарушило стандартную первую линию противовирусной защиты [78]. Связи с этим, проявляется бесконтрольное действие цитокиновой системы в роли «пожарной кнопки» для отпора Sars-CoV-2 [78]. Инфицированные клетки первой линии не имеют возможности передавать сигнатуры по IFN-I/III путям, в целях информирования последующего защитного «рубежа». Данный дисбаланс между система иммунного коммуницирования приводит к бесконтрольному выбросу цитокинов [77].
Поток цитокинов приводит к локальному и (или) системному повреждению тканей с параллельно снижающимся уровнем CD4+; CD8+ и Т-клеток [79, 80], в период реконвалисценции баланс между цитокинами и CD4/CD8 восстанавливается [81, 82].
Помимо клеточного механизма, в период инфицирования, активно проявляется гуморальный иммунитет, что отражается активным синтезированием IgM, IgA и IgG с первого дня инфицирования [83–85].
Гипервоспалительная реакция при COVID-19
Прямое повреждение тканей первичной воспалительной реакцией на Sars-CoV-2 активирует, и в последующем, стимулирует миграцию гранулоцитов и макрофагов [85].
Макрофаги, в зоне поражения, увеличивают секрецию цитокинов и дополнительному привлечению лейкоцитов, приводя к системной воспалительной реакции [86].
В ряде исследований показано, что концентрация провоспалительных цитокинов и тяжесть клинического состояния, имеют прямую корреляционную связь [79, 87, 88].
ИЛ-1β, ИЛ-7, ИЛ-8, ИЛ-10 и TNF-α повышаются у инфицированных лиц [89]. В исследованиях Cin S. et al. и Ye Q. et al., ИЛ-2 и ИЛ-6 – определяются как маркер неблагоприятного течения заболевания и формируют клиническое представление об общем клиническом состоянии пациента [79, 90].
Механизм гипервоспалительной реакции отображен на Рисунке 2 [91].
Иммунологический механизм действия цитокинов, хемокинов и интерферонов при инфицировании HCoV
Sars-CoV-2, как источник цитокинового шторма, по иммунологическим механизмам имеет сродство с другими известными коронавирусами человека [92].
Экспериментальные исследования на культуре респираторного эпителия, показало задержку высвобождения иммуномодуляторов и низкие значения противовирусных IFN, а также, значительное повышение провоспалительных цитокинов (ИЛ-1β, ИЛ-6, ФНО-α), хемокинов (CLL-3 и CLL-5) [93-95].
Для коронавирусов человека характерно, повышенная концентрация нейтрофилов/моноцитов в паренхиме легких и периферической крови инфицированных, что коррелирует с уровнями провоспалительных цетокинов и хемокинов, что свидетельствует об участии их в развитии воспаления паренхимы при COVID-19 [96, 97].
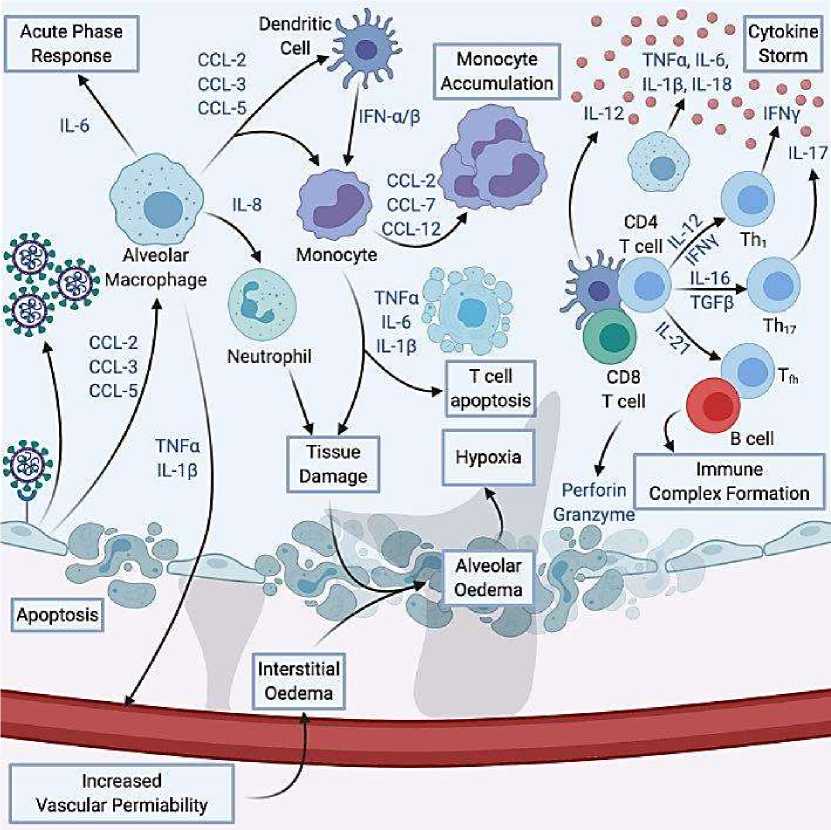
Рисунок 2. Иммунологические механизмы гипервоспаления при COVID-19
Синтез IFN-1 и IFN-α/β, как главных факторов естественного противовирусного иммунитета [98], при HCoV, замедляется, что препятствует формированию защитных механизмов на ранних стадиях заболевания [99]. Параллельное повышения уровня цитокинов и хемокинов, привлекают в очаг нейтрофилов и моноцитов, как итог наблюдается непропорциональное инфильтрация альвеолярной ткани и ее повреждение [92, 100].
Привлеченные мононуклеарные макрофаги активируются посредством рецептора IFN-α/β, секретируют CLL-2 и СLL-7, что приводит к агрегации макрофагов с последующим повышением концентрации цитокинов, хемоцинов и свободных радикалов. В комплексе: цитокины-хемокины-рецептор IFN-α/β приводит к активации рецептора TRAIL-смерть, запуская механизм апоптоза [101–103].
Как обобщенный итог, дисбаланс цитокиновой, хемокиновой и интерфероной системы приводит к активации множества механизмов смерти клеток легочной ткани [92, 100].
Функционирование лейкоцитов при COVID-19
Общим свойством лейкоцитов при COVID-19, явилось их тенденция к снижению. Так в ряде исследований отмечалось популяционное снижение CD4+, CD8+, B-лимфоцитов и NK-клеток [104, 105].
Базофилы и эозинофилы так же отмечались на низких значениях [105]. Нейтрофилы же имели тенденцию к росту, с увеличением отношения нейтрофилы/лимфоциты [105, 106]. Тяжесть состояния также как и в случае с цитокинами, коррелировало по отношению к отношению нейтрофилы/лимфоциты [106].
Логично, что значения лейкоцитов и их отношения не имело диагностического смысла при легких случаях COVID-19. Прогностический характер они обретают в тяжелом клиническом течении в сочетании эозинопенией и цитозом гранулоцитов [107–110].
Обобщая полученные данные, мы можем сказать что иммунные «качели» в лейкоцитах свидетельствуют о дисбалансе в механизме клеточной защиты против Sars-CoV-2 [111].
Иммунологические механизмы в сосудистом русле при COVID-19
Нарушение свертывающей системы крови с повышением уровня D-димера, один из главных маркеров клинического состояния пациентов [112].
Тромбозы, микротромбы легких и ДВС проявляющихся как результат аномального функционирования цитокиновой системы [113, 114]. Главным путем активации макрофагов в сосудистом русле выступают оксиленные фосфолипиды по пути TL-TRIF-TRAF6-NF-кВ, что характерно для HCoV [115-117].
Таким образом, в настоящее время, говорится об активации ACE2 рецептора на стенках сосудистого русла, с последующим развитием воспалительной реакции, ввиду снижения положительного эффекта ACE2 как противовоспалительного компонента в организме человека [116, 117].
Гипотезу подтверждает ряд наблюдений за детьми в период пандемии. Так дети менее подвержены COVID-19, за тот же период отмечается рост заболеваемости различного рода васкулитов [118–121].
Вопросы связанные с иммунопатологией в сосудистом русле остаются актуальными для обсуждения, в настоящем времени.
Дополнительные механизмы поддержания негативных эффектов COVID-19
Помимо основных патологических механизмов становления нозологии, COVID-19 поддерживается механизмами свободно-радикального механизма воспаления на основе перекисного окисления липидов и дисбаланса регуляционного/контррегуляционного механизмов влияния ACE2.
Роли перекисного окисления липидов при COVID-19
Ряд авторов выдвинули идеи касательно роли перекисного окисления липидов (ПОЛ) в патогенезе COVID-19, ввиду его участия в большинстве воспалительных реакций сопровождающихся гипоксиемией [122-126].
В Испании проведено когортное исследование, результатом которого явилось, что у пациентов с COVID-19 низкий уровень антиоксидантной защиты и более высокие значения антиоксидантных ферментов [127].
Стимулирование ПОЛ при COVID-19, опосредуется ее особенностью к агрегации
ACE2, что приводит к дефициту ACE2 и снижению антиоксилантной функции организма в целом. Снижение защиты приводит к накоплению супероксидов и как следствие запускается каскад ПОЛ [128].
Активация ПОЛ и ее циклично замкнутой системой самовоспроизведения приводит к стойкой дисфункции клеток в области поражения отягощая общее состояние пациентов [127–130].
Также нельзя не отметить роль ПОЛ в прогрессировании хронических заболеваний, таких как заболевания ССС, ХОБЛ, СД и других хронических патологий [131–136].
В настоящее время выявлено что высокий уровень активности ПОЛ при COVID-19 ухудшают прогноз выживаемости в течение 28 дней пребывания в госпитале [127].
Роль дефицита ACE2 в патогенезе COVID-19
В результате проведенных исследований по всему миру, доказано сродность S-пептида Sars-CoV-2 к ангиотензин превращающему ферменту 2 типа (ACE2) экспрессируемом на поверхности множества клеток человека [137, 138].
Проведено существенное количество исследований по изучению осей взаимодействия ACE2 на течение COVID-19 и на ряд других заболеваний. Так в исследовании Magalhaes GS et al. отображена ось: ACE2 – ангиотензин 1–7 – Mas рецептор – легкие. Где показано что, ACE2, преобразуя ангиотензин 2 в ангиотензин 1–7 улучшает течение патологических состояний и предупреждает развития фиброза легких [139–142].
Так же связывание ACE2 с брадикинином в легких не допускает попадание последнего на брадикининовый рецептор B1, что блокирует отрицательные эффекты брадикининовых механизмов [143, 144].
В проведенном исследовании в Германии определены механизмы влияния ACE2 на тромбоцитарную систему. Результатом исследования явилось экспрессия рецепторов Mas на поверхности тромбоцитов, активация первых ангиотензином 1–7, приводит к высвобождению простациклина и NO, что сопровождает выраженный антитромботический эффект [145–147].
Sars-CoV-2 связываясь с ACE2, проникает в клетку вместе с ACE2, что уменьшает общее количество ACE2 – это приводит к ослаблению оси формирования ангиотензина 1–7 с воздействием на Mas рецепторы и усилению оси синтеза ангиотензина 2 с воздействием на ангиотензиновые рецепторы 1 типа [148–150].
Отходя от фундаментальной физиологической функции ACE2, интересно заметить, что дефицит ACE2 усугубляется с возрастом и наблюдается чаще у мужчин чем женщин [151] коррелируя с показателями смертности от COVID-19 в гендерном аспекте [152].
В особенности нас интересует связь дефицита ACE2 с гипертензией. Так в исследовании Чжун Дж и др. дефицит ACE2 был связан с обострением гипертензии и гипертрофией сердца посредством накопления ангиотензина 2 [153].
Научные сообщества активно обсуждают о возможности пагубного влияния ACE2 на течение COVID-19 ввиду того что ACE2 «входные ворота» для Sars-CoV-2, но высокое сродство S-пептида к ACE2 говорит о малой значимости количества ACE2 на поверхности клеток [154].
К дополнению, дефицит ACE2 вызванный действием Sars-CoV-2, усиливает дисбаланс: по оси ангиотензина 2 (усиление пагубного влияния), по оси ангиотензина 1–7 (ослабление защитных механизмов), что уменьшает резерв компенсаторных механизмов при сопутствующей гипертензии [155, 156].
Влияние климатических факторов на COVID-19.
Климатические и географические факторы окружающей среды оказывают разностороннее влияние на биологические процессы в организме человека. Так, патологические процессы при COVID-19, в различных условиях окружающей среды претерпевают изменения «классических» механизмов [157].
Первые предположения о влиянии климата на распространение COVID-19 выдвинуты в Италии. Исследователи из Университета Калабрии отметили что относительная влажность и температура воздуха в течение дня оказывают влияние на статистику выявленных случаев [158].
Аналогичные наблюдения описали в исследовании из Университета Джоржии, иллюстрируя зависимость температуры, концентрации озона, относительной влажности и облачности с выявленными случаями в штате Нью-Йорк [159].
Katherine Li опубликовала тезис: «смертность от COVID-19 во всех 50 штатах США напрямую коррелируют с влажностью окружающей среды, которую испытывает COVID-19» [160].
Касаясь предметов исследования климатического влияния на COVID-19, стоит отметить что роль отводилась показателям: температуры, влажности, количества осадков, атмосферного давления, скорости ветра, уровню ультрафиолетового излучения и др. [161– 163].
Из 300 статей посвященным, данной тематике, более половины оценивают температурный фактор и влажность, с меньшим вниманием на атмосферное давление [157].
В Китае и Сингапуре, наблюдалось слабая отрицательная корреляционна я зависимость между заболеваемостью COVID-19 и атмосферным давлением [163, 164].
В индийском штате Мумбай, коэффициент корреляции ограничился на значениях R=-0,20; -0,29 [165]. Исследование в трех других штатах Индии, оценило влияние атмосферного давления на заболеваемость в пределах R=-0,56, -0,80 [166].
Исходя из вышесказанного, можно прийти к выводу что заболеваемость и смертность от COVID-19 имеет обратную корреляционную связь относительно атмосферного давления [157]. Противовесом вышесказанному тезису, приходится работа Nicole Y. Leung et all, в исследовании отражается более динамичное распространение COVID-19 в высокогорных регионах мира, тогда как высокогорье характеризуется низким атмосферным давлением и высоким уровнем УФ-излучения [157, 167].
В ряде исследований, выдвинули предположение, что COVID-19, как и другие воздушно-капельные респираторные инфекции лучше распространяется в холодном и сухом климате [168].
Оппозитно гипотезе «низкая температура-низкая влажность» выступают данные исследований проведенных во всех климатических регионах Китая, где высокогорный регион показал наименьшие значения заболеваемости и высокий коэффициент смертности [169, 170].
В исследованиях, по комплексной оценке, влияния климата, выявлено, что сочетание низкого атмосферного давления с высокой амплитудой колебаний температуры воздуха вызывает рост смертности и замедление заболеваемости. Данная концепция приемлема и для эпидемиологической картины Кыргызской Республики в период пандемии COVID-19 [171].
Выводы
Исходя из вышесказанного, следует, что исследования по коронавирусной инфекции (COVID-19) во всем мире ведутся в быстром режиме, ввиду ее социально-экономической значимости. Однако, мало информации об особенностях патогенетических и иммунологических механизмах развития COVID-19 в условиях низко-, средне- и высокогорья. Клинические проявления и интенсивность обострения хронических патологий при гипоксической гипоксии так же остаются малоизученными. В литературных данных слаба отображена роль продуктов ПОЛ в создании и/или поддержании патологических состояний, влияние на терапию и влияние терапии на ПОЛ при COVID-19. Важным вопросом остается дефицит/профицит ACE2 в условиях высокогорного климата. Крайне мало исследований относительно влияния гипоксической гипоксии на скорость развития и тяжесть течения ОРДС при COVID-19. Особенности терапии хронических патологий, свойственным жителям высокогорного региона, при COVID-19 так же остаются неточными.
Автор заявляет об отсутствии конфликта интересов.
Исследование не финансировалось.
Список литературы Современные представления об эпидемиологии, клинико-патогенезу, иммунопатологии, дополнительных факторах поддержания воспаления, диагностике, лечению COVID-19 в условиях высокогорья (обзор литературы)
- 1. Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China // The lancet. 2020. V. 395. №10223. P. 497-506. https://doi.org/10.1016/S0140-6736(20)30183-5
- Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction // Bmj. 2020. V. 368. №8. P. 1036. https://doi.org/10.1136/bmj.m1036
- Guo Y. R., Cao Q. D., Hong Z. S., Tan Y. Y., Chen S. D., Jin H. J., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status // Military medical research. 2020. V. 7. P. 1-10. https://doi.org/10.1186/s40779-020-00240-0
- Chakraborty I., Maity P. COVID-19 outbreak: Migration, effects on society, global environment and prevention // Science of the total environment. 2020. V. 728. P. 138882. https://doi.org/10.1016/j.scitotenv.2020.138882
- Ye Z. W., Yuan S., Yuen K. S., Fung S. Y., Chan C. P., Jin D. Y. Zoonotic origins of human coronaviruses // International journal of biological sciences. 2020. V. 16. №10. P. 1686. https://doi.org/10.7150%2Fijbs.45472
- Li H., Liu S. M., Yu X. H., Tang S. L., Tang C. K. Coronavirus disease 2019 (COVID-19): current status and future perspectives // International journal of antimicrobial agents. 2020. V. 55. №5. P. 105951. https://doi.org/10.1016/j.ijantimicag.2020.105951
- Mackenzie J. S., Smith D. W. COVID-19: a novel zoonotic disease caused by a coronavirus from China: what we know and what we don’t // Microbiology Australia. 2020. V. 41. №1. P. 45-50. https://doi.org/10.1071/MA20013
- Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q., Wu T. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China // Jama. 2020. V. 323. №19. P. 1915-1923. https://doi.org/10.1001/jama.2020.6130
- Global guidance for surgical care during the COVID-19 pandemic // Journal of British Surgery. 2020. V. 107. №9. P. 1097-1103. https://doi.org/10.1002/bjs.11646
- Nuccetelli M., Pieri M., Grelli S., Ciotti M., Miano R., Andreoni M., Bernardini S. SARSCoV- 2 infection serology: a useful tool to overcome lockdown? // Cell Death Discovery. 2020. V. 6. №1. P. 38. https://doi.org/10.1038/s41420-020-0275-2
- Paules C. I., Marston H. D., Fauci A. S. Coronavirus infections—more than just the common cold // Jama. 2020. V. 323. №8. P. 707-708. https://doi.org/10.1001/jama.2020.0757
- Pal M., Berhanu G., Desalegn C., Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update // Cureus. 2020. V. 12. №3. https://doi.org/10.7759/cureus.7423
- Wang Q., Qiu Y., Li J. Y., Zhou Z. J., Liao C. H., Ge X. Y. A unique protease cleavage site predicted in the spike protein of the novel pneumonia coronavirus (2019-nCoV) potentially related to viral transmissibility // Virologica Sinica. 2020. V. 35. P. 337-339. https://doi.org/10.1007/s12250-020-00212-7
- Lan J. et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor // Nature. 2020. V. 581. №7807. P. 215-220. https://doi.org/10.1038/s41586-020-2180-5
- Millet J. K., Whittaker G. R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis // Virus research. 2015. V. 202. P. 120-134. https://doi.org/10.1016/j.virusres.2014.11.021
- Wang N., Shang J., Jiang S., Du L. Subunit vaccines against emerging pathogenic human coronaviruses // Frontiers in microbiology. 2020. V. 11. P. 298. https://doi.org/10.3389/fmicb.2020.00298
- Kim D., Lee J. Y., Yang J. S., Kim J. W., Kim V. N., Chang H. The architecture of SARS-CoV-2 transcriptome // Cell. 2020. V. 181. №4. P. 914-921. e10. https://doi.org/10.1016/j.cell.2020.04.011
- Duan L., Zheng Q., Zhang H., Niu Y., Lou Y., Wang H. The SARS-CoV-2 spike glycoprotein biosynthesis, structure, function, and antigenicity: implications for the design of spikebased vaccine immunogens // Frontiers in immunology. 2020. V. 11. P. 576622. https://doi.org/10.3389/fimmu.2020.576622
- Schoeman D., Fielding B. C. Coronavirus envelope protein: current knowledge // Virology journal. 2019. V. 16. №1. P. 1-22. https://doi.org/10.1186/s12985-019-1182-0
- Chang C. K., Hou M. H., Chang C. F., Hsiao C. D., Huang T. H. The SARS coronavirus nucleocapsid protein–forms and functions // Antiviral research. 2014. V. 103. P. 39-50. https://doi.org/10.1016/j.antiviral.2013.12.009
- Hoffmann M., Kleine-Weber H., Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells // Molecular cell. 2020. V. 78. №4. P. 779-784. e5. https://doi.org/10.1016/j.molcel.2020.04.022
- Hayashi T., Ura T., Abiko K., Mandan M., Yaegashi N., Konishi I. Reasons why new coronavirus, SARS-CoV-2 infections are likely to spread // Journal of Genetic Medicine and Gene Therapy. 2020. V. 3. №1. P. 001-003. https://dx.doi.org/10.29328/journal.jgmgt.1001005
- Wrapp D., Wang N., Corbett K. S., Goldsmith J. A., Hsieh C. L., Abiona O., McLellan J. S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation // Science. 2020. V. 367. №6483. P. 1260-1263.
- Li F, Li W, Farzan M, Harrison S. C. Structure of SARS coronavirus spike receptorbinding domain complexed with receptor.Science. 2005. September 16;309(5742):1864–1868. https://doi.org/10.1126/science.abb2507
- Park J. E., Li K., Barlan A., Fehr A. R., Perlman S., McCray Jr P. B., Gallagher T. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism // Proceedings of the National Academy of Sciences. 2016. V. 113. №43. P. 12262-12267. https://doi.org/10.1073/pnas.1608147113
- Zhou P., Yang X. L., Wang X. G., Hu B., Zhang L., Zhang W., Shi Z. L A pneumonia outbreak associated with a new coronavirus of probable bat origin // Nature. 2020. V. 579. №7798. P. 270-273. https://doi.org/10.1038/s41586-020-2012-7
- Hamming I., Timens W., Bulthuis M. L. C., Lely A. T., Navis G. V., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis // The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2004. V. 203. №2. P. 631-637. https://doi.org/10.1002/path.1570
- Watanabe Y., Bowden T. A., Wilson I. A., Crispin M. Exploitation of glycosylation in enveloped virus pathobiology // Biochimica et Biophysica Acta (BBA)-General Subjects. 2019. V. 1863. №10. P. 1480-1497. https://doi.org/10.1016/j.bbagen.2019.05.012
- Barile E., Baggio C., Gambini L., Shiryaev S. A., Strongin A. Y., Pellecchia M. Potential therapeutic targeting of coronavirus spike glycoprotein priming // Molecules. 2020. V. 25. №10. P. 2424. https://doi.org/10.3390/molecules25102424
- Tang T., Bidon M., Jaimes J. A., Whittaker G. R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development // Antiviral research. 2020. V. 178. P. 104792. https://doi.org/10.1016/j.antiviral.2020.104792
- Astuti I. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response // Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020. V. 14. №4. P. 407-412. https://doi.org/10.1016/j.dsx.2020.04.020
- Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS‐CoV, and 2019‐nCoV // Journal of medical virology. 2020. V. 92. №5. P. 491-494. https://doi.org/10.1002/jmv.25709
- Sorci G., Faivre B., Morand S. Explaining among-country variation in COVID-19 case fatality rate // Scientific reports. 2020. V. 10. №1. P. 18909. https://doi.org/10.1038/s41598-020-75848-2
- Riou J., Althaus C. L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020 // Eurosurveillance. 2020. V. 25. №4. P. 2000058. https://doi.org/10.2807/1560-7917.es.2020.25.7.20200220c
- Zhao S., Lin Q., Ran J., Musa S. S., Yang G., Wang W., Wang M. H. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak // International journal of infectious diseases. 2020. V. 92. P. 214-217. https://doi.org/10.1016/j.ijid.2020.01.050
- Zhou T., Liu Q., Yang Z., Liao J., Yang K., Bai W., Zhang W. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019‐nCoV // Journal of Evidence‐ Based Medicine. 2020. V. 13. №1. P. 3-7. https://doi.org/10.1111/jebm.12376
- Chan J. F. W., Yuan S., Kok K. H., To K. K. W., Chu H., Yang J., Yuen K. Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster // The lancet. 2020. V. 395. №10223. P. 514-523. https://doi.org/10.1016/S0140-6736(20)30154-9
- Okada P., Phuygun S., Thanadachakul T., Parnmen S., Wongboot W., Waicharoen S., Maurer-Stroh S. Early transmission patterns of coronavirus disease 2019 (COVID-19) in travelers from Wuhan to Thailand, January 2020 // Eurosurveillance. 2020. V. 25. №8. P. 2000097. https://doi.org/10.2807/1560-7917.ES.2020.25.8.2000097
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients // New England journal of medicine. 2020. V. 382. №12. P. 1177-1179. https://doi.org/10.1056/NEJMc2001737
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia // New England journal of medicine. 2020. V. 382. №13. P. 1199-1207. https://doi.org/10.1056/NEJMoa2001316
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J., Xu W. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) // China CDC weekly. 2020. V. 2. №8. P. 123-124. https://weekly.chinacdc.cn/en/article/doi/10.46234/ccdcw2020.033
- Karimi-Zarchi M., Neamatzadeh H., Dastgheib S. A., Abbasi H., Mirjalili S. R., Behforouz A., Bahrami R. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review // Fetal and pediatric pathology. 2020. V. 39. №3. P. 246-250. https://doi.org/10.1080/15513815.2020.1747120
- Alzamora M. C., Paredes T., Caceres D., Webb C., Valdez L., Huang C., Moss T. Severe COVID-19 during pregnancy and possible vertical transmission // American journal of perinatology. 2020. V. 37. №08. P. 861-865. https://doi.org/10.1055/s-0040-1710050
- Kalyanasundaram S., Krishnamurthy K., Sridhar A., Narayanan V. K., Rajendra Santosh A. B., Rahman S. Novel corona virus pandemic and neonatal care: it’s too early to speculate on impact! // SN Comprehensive Clinical Medicine. 2020. V. 2. №9. P. 1412-1418. https://doi.org/10.1007/s42399-020-00440-8
- Martínez-Perez O., Vouga M., Melguizo S. C., Acebal L. F., Panchaud A., Muñoz- Chápuli M., Baud D. Association between mode of delivery among pregnant women with COVID-19 and maternal and neonatal outcomes in Spain // Jama. 2020. V. 324. №3. P. 296-299. https://doi.org/10.1001/jama.2020.10125
- Dong L. et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn // Jama. 2020. V. 323. №18. P. 1846-1848. https://doi.org/10.1001/jama.2020.4621
- Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H., Liu Y. Infants born to mothers with a new coronavirus (COVID-19) // Frontiers in pediatrics. 2020. V. 8. P. 104.
- Arnaez J., Montes M. T., Herranz-Rubia N., Garcia-Alix A. The impact of the current SARS-CoV-2 pandemic on neonatal care // Frontiers in Pediatrics. 2020. V. 8. P. 247. https://doi.org/10.3389/fped.2020.00247
- Yi Y., Lagniton P. N., Ye S., Li E., Xu R. H. COVID-19: what has been learned and to be learned about the novel coronavirus disease // International journal of biological sciences. 2020. V. 16. №10. P. 1753. https://doi.org/10.7150%2Fijbs.45134
- Hoffmann M. et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells //BioRxiv. – 2020. – С. 2020.01. 31.929042. https://doi.org/10.1101/2020.01.31.929042
- Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia // Emerging microbes & infections. 2020. V. 9. №1. P. 727-732. https://doi.org/10.1080/22221751.2020.1746199
- Wu Y., Huang X., Sun J., Xie T., Lei Y., Muhammad J., Zhang Q. Clinical characteristics and immune injury mechanisms in 71 patients with COVID-19 // Msphere. 2020. V. 5. №4. P. 10.1128/msphere. 00362-20. https://doi.org/10.1128/msphere.00362-20
- Coccia E. M., Battistini A. Early IFN type I response: Learning from microbial evasion strategies // Seminars in immunology. Academic Press, 2015. V. 27. №2. P. 85-101. https://doi.org/10.1016/j.smim.2015.03.005
- Hu G., Christman J. W. Alveolar macrophages in lung inflammation and resolution // Frontiers in immunology. 2019. V. 10. P. 2275. https://doi.org/10.3389/fimmu.2019.02275
- Abdulkhaleq L. A., Assi M. A., Abdullah R., Zamri-Saad M., Taufiq-Yap Y. H., Hezmee M. N. M. The crucial roles of inflammatory mediators in inflammation: A review // Veterinary world. 2018. V. 11. №5. P. 627. https://doi.org/10.14202%2Fvetworld.2018.627-635
- Fahey E., Doyle S. L. IL-1 family cytokine regulation of vascular permeability and angiogenesis // Front Immunol. 2019. V. 10. P. 1426.
- Gonzales J. N., Lucas R., Verin A. D. The acute respiratory distress syndrome: mechanisms and perspective therapeutic approaches // Austin journal of vascular medicine. 2015. V. 2. №1.
- Rahman S., Montero M. T. V., Rowe K., Kirton R., Kunik Jr, F. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: a review of current evidence // Expert review of clinical pharmacology. 2021. V. 14. №5. P. 601-621. https://doi.org/10.1080/17512433.2021.1902303
- Bustin S. A., Mueller R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis // Clinical Science. 2005. V. 109. №4. P. 365-379. https://doi.org/10.1042/CS200500860050086
- Tang Y. W., Schmitz J. E., Persing D. H., Stratton C. W. Laboratory diagnosis of COVID- 19: current issues and challenges // Journal of clinical microbiology. 2020. V. 58. №6. P. 10.1128/jcm. 00512-20. https://doi.org/10.1128/jcm.00512-20
- Peeling R. W., Wedderburn C. J., Garcia P. J., Boeras D., Fongwen N., Nkengasong J., Heymann D. L. Serology testing in the COVID-19 pandemic response // The Lancet Infectious Diseases. 2020. V. 20. №9. P. e245-e249. https://doi.org/10.1016/S1473-3099(20)30517-X
- Freeman B., Lester S., Mills L., Rasheed M. A. U., Moye S., Abiona O., Thornburg N. J. Validation of a SARS-CoV-2 spike protein ELISA for use in contact investigations and serosurveillance // Biorxiv. 2020. https://doi.org/10.1101%2F2020.04.24.057323
- Ospina-Tascon G. A., Calderón-Tapia L. E., García A. F., Zarama V., Gómez-Álvarez F., Álvarez-Saa T., .Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a randomized clinical trial // Jama. 2021. V. 326. №21. P. 2161-2171. https://doi.org/10.1001/jama.2021.20714
- Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19 // Jama. 2020. V. 324. №6. P. 603-605. https://doi.org/10.1001/jama.2020.12603
- Halpin S. J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: A crosssectional evaluation // Journal of medical virology. 2021. V. 93. №2. P. 1013-1022. https://doi.org/10.1002/jmv.26368
- Bowles K. H., McDonald M., Barron Y., Kennedy E., O’Connor M., & Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients // Annals of internal medicine. 2021. V. 174. №3. P. 316-325. https://doi.org/10.7326/M20-5206
- Wilder-Smith A., Freedman D. O. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019- nCoV) outbreak // Journal of travel medicine. 2020. V. 27. №2. P. taaa020. https://doi.org/10.1093/jtm/taaa020
- Güner H. R., Hasanoğlu İ., Aktaş F. COVID-19: Prevention and control measures in community // Turkish Journal of medical sciences. 2020. V. 50. №9. P. 571-577. https://doi.org/10.3906/sag-2004-146
- Cirrincione L., Plescia F., Ledda C., Rapisarda V., Martorana D., Moldovan R. E., Cannizzaro E. COVID-19 pandemic: Prevention and protection measures to be adopted at the workplace // Sustainability. 2020. V. 12. №9. P. 3603. https://doi.org/10.3390/su12093603
- Yao J. S., Paguio J. A., Dee E. C., Tan H. C., Moulick A., Milazzo C., Celi L. A. The minimal effect of zinc on the survival of hospitalized patients with COVID-19: an observational study // Chest. 2021. V. 159. №1. P. 108-111. https://doi.org/10.1016/j.chest.2020.06.082
- Krause P., Fleming T. R., Longini I., Henao-Restrepo A. M., Peto R., Dean N. E., Henao- Restrepo A. M. COVID-19 vaccine trials should seek worthwhile efficacy // The Lancet. 2020. V. 396. №10253. P. 741-743. https://doi.org/10.1016/S0140-6736(20)31821-3
- Koo J. R., Cook A. R., Park M., Sun Y., Sun H., Lim J. T., Dickens B. L. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study // The Lancet Infectious Diseases. 2020. V. 20. №6. P. 678-688. https://doi.org/10.1016/S1473-3099(20)30162-6
- Rodriguez-Morales A. J., Cardona-Ospina J. A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J. P., Sah R. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis // Travel medicine and infectious disease. 2020. V. 34. P. 101623. https://doi.org/10.1016/j.tmaid.2020.101623
- Shim E., Tariq A., Choi W., Lee Y., Chowell G.Transmission potential and severity of COVID-19 in South Korea // International Journal of Infectious Diseases. 2020. V. 93. P. 339-344. https://doi.org/10.1016/j.ijid.2020.03.031
- Bavli I., Sutton B., Galea S. Harms of public health interventions against covid-19 must not be ignored // Bmj. 2020. V. 371. https://doi.org/10.1136/bmj.m4074
- Iwasaki A., Grubaugh N. D. Why does Japan have so few cases of COVID‐19? // EMBO molecular medicine. 2020. V. 12. №5. P. e12481. https://doi.org/10.15252/emmm.202012481
- Felsenstein S., Herbert J. A., McNamara P. S., Hedrich C. M. COVID-19: Immunology and treatment options // Clinical immunology. 2020. V. 215. P. 108448. https://doi.org/10.1016/j.clim.2020.108448
- Vabret N., Britton G. J., Gruber C., Hegde S., Kim J., Kuksin M., Laserson U. Immunology of COVID-19: current state of the science // Immunity. 2020. V. 52. №6. P. 910-941. https://doi.org/10.1016/j.immuni.2020.05.002
- Chuan Q., Luoqi Z., Ziwei H., Shuoqi Z., Sheng Y., Yu T., Dai-Shi T. Dysregulation of immune response in patients with COVID-19 in Wuhan, China // Clin Infect Dis. 2020. V. 10.
- Tan M., Liu Y., Zhou R., Deng X., Li F., Liang K., Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China // Immunology. 2020. V. 160. №3. P. 261-268. https://doi.org/10.1111/imm.13223
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) // Frontiers in immunology. 2020. P. 827. https://doi.org/10.3389/fimmu.2020.00827
- Wan S., Xiang Y. I., Fang W., Zheng Y., Li B., Hu Y., Yang R. Clinical features and treatment of COVID‐19 patients in northeast Chongqing // Journal of medical virology. 2020. V. 92. №7. P. 797-806. https://doi.org/10.1002/jmv.25783
- Haveri A., Smura T., Kuivanen S., Österlund P., Hepojoki J., Ikonen N., Savolainen- Kopra C. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020 // Eurosurveillance. 2020. V. 25. №11. P. 2000266. https://doi.org/10.2807/1560-7917.ES.2020.25.11.2000266
- Lou B., Li T. D., Zheng S. F., Su Y. Y., Li Z. Y., Liu W., Chen Y. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset // European Respiratory Journal. 2020. V. 56. №2. https://doi.org/10.1183/13993003.00763-2020
- Wu Y. C., Chen C. S., Chan Y. J. The outbreak of COVID-19: An overview // Journal of the Chinese medical association. 2020. V. 83. №3. P. 217. https://doi.org/10.1097%2FJCMA.0000000000000270
- McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease // Autoimmunity reviews. 2020. V. 19. №6. P. 102537. https://doi.org/10.1016/j.autrev.2020.102537
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China // The lancet. 2020. V. 395. №10223. P. 497-506. https://doi.org/10.1016/S0140-6736(20)30183-5
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia // The Journal of infectious diseases. 2020. V. 221. №11. P. 1762-1769. https://doi.org/10.1093/infdis/jiaa150
- Tufan A., Güler A. A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposingantirheumatic drugs // Turkish journal of medical sciences. 2020. V. 50. №9. P. 620-632. https://doi.org/10.3906/sag-2004-168
- Crayne C. B., Albeituni S., Nichols K. E., Cron R. Q.The immunology of macrophage activation syndrome // Frontiers in immunology. 2019. V. 10. P. 119. https://doi.org/10.3389/fimmu.2019.00119
- Anka A. U., Tahir M. I., Abubakar S. D., Alsabbagh M., Zian Z., Hamedifar H., Azizi G. Coronavirus disease 2019 (COVID‐19): An overview of the immunopathology, serological diagnosis and management // Scandinavian journal of immunology. 2021. V. 93. №4. P. e12998. https://doi.org/10.1111/sji.12998
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID- 19 // Journal of infection. 2020. V. 80. №6. P. 607-613. https://doi.org/10.1016/j.jinf.2020.03.037
- Law H. K., Cheung C. Y., Ng H. Y., Sia S. F., Chan Y. O., Luk W., Lau Y. L. Chemokine up-regulation in SARS-coronavirus–infected, monocyte-derived human dendritic cells // Blood. 2005. V. 106. №7. P. 2366-2374. https://doi.org/10.1182/blood-2004-10-4166
- Tynell J., Westenius V., Rönkkö E., Munster V. J., Melen K., Österlund P., Julkunen, I. Middle East respiratory syndrome coronavirus shows poor replication but significant induction of antiviral responses in human monocyte-derived macrophages and dendritic cells // The Journal of general virology. 2016. V. 97. №Pt 2. P. 344. https://doi.org/10.1099%2Fjgv.0.000351
- Scheuplein V. A., Seifried J., Malczyk A. H., Miller L., Höcker L., Vergara-Alert J., Mühlebach M. D. High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus // Journal of virology. 2015. V. 89. №7. P. 3859-3869. https://doi.org/10.1128/jvi.03607-14
- Kim E. S., Choe P. G., Park W. B., Oh H. S., Kim E. J., Nam E. Y., Oh M. D. Clinical progression and cytokine profiles of Middle East respiratory syndrome coronavirus infection // Journal of Korean medical science. 2016. V. 31. №11. P. 1717-1725. https://doi.org/10.3346/jkms.2016.31.11.1717
- Wang C. H., Liu C. Y., Wan Y. L., Chou C. L., Huang K. H., Lin H. C., Kuo H. P. Persistence of lung inflammation and lung cytokines with high-resolution CT abnormalities during recovery from SARS // Respiratory research. 2005. V. 6. P. 1-12. https://doi.org/10.1186/1465-9921-6-42
- García-Sastre A., Biron C. A. Type 1 interferons and the virus-host relationship: a lesson in detente // Science. 2006. V. 312. №5775. P. 879-882. https://doi.org/10.1126/science.1125676
- Channappanavar R., Fehr A. R., Zheng J., Wohlford-Lenane C., Abrahante J. E., Mack, M., Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes // The Journal of clinical investigation. 2019. V. 129. №9. P. 3625-3639. https://doi.org/10.1172/JCI126363
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system // Cytokine & growth factor reviews. 2020. V. 53. P. 25-32. https://doi.org/10.1016/j.cytogfr.2020.05.003
- Channappanavar R., Fehr A. R., Vijay R., Mack M., Zhao J., Meyerholz D. K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice // Cell host & microbe. 2016. V. 19. №2. P. 181-193. http://dx.doi.org/10.1016/j.chom.2016.01.007
- Högner K., Wolff T., Pleschka S., Plog S., Gruber A. D., Kalinke U., Herold S. Macrophage-expressed IFN-β contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia // PLoS pathogens. 2013. V. 9. №2. P. e1003188. https://doi.org/10.1371/journal.ppat.1003188
- Rodrigue-Gervais I. G., Labbé K., Dagenais M., Dupaul-Chicoine J., Champagne C., Morizot A., Saleh M. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival // Cell host & microbe. 2014. V. 15. №1. P. 23-35. http://dx.doi.org/10.1016%2Fj.chom.2013.12.003
- Wu F., Zhao S., Yu B., Chen Y. M., Wang W., Song Z. G., Zhang Y. Z. A new coronavirus associated with human respiratory disease in China // Nature. 2020. V. 579. №7798. P. 265-269. https://doi.org/10.1038/s41586-020-2008-3
- Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Xu G. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study // American journal of
- respiratory and critical care medicine. 2020. V. 201. №11. P. 1372-1379. https://doi.org/10.1164/rccm.202003-0543OC
- Cao X. COVID-19: immunopathology and its implications for therapy // Nature reviews immunology. 2020. V. 20. №5. P. 269-270. https://doi.org/10.1038/s41577-020-0308-3
- Xu, Z., Shi, L., Wang, Y., Zhang, J., Huang, L., Zhang, C., ... & Wang, F. S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome // The Lancet respiratory medicine. 2020. V. 8. №4. P. 420-422. https://doi.org/10.1016/S2213-2600(20)30076-X
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China // Jama. 2020. V. 323. №11. P. 1061-1069. https://doi.org/10.1001/jama.2020.1585
- Li Y. X., Wu W., Yang T., Zhou W., Fu Y. M., Feng Q. M., Ye J. M. Characteristics of peripheral blood leukocyte differential counts in patients with COVID-19 // Zhonghua nei ke za zhi. 2020. V. 59. №5. P. 372-374. https://doi.org/10.3760/cma.j.cn112138-20200221-00114
- Lindsley A. W., Schwartz J. T., Rothenberg M. E. Eosinophil responses during COVID- 19 infections and coronavirus vaccination // Journal of Allergy and Clinical Immunology. 2020. V 146. №1. P. 1-7. https://doi.org/10.1016%2Fj.jaci.2020.04.021
- Tabachnikova A., Chen S. T. Roles for eosinophils and basophils in COVID-19? // Nature Reviews Immunology. 2020. V. 20. №8. P. 461-461. https://doi.org/10.1038/s41577-020-0379-1
- Xiang-Hua Y., Le-Min W., Ai-Bin L., Zhu G., Riquan L., Xu-You Z., Ye-Nan W.Severe acute respiratory syndrome and venous thromboembolism in multiple organs // American journal of respiratory and critical care medicine. 2010. V. 182. №3. P. 436-437. https://doi.org/10.1164/ajrccm.182.3.436
- Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19 // New England Journal of Medicine. 2020. V. 382. №17. P. e38. https://doi.org/10.1056/NEJMc2007575
- Levi M., Nieuwdorp M., van der Poll T., Stroes E. Metabolic modulation of inflammation-induced activation of coagulation // Seminars in thrombosis and hemostasis // Thieme Medical Publishers. 2008. V. 34. №01. P. 026-032. https://doi.org/10.1055/s-2008-1066020
- Imai Y., Kuba K., Neely G. G., Yaghubian-Malhami R., Perkmann T., van Loo G., Penninger J. M. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury // Cell. 2008. V. 133. №2. P. 235-249. https://doi.org/10.1016/j.cell.2008.02.043
- Hamming I., Timens W., Bulthuis M. L. C., Lely A. T., Navis G. V., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis // The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2004. V. 203. №2. P. 631-637. https://doi.org/10.1002/path.1570
- Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2 // Cardiovascular research. 2020. V. 116. №6. P. 1097-1100. https://doi.org/10.1093/cvr/cvaa078
- CDC Covid-19 Response Team et al. Coronavirus disease 2019 in children—United States, february 12–april 2, 2020 // Morbidity and Mortality Weekly Report. 2020. V. 69. №14. P. 422-426.
- Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study // The Lancet. 2020. V. 395. №10239. P. 1771-1778. https://doi.org/10.1016/S0140-6736(20)31103-X
- Viner R. M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic // The Lancet. 2020. V. 395. №10239. P. 1741-1743. https://doi.org/10.1016/S0140-6736(20)31129-6
- Kato H., Sugimura T., Akagi T., Sato N., Hashino K., Maeno Y., Yamakawa R. Longterm consequences of Kawasaki disease: a 10-to 21-year follow-up study of 594 patients // Circulation. 1996. V. 94. №6. P. 1379-1385. https://doi.org/10.1161/01.CIR.94.6.1379
- Aykac K., Ozsurekci Y., Yayla B. C. C., Gurlevik S. L., Oygar P. D., Bolu N. B., Ceyhan M. Oxidant and antioxidant balance in patients with COVID‐19 // Pediatric pulmonology. 2021. V. 56. №9. P. 2803-2810. https://doi.org/10.1002/ppul.25549
- Cecchini R., Cecchini A. L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression // Medical hypotheses. 2020. V. 143. P. 110102. https://doi.org/10.1016/j.mehy.2020.110102
- Pincemail J., Cavalier E., Charlier C., Cheramy–Bien J. P., Brevers E., Courtois A., Rousseau A. F. Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A pilot study // Antioxidants. 2021. V. 10. №2. P. 257. https://doi.org/10.3390/antiox10020257
- Karkhanei B., Ghane E. T., Mehri F. Evaluation of oxidative stress level: Total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19 // New Microbes and New Infections. 2021. V. 42. P. 100897. https://doi.org/10.1016/j.nmni.2021.100897
- Gadotti A. C., Lipinski A. L., Vasconcellos F. T., Marqueze L. F., Cunha E. B., Campos A. C., Pinho R. A. Susceptibility of the patients infected with Sars-Cov2 to oxidative stress and possible interplay with severity of the disease // Free Radical Biology and Medicine. 2021. V. 165. P. 184-190. https://doi.org/10.1016/j.freeradbiomed.2021.01.044
- Martín-Fernández M., Aller R., Heredia-Rodríguez M., Gómez-Sánchez E., Martínez- Paz P., Gonzalo-Benito H., Tamayo-Velasco Á. Lipid peroxidation as a hallmark of severity in COVID-19 patients // Redox Biology. 2021. V. 48. P. 102181. https://doi.org/10.1016/j.redox.2021.102181
- Sena C. M., Leandro A., Azul L., Seica R., Perry G. Vascular oxidative stress: impact and therapeutic approaches // Frontiers in physiology. 2018. V. 9. P. 1668. https://doi.org/10.3389/fphys.2018.01668
- Suhail S., Zajac J., Fossum C., Lowater H., McCracken C., Severson N., Hati S.. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review // The protein journal. 2020. V. 39. P. 644-656. https://doi.org/10.1007/s10930-020-09935-8
- Lovren F., Pan Y., Quan A., Teoh H., Wang G., Shukla P. C., Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis // American Journal of Physiology-Heart and Circulatory Physiology. 2008. V. 295. №4. P. H1377-H1384. https://doi.org/10.1152/ajpheart.00331.2008
- Daiber A., Hahad O., Andreadou I., Steven S., Daub S., Münzel T. Redox-related biomarkers in human cardiovascular disease-classical footprints and beyond // Redox biology.2021. V. 42. P. 101875. https://doi.org/10.1016/j.redox.2021.101875
- Chen X., Kang R., Kroemer G., Tang D. Broadening horizons: the role of ferroptosis in cancer // Nature reviews Clinical oncology. 2021. V. 18. №5. P. 280-296. https://doi.org/10.1038/s41571-020-00462-0
- Chang Y. T., Chang W. N., Tsai N. W., Huang C. C., Kung C. T., Su Y. J., Lu C. H. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer’s disease: a systematic review // BioMed research international. 2014. V. 2014. https://doi.org/10.1155/2014/182303
- Notarnicola M., Osella A. R., Caruso M. G., Pesole P. L., Lippolis A., Tutino V., Messa C. Nonalcoholic fatty liver disease: Focus on new biomarkers and lifestyle interventions // International Journal of Molecular Sciences. 2021. V. 22. №8. P. 3899. https://doi.org/10.3390/ijms22083899
- Gonzalo Benito H., Brieva Ruiz L., Tatzber F., Jové Font M., Cacabelos Barral D., Cassanyé A., Portero Otín M. Lipidome analysis in multiple sclerosis reveals protein lipoxidative damage as a potential pathogenic mechanism // Journal of Neurochemistry. 2012. V. 123. №4. P. 622-634. http://hdl.handle.net/10459.1/58574
- Paliogiannis P., Fois A. G., Sotgia S., Mangoni A. A., Zinellu E., Pirina P., Zinellu A.Circulating malondialdehyde concentrations in patients with stable chronic obstructive pulmonary disease: A systematic review and meta-analysis // Biomarkers in Medicine. 2018. V. 12. №7. P. 771-781. https://doi.org/10.2217/bmm-2017-0420
- Chan-Yeung M., Xu R. H. SARS: epidemiology. Respirology 8 (Suppl): S9–S14. 2003.
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor // Cell. 2020. V. 181. №2. P. 271-280. e8. https://doi.org/10.1016/j.cell.2020.02.052
- Magalhães G. S., Rodrigues‐Machado M. G., Motta‐Santos D., Silva A. R., Caliari M. V., Prata L. O., Campagnole‐Santos M. J. A ngiotensin‐(1‐7) attenuates airway remodelling and hyperresponsiveness in a model of chronic allergic lung inflammation // British Journal of Pharmacology. 2015. V. 172. №9. P. 2330-2342. https://doi.org/10.1111/bph.13057
- Chen Q., Yang Y., Huang Y., Pan C., Liu L., Qiu H. Angiotensin-(1-7) attenuates lung fibrosis by way of Mas receptor in acute lung injury // Journal of surgical research. 2013. V. 185. №2. P. 740-747. https://doi.org/10.1016/j.jss.2013.06.052
- Li Y., Cao Y., Zeng Z., Liang M., Xue Y., Xi C., Jiang W. Angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis prevents lipopolysaccharide–induced apoptosis of pulmonary microvascular endothelial cells by inhibiting JNK/NF–κB pathways // Scientific reports. 2015. V. 5. №1. P. 8209. https://doi.org/10.1038/srep08209
- Meng Y., Yu C. H., Li W., Li T., Luo W., Huang S., Li X. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway // American journal of respiratory cell and molecular biology. 2014. V. 50. №4. P. 723-736. https://doi.org/10.1165/rcmb.2012-0451OC
- Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase // Journal of Biological Chemistry. 2002. V. 277. №17. P. 14838-14843. https://doi.org/10.1074/jbc.M200581200
- Sodhi C. P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W. B., Wang S., Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration // American Journal of Physiology-Lung Cellular and Molecular Physiology. 2018. V. 314. №1. P. L17-L31. https://doi.org/10.1152/ajplung.00498.2016
- Fraga-Silva R. A., Costa-Fraga F. P., Sousa F. B. D., Alenina N., Bader M., Sinisterra R. D., Santos R. A. An orally active formulation of angiotensin-(1-7) produces an antithrombotic effect // Clinics. 2011. V. 66. P. 837-841. https://doi.org/10.1590/S1807-59322011000500021
- Marques F. D., Ferreira A. J., Sinisterra R. D., Jacoby B. A., Sousa F. B., Caliari M. V., Santos R. A. An oral formulation of angiotensin-(1-7) produces cardioprotective effects in infarcted and isoproterenol-treated rats // Hypertension. 2011. V. 57. №3. P. 477-483. https://doi.org/10.1161/HYPERTENSIONAHA.110.167346
- Fraga-Silva R. A., Pinheiro S. V. B., Gonçalves A. C. C., Alenina N., Bader M., Souza Santos R. A. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets // Molecular Medicine. 2008. V. 14. P. 28-35. https://doi.org/10.2119/2007-00073.Fraga-Silva
- Zhang H., Penninger J. M., Li Y., Zhong N., Slutsky A. S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target // Intensive care medicine. 2020. V. 46. P. 586-590. https://doi.org/10.1007/s00134-020-05985-9
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Penninger J. M. crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury // Nature medicine. 2005. V. 11. №8. P. 875-879. https://doi.org/10.1038/nm1267
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Penninger J. M. Angiotensinconverting enzyme 2 protects from severe acute lung failure // Nature. 2005. V. 436. №7047. P. 112-116. https://doi.org/10.1038/nature03712
- Xie X., Chen J., Wang X., Zhang F., Liu Y. Erratum to “Age-and gender-related difference of ACE2 expression in rat lung” // Life Sciences. 2006. V. 26. №79. P. 2499. http://dx.doi.org/10.1016%2Fj.lfs.2006.09.028
- Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection // European journal of internal medicine. 2020. V. 76. P. 14-20.
- Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. 718 p following 728. https://doi.org/10.1016/j.ejim.2020.04.037
- Trask A. J., Averill D. B., Ganten D., Chappell M. C., Ferrario C. M. Primary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1–7) in transgenic Ren-2 hypertensive rats // American Journal of Physiology-Heart and Circulatory Physiology. 2007. V. 292. №6. P. H3019-H3024. https://doi.org/10.1152/ajpheart.01198.2006
- Mehta P., McAuley D. F., Brown M., Sanchez E., Tattersall R. S., Manson J. J. COVID- 19: consider cytokine storm syndromes and immunosuppression // The lancet. 2020. V. 395. №10229. P. 1033-1034. https://doi.org/10.1016/S0140-6736(20)30628-0
- Akhmerov A., Marbán E. COVID-19 and the heart // Circulation research. 2020. V. 126. №10. P. 1443-1455. https://doi.org/10.1161/CIRCRESAHA.120.317055
- Кривошеев В. В., Столяров А. И. Атмосферное давление и COVID-19 // Санитарный врач. 2021. №7. С. 8-17. EDN: KGDRBD. https://doi.org/10.33920/med-08-2107-01
- Pirouz B., Shaffiee Haghshenas S., Shaffiee Haghshenas S., Piro P. Investigating a serious challenge in the sustainable development process: analysis of confirmed cases of COVID-19 (new type of coronavirus) through a binary classification using artificial intelligence and regression analysis // Sustainability. 2020. V. 12. №6. P. 2427. https://doi.org/10.3390/su12062427
- Adhikari A., Yin J. Short-term effects of ambient ozone, PM2. 5, and meteorological factors on COVID-19 confirmed cases and deaths in Queens, New York // International journal of environmental research and public health. 2020. V. 17. №11. P. 4047. https://doi.org/10.3390/ijerph17114047
- Li K. The link between humidity and COVID-19 caused death // Journal of Biosciences and Medicines. 2020. V. 8. №6. P. 50-55. https://doi.org/10.4236/jbm.2020.86005
- Bashir M. F., Ma B., Komal B., Bashir M. A., Tan D., Bashir M. Correlation between climate indicators and COVID-19 pandemic in New York, USA // Science of the Total Environment. 2020. V. 728. P. 138835. https://doi.org/10.1016/j.scitotenv.2020.138835
- Gupta A., Banerjee S., Das S. Significance of geographical factors to the COVID-19 outbreak in India // Modeling earth systems and environment. 2020. V. 6. P. 2645-2653. https://doi.org/10.1007/s40808-020-00838-2
- Pani S. K., Lin N. H., RavindraBabu S. Association of COVID-19 pandemic with meteorological parameters over Singapore // Science of the Total Environment. 2020. V. 740. P. 140112. https://doi.org/10.1016/j.scitotenv.2020.140112
- Cai Q. C., Lu J., Xu Q. F., Guo Q., Xu D. Z., Sun Q. W., Jiang Q. W. Influence of meteorological factors and air pollution on the outbreak of severe acute respiratory syndrome // Public health. 2007. V. 121. №4. P. 258-265. https://doi.org/10.1016/j.puhe.2006.09.023
- Kumar G., Kumar R. R. A correlation study between meteorological parameters and COVID-19 pandemic in Mumbai, India // Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020. V. 14. №6. P. 1735-1742. https://doi.org/10.1016/j.dsx.2020.09.002
- Deyal N., Tiwari V., Bisht N. Impact of climatic parameters on COVID-19 pandemic progression in India: analysis and prediction. 2020. https://doi.org/10.1101/2020.07.25.20161919
- Leung N. Y., Bulterys M. A., Bulterys P. L. Predictors of COVID-19 incidence, mortality, and epidemic growth rate at the country level // MedRxiv. 2020. P. 2020.05. 15.20101097. https://doi.org/10.1101/2020.05.15.20101097
- Jaakkola K., Saukkoriipi A., Jokelainen J., Juvonen R., Kauppila J., Vainio O. Decline in temperature and humidity increases the occurrence of influenza in cold climate // Environmental Health. 2014. V. 13. P. 1-8. https://doi.org/10.1186/1476-069X-13-22
- Liu J., Zhou J., Yao J., Zhang X., Li L., Xu X., Zhang K. (Impact of meteorological factors on the COVID-19 transmission: A multi-city study in China // Science of the total environment. 2020. V. 726. P. 138513. https://doi.org/10.1016/j.scitotenv.2020.138513
- Luo W., Majumder M. S., Liu D., Poirier C., Mandl K. D., Lipsitch M., Santillana M. The role of absolute humidity on transmission rates of the COVID-19 outbreak // MedRxiv. 2020. P. 2020.02. 12.20022467. https://doi.org/10.1101/2020.02.12.20022467
- Briz-Redón Á., Serrano-Aroca Á. A spatio-temporal analysis for exploring the effect of temperature on COVID-19 early evolution in Spain // Science of the total environment. 2020. V. 728. P. 138811. https://doi.org/10.1016/j.scitotenv.2020.138811


