Сравнительный анализ методов полностью эндоскопической декомпрессии плечевого сплетения и мини-инвазивной техники с эндоскопической ассистенцией в лечении пациентов с травматической брахиоплексопатией
Автор: Беляк Е. А., Сагдиев Р. Х., Лазко Ф. Л., Суфианов А. А., Пасхин Д. Л., Призов А. П., Лазко М. Ф., Загородний Н. В.
Журнал: Гений ортопедии @geniy-ortopedii
Рубрика: Оригинальные статьи
Статья в выпуске: 2 т.30, 2024 года.
Бесплатный доступ
Введение. Брахиоплексопатия является актуальным полиэтиологичным заболеванием со встречаемостью, по данным разных авторов, от 0,17 до 1,6 на 100 тыс. населения в год. Существуют две основные эндоскопические методики лечения брахиоплексопатии путем невролиза плечевого сплетения: мини-инвазивный трансаксиллярный метод с эндоскопической ассистенцией и полностью эндоскопический невролиз в ассоциации с артроскопией плечевого сустава.Цель работы - сравнить два основных существующих на данный момент метода проведения невролиза плечевого сплетения с использованием эндоскопа.Материалы и методы. В исследование включено 22 пациента с установленным диагнозом «посттравматическая брахиоплексопатия»: в группу 1 - 8 пациентов, в группу 2 - 14. Всем пациентам проведено клинические и инструментальные обследования. Статистический анализ проведен с использованием непараметрического U-критерия Манна - Уитни. Различия считали значимыми при p
Брахиоплексопатия, декомпрессия, невролиз, эндоскопия, артроскопия, плечевой сустав, синдром грудной апертуры
Короткий адрес: https://sciup.org/142240815
IDR: 142240815 | УДК: 616.833.34-009.7-089.853 | DOI: 10.18019/1028-4427-2024-30-2-171-181
Текст научной статьи Сравнительный анализ методов полностью эндоскопической декомпрессии плечевого сплетения и мини-инвазивной техники с эндоскопической ассистенцией в лечении пациентов с травматической брахиоплексопатией
Brachioplexopathies (BPPs) are a group of conditions of various etiologies accompanied by damage to the brachial plexus at any level that leads to dysfunction of the upper limb due to the development of motor, sensory and autonomic disorders. According to recent studies and of different authors, the incidence of BPP varies from 0.17 to 1.6 per 100,000 per year [1–7]. Etiology includes both trauma, open and closed, as well as non-traumatic causes, including congenital anomalies, post-radiation plexitis, and idiopathic BPP. It is generally accepted that when symptoms of BPP develop, neuroimaging should be performed to verify the presence or absence of anatomical integrity break of the nervous structures. If there is an anatomical break in the nerve, its spontaneous recovery is considered impossible and requires immediate surgical treatment involving plastic surgery or neurotization of the damaged nerves [8–10]. If the break of anatomical integrity of the nerves is not reliably verified, it is believed that it is possible to restore conduction along the nerves with conservative treatment for 3–6 months, and if recovery does not occur within this period of time, surgical treatment should be performed [11–13]. One of the options for surgical treatment in such cases is neurolysis, the essence of which is to free the nerves from scars and adhesions. It can be carried out using low-invasive endoscopic techniques. Over the past two decades, this operation has developed, and two main approaches to its implementation have emerged:
-
• neurolysis of the brachial plexus under video-endoscopic assistance [14–16];
-
• completely endoscopic neurolysis of the brachial plexus, including robot-assisted [17–20].
A few published works prove the effectiveness of both methods, but their comparison has not been previously carried out, which determines the relevance of this study.
The purpose was to compare the two main currently used methods for performing neurolysis of the brachial plexus using an endoscope.
MATERIALS AND METHODS
Our study included 22 patients (18 men, 4 women) diagnosed with post-traumatic brachioplexopathy established on the basis of complaints, anamnesis, examination data and instrumental research methods. They underwent surgery at the Department of Traumatology and Orthopaedics of the Buyanov City Clinical Hospital (Moscow) and the Department of Neurosurgery No. 5 of the Federal Center for Neurosurgery (Tyumen) from 2015 to 2022. The study was retrospective in nature.
Criteria for inclusion of patients in the study:
-
1) verified diagnosis of post-traumatic brachioplexopathy based on complaints, anamnesis, and instrumental research data ;
-
2) absence of reliable signs of anatomical integrity break of the brachial plexus structures according to examination findings;
-
3) incomplete restoration of affected limb function after adequate conservative treatment for 6 months or more prior to hospitalization;
-
4) significant impairment of upper limb function, impairing the patient’s quality of life;
-
5) patient’s age 18 years and older.
Criteria for non-inclusion of patients into the study:
-
1) non-traumatic nature of the brachial plexus injury;
-
2) muscle paresis according to the British scale < 2 points;
-
3) patient’s refusal to participate in the study.
Group 1 included 8 patients who underwent arthroscopy of the shoulder joint according to indications and all endoscopic decompression of the brachial plexus. Group 2 included 14 patients who underwent revision and neurolysis of the brachial plexus under video-endoscopic assistance.
All patients underwent examinations according to generally accepted standards prior to hospitalization. A neurological examination was carried out that determined muscle strength according to the British Medical Research Council Scale (BMRC, M5–M0). The following scales and questionnaires were used: the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire, the Numerical Pain Rating Scale (NRS) [21]. Stimulation ENMG and MRI of the brachial plexus were performed to exclude violations of the anatomical integrity of its structures.
Statistical data processing was carried out using the Microsoft Excel (Microsoft Office 365) and Stattech 2.0 software packages. For quantitative signs, the arithmetic mean (M) and standard error of the mean (SEM) were calculated. To assess the statistical significance of the results obtained, the Shapiro – Wilk test was used to assess the normality of the distribution of the parameter; the nonparametric Mann – Whitney U test was used in non-normal distribution. Differences were considered significant at p < 0.05. The resulting non-integer values were rounded to the nearest tenth.
Surgical technique
Surgical intervention in group 1
The operation was performed with the patient in the “beach chair” position under endotracheal anesthesia. The first stage included therapeutic and diagnostic arthroscopy of the shoulder joint with examination of intra-articular structures and identification of intra-articular pathology. If signs of chronic tenosynovitis of the long head of the biceps tendon (LHBT) were detected, tenotomy was performed. If degenerative changes in the fibrous labrum of the glenoid were detected, debridement of the changed areas of the fibrous labrum was performed. Areas of chondromalacia of the articular cartilage of the humeral head and glenoid were treated with a shaver and ablator. If degenerative changes in the rotator cuff tendons (RCT) were detected, debridement of the changed sections of the RC was performed. If a massive irreparable injury to the supraspinatus tendon was detected, a subacromial spacer was installed (Fig. 1).
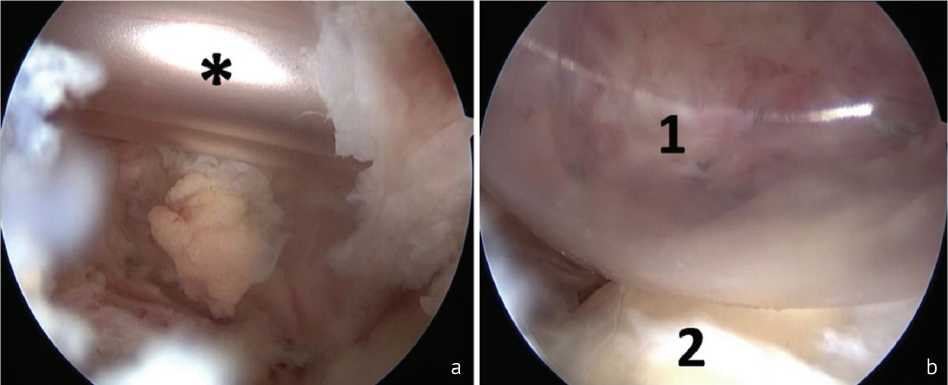
Fig. 1 Installation of a subacromial spacer in a massive injury to the rotator cuff: a spacer (*) inserted in a folded state; b spacer (1) straightened, head of the humerus (2)
Next, the tissues of the rotator interval were excised, the coracoid process of the scapula was visualized, and the tissues around the coracoid process were released, identifying the coracoacromial ligament, conjoint tendon, and pectoralis minor muscle. Further on, the pectoralis minor muscle was cut off from the coracoid process and displaced medially, which was a decompression component of the neurovascular bundle in the area of the pectoralis minor muscle. Through the “window” thus formed, tissue dissection was carried out and the components of the brachial plexus and vessels were visualized. By cutting the scaradhesive tissue in that area, neurolysis and decompression of the neurovascular bundle were performed (Fig. 2).
Next, tissue dissection was performed at the base of the coracoid process and medially from it. The subclavian muscle was visualized and the lateral portion of the muscle was cut off from the clavicle, forming a “window” to the thoracic outlet. Next, tissue dissection was performed in the area of the thoracic outlet, dissecting the scar-adhesive tissue around the plexus and between its components, and the BP components and the subclavian artery were visualized (Fig. 3).
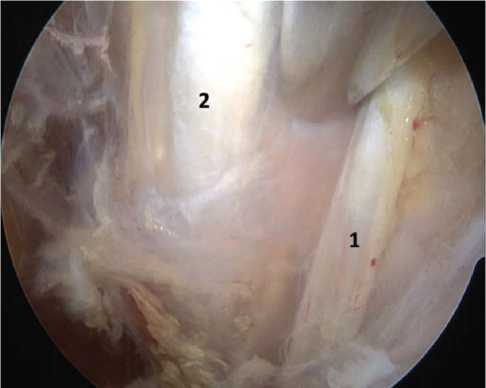
Fig. 2 Musculocutaneous nerve (1) and median nerve (2) in the area of the coracoid process after cutting off the pectoralis minor muscle
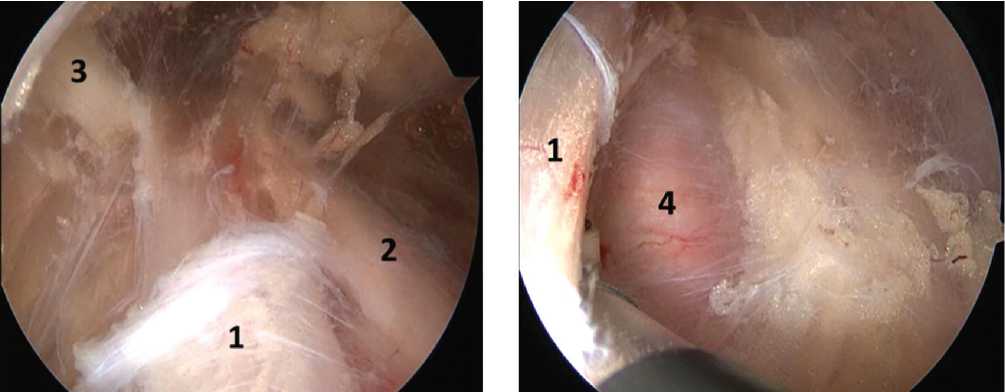
Fig. 3 Components of the brachial plexus (BP) in the area of the thoracic outlet after decompression: (1) is the upper BP trunk, (2) is the posterior division of the upper BP trunk, (3) is the suprascapular nerve, and (4) is the subclavian artery
Next, supraclavicular ports were formed, into which the arthroscope and working instrument were transferred (Fig. 7); tissue dissection was performed, and the interscalene space was accessed. The scar-adhesive tissues around the trunks of the brachial plexus and between them were dissected, thus achieving decompression and neurolysis. The upper, middle and lower BP trunks, the subclavian artery and the middle scalene muscle were visualized (Fig. 4, 5, 6).
The final stage of the operation was to suture the postoperative wounds, apply aseptic dressings, and immobilize the upper limb in a head scarf orthosis (Fig. 7).
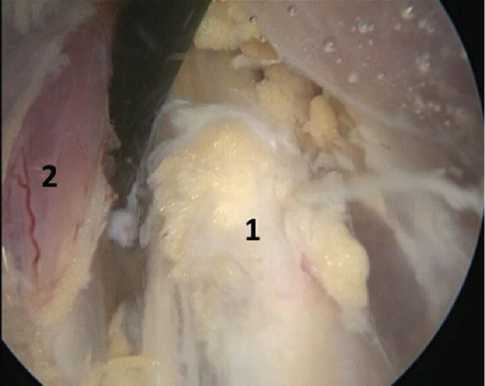
Fig. 4 Area of the interscalene space, upper trunk of the brachial plexus (1), middle scalene muscle (2)
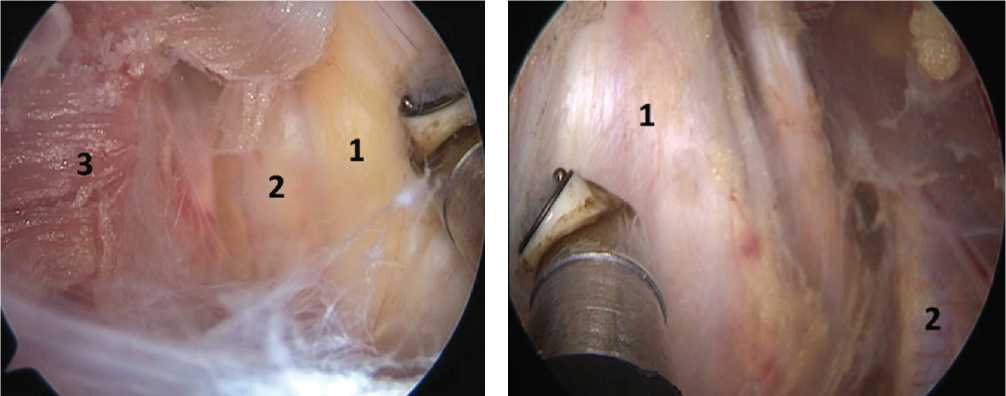
Fig. 6 Relative position of the superior trunk of the brachial plexus (1) and the subclavian artery (2) in the area of the interscalene space
Fig. 5 Components of the brachial plexus (BP) after decompression: (1) is the middle BP trunk, (2) is the lower BP trunk, (3) is middle scalene muscle
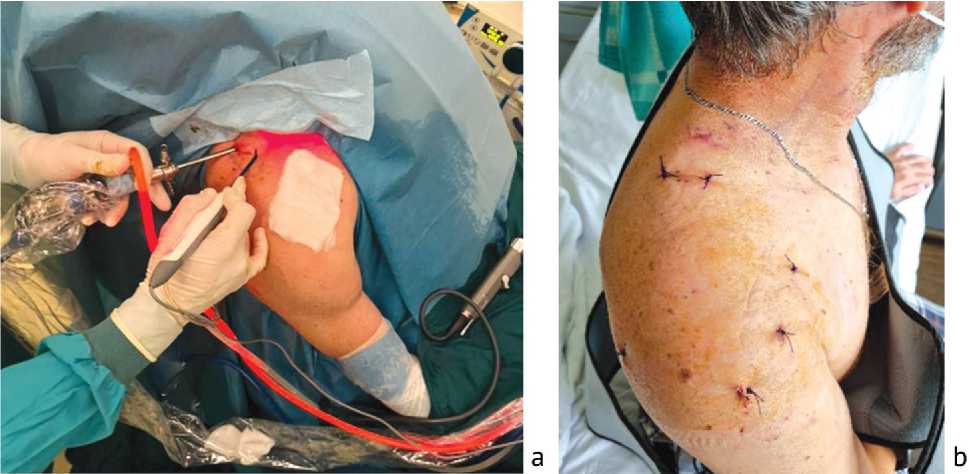
Fig. 7 View of intraoperative location of instruments ( a ); postoperative endoscopic approaches ( b )
Surgical intervention in group 2
Under endotracheal anesthesia, with the patient lying on the back with the arm retracted to the side, an incision was made in the armpit in the skin fold so that the projection of the neurovascular bundle of the shoulder was located in the center of the incision (Fig. 8).
After opening the axillary fascia, the neurovascular bundle was exposed; nerves, arteries and veins were identified in it, and they were fixed on holders. Next, a retractor with optics was inserted into the wound parallel to the neurovascular bundle. Further actions were carried out under the control of endoscopic optics and with the help of the instruments for endoneurolysis.
Dissection of neurovascular structures was carried out in the proximal direction until the costoclavicular space was reached. Then the upper limb on the side under study was moved by the assistant upwards by the shoulder and held in this position (Fig. 9), which ensured protraction of the clavicle and expansion of the costoclavicular space (Fig. 10).
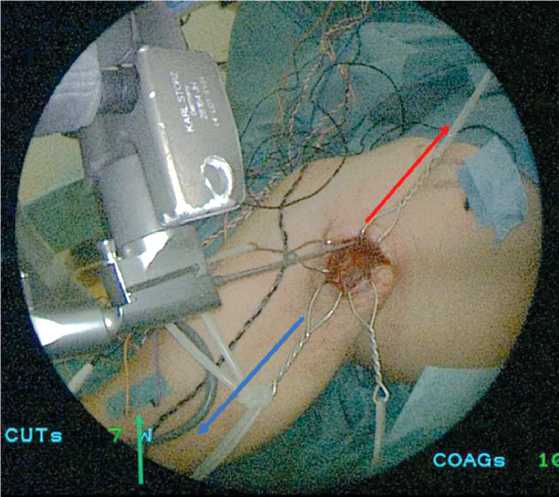
Fig. 8 View of the wound before inserting the endoscope into the endoscopic retractor. The red arrow indicates the proximal direction, the blue arrow indicates the distal direction. The green arrow indicates electrodes for neurophysiological monitoring
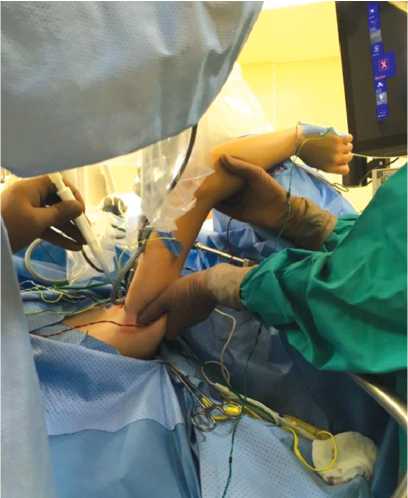
Fig. 9 Traction of the upper limb on the studied side upwards; the assistant holds the arm in the achieved position
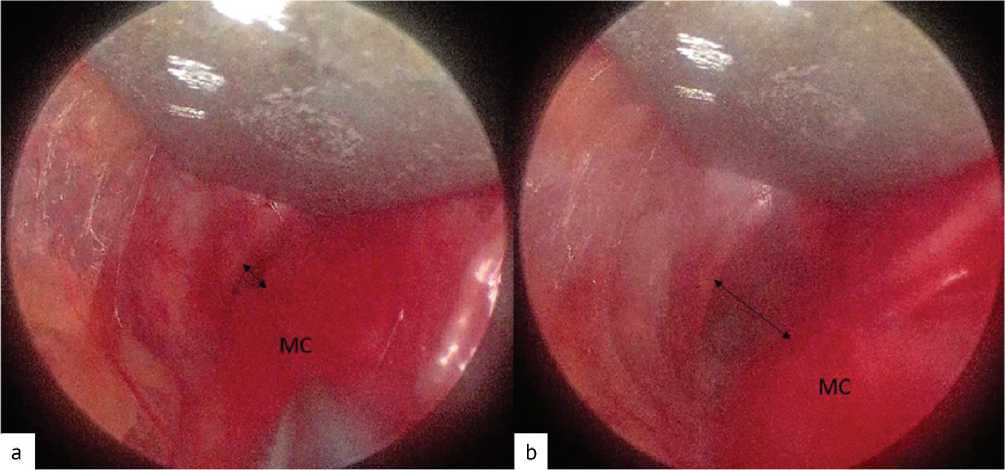
Fig. 10 Endoscopic view of the costoclavicular space before traction of the upper limb ( a ) and at the moment of traction ( b ). The black arrow indicates the costoclavicular space, MC — medial fascicle
An endoscopic retractor passed behind the clavicle followed by revision and neurolysis of the supraclavicular part of the brachial plexus which was carried out to the point where the roots entered the intervertebral foramina. Gradually moving the endoscopic retractor along the neurovascular structures, the adhesions were separated and the brachial plexus structures were freed from the surrounding tissues, starting distally at the level of the branch of the terminal branches and ending proximally, at the point where the roots entered the intervertebral foramina (Fig. 11). Neurophysiological monitoring conducted during the operation.
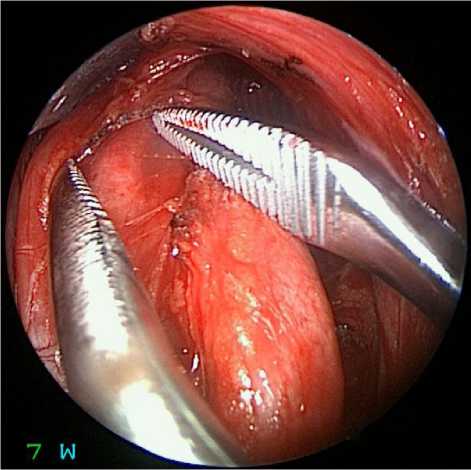
Fig. 11 Endoscopic view (diameter of the working part 4 mm, angle of direction of the optics 0 degrees) of the structures of the brachial plexus during the main stage of the operation
Interpretation of possible results
The results of treatment were assessed based on data from scales and questionnaires, a neurological examination, and were confirmed by functional diagnostic data (stimulation ENMG) 6 months after surgery. During the neurological examination, two parameters were assessed separately: strength and pain. An increase in strength in the affected muscles by 1 point or more was considered a positive result. Pain was assessed using the BMRC scale; a decrease in pain by 50 % or more was considered a positive result.
RESULTS
In group 1, positive treatment results were achieved in 87.5 % of cases ( n = 7). In one case, a positive result was not achieved; no deterioration of the condition was observed after the operation. In group 2, positive treatment results were achieved in 92.9 % of cases ( n = 13). In one case, a positive result was not achieved; no deterioration of the condition was observed after the operation. The results of patient treatment are presented in Table 1.
Table 1
Data of assessment before and after surgery
|
Scales of assessment |
Before surgery (М ± SEM) |
6 months after surgery (М ± SEM) |
p -value |
|||
|
group 1 |
group 2 |
group 1 |
group 2 |
group 1 |
group 2 |
|
|
BMRC grade of paresis |
3.1 ± 0.3 |
2.6 ± 0.7 |
4.4 ± 0.3 |
3.7 ± 1.0 |
< 0.05 |
< 0.05 |
|
NRS |
6.8 ± 1.1 |
2.6 ± 1.0 |
1.9 ± 0.6 |
0.4 ± 0.3 |
< 0.05 |
< 0.05 |
|
DASH |
52.3 ± 2.2 |
47.9 ± 4.4 |
28.8 ± 3.8 |
26.6 ± 4.3 |
< 0.05 |
< 0.05 |
Due to significant differences in surgical techniques, comparisons were made using the universal DASH scale. To do this, the difference in the indicator before and after surgery in each group was calculated, and then the arithmetic mean and standard error of the mean were calculated for the data obtained. In the first group, the value (M ± SEM) was 23.5 ± 3.6, in the second group — 19.4 ± 5.4, with p > 0.05.
According to stimulation ENMG data, patients noted a reduction in the latent period and an increase in the amplitude of the M-response (Fig. 12, 13).
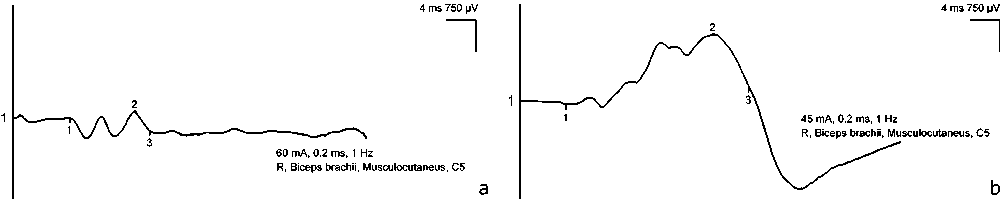
Fig. 12 Amplitude of the M-response upon stimulation of the musculocutaneous nerve in patient G. (male, 38 years old, endoscopic neurolysis group) before ( a ) and 6 months after surgery ( b ) (significant increase is noted)
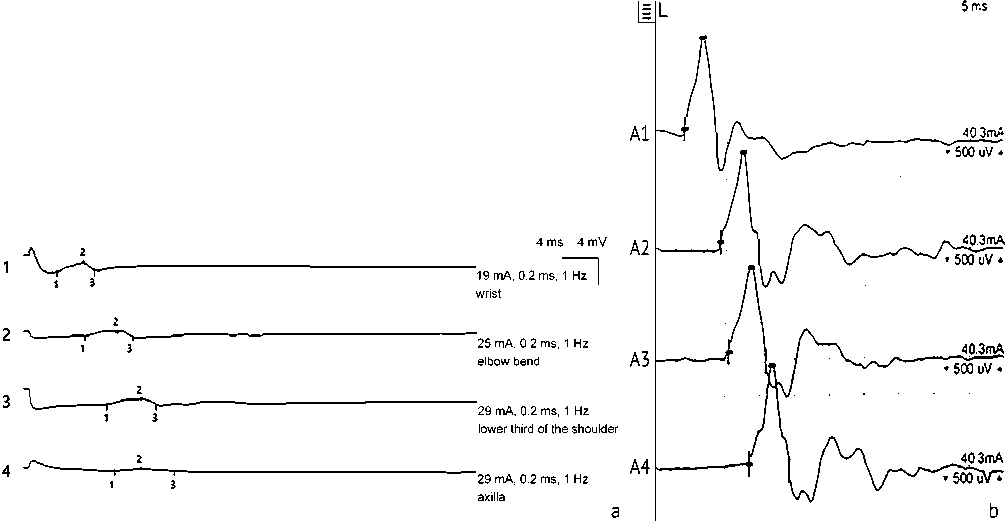
Fig. 13 Increase in the amplitude of the M-response during stimulation electroneuromyography of the brachial plexus in patient P. (male, 53 years old, group with endoscopic assistance) before ( a ) and after 6 months after surgery ( b )
DISCUSSION
The current principles of surgery of peripheral nerves and the brachial plexus have established after the introduction of minimally invasive methods in peripheral nerve surgery in the 60s of the last century. Since then, the main directions in this area are:
-
• neurotization of the proximal parts of the brachial plexus with nerves passing near them, such as accessory, phrenic, intercostal, and other nerves;
-
• neurotization of the terminal and short branches of the brachial plexus with the nerves functioning nearby, and namely, neurotization of the median nerve by the radial nerve, neurotization of the musculocutaneous nerve by the ulnar nerve, etc.;
-
• muscle transposition.
The role of brachial plexus neurolysis as a self-sufficient method of surgical treatment has not been taken seriously for a long time. It was considered, rather, as one of the stages of plastic surgery or neurotization of nerves, allowing injury analysis, final diagnosis of the injury to the nerve structures and determination of their level [22].
In 1973, a literature review was published that provided information on the effectiveness of brachial plexus neurolysis and stated that neurolysis is an effective treatment for local fibrotic lesions, but is not considered a panacea [23].
The introduction of endoscopic technology into the arsenal of methods for the surgical treatment of peripheral neuropathies started not long ago, in the 80s of the 20th century, when it was proposed for the first time to decompress the median nerve at the level of the carpal tunnel under arthroscope control [24]. The use of an endoscope in surgery on the brachial plexus itself was proposed later, in the 90s, when an experiment was conducted for the first time to examine the roots of the brachial plexus under endoscopic assistance [14]. Later, Krishnan et al., having conducted studies on corpses, was one of the first to propose a revision of the brachial plexus as a diagnostic operation aimed at identifying the severity of brachial plexus injury and planning further treatment tactics. In addition, the main anatomical landmarks were identified when it is performed through the supraclavicular and subclavian approaches [14]. A similar study, but with the use of robotic technology, was carried out by Mantovani et al. in 2011 on two brachial plexuses on one fresh cadaver [17].
The first case of endoscopic revision of the brachial plexus on a living person was described in the literature in 2006. A patient with a closed brachial plexus injury due to an accident underwent this operation after which there was a complete restoration of strength and sensitivity in the affected arm 6 months later [15]. Subsequently in 2007, it was first proposed to perform resection of the first rib for upper outlet syndrome that was carried out since 1910 under video endoscopic assistance [26]. In 2017, Lafosse et al. offered an original method for performing neurolysis of all parts of the brachial plexus for upper thoracic outlet syndrome [18]. A little later, in 2020, the same group of scientists proved the effectiveness of this method in the treatment of brachioplexopathies in adults resulting from dislocation of the shoulder joint, and proposed an algorithm for the management of patients with this pathology [19]. In 2021, a group of scientists proposed a method of endoscopic revision and neurolysis of the brachial plexus, which allows, if necessary, to carry out interventions on the shoulder joint, which is relevant for patients with concomitant pathology [20]. In 2023, positive results were published in treated patients with brachioplexopathies of various etiologies using the method of neurolysis under video endoscopic assistance, which allows the operation to be performed through one transaxillary approach, especially when the technique for expanding the costoclavicular space [16, 27].
Despite the development of the endoscopic treatment of patients with brachioplexopathies, its widespread use is still limited. In the latest, most comprehensive, guidelines for the management and surgical treatment of patients with brachioplexopathies dated issued in 2021, brachial plexus neurolysis is shown only in a historical aspect, and its use with an endoscope is not described at all [21].
Based on the studies described above, it is currently possible to divide the methods of endoscopic neurolysis of the brachial plexus into several types:
-
• neurolysis of the brachial plexus under video endoscopic assistance;
-
• all-endoscopic neurolysis of the brachial plexus, including robot-assisted.
Due to the emergence of different options for performing brachial plexus neurolysis operations using an endoscope and positive results, we can conclude that there is no optimal generally accepted method of treatment and that this area of surgery is actively developing.
In general, based on the study, it follows that neurolysis using an endoscope is a low-traumatic method of treating post-traumatic brachioplexopathies in patients with not deep paresis in the affected muscle groups when conservative treatment appeared ineffective. In this study, the results of treatment of patients in group 1, in which, shoulder joint reconstruction was performed in combination with brachial plexus neurolysis, were not statistically different from the results in group 2 that underwent only brachial plexus neurolysis. This means that in case of isolated damage to the brachial plexus, both methods are effective and have approximately similar and expected treatment outcomes. However, in the association of plexopathy with the pathology of the shoulder joint, a one-stage combined intervention with correction of intra-articular pathology and neurolysis is acceptable and contributes to a more complete and early restoration of upper limb function.
The team of the authors suggests a further study of the topic in order to create an optimal treatment and diagnostic algorithm for patients with brachioplexopathies.
CONCLUSION
The techniques of performing brachial plexus neurolysis with an endoscope are effective in the treatment of post-traumatic brachioplexopathies in adults if the strength in the affected muscles is two or more BMRC points. In this study, the methods of endoscopic neurolysis of the brachial plexus in combination with arthroscopy of the shoulder joint and isolated mini-invasive neurolysis of the brachial plexus under video-endoscopic assistance have resulted equally effective. Treatment of patients with post-traumatic brachioplexopathy is challenging and requires a multidisciplinary approach.
Competing interest The authors declare that they have no competing interests.
Funding source The study had no sponsorship.
Список литературы Сравнительный анализ методов полностью эндоскопической декомпрессии плечевого сплетения и мини-инвазивной техники с эндоскопической ассистенцией в лечении пациентов с травматической брахиоплексопатией
- Kaiser R, Waldauf P, Ullas G, Krajcová A. Epidemiology, etiology, and types of severe adult brachial plexus injuries requiring surgical repair: systematic review and meta-analysis. Neurosurg Rev. 2020;43(2):443-452. doi: 10.1007/ s10143-018-1009-2
- Tapp M, Wenzinger E, Tarabishy S, Ricci J, Herrera FA. The Epidemiology of Upper Extremity Nerve Injuries and Associated Cost in the US Emergency Departments. Ann Plast Surg. 2019;83(6):676-680. doi: 10.1097/SAP.0000000000002083
- Narakas AO. The treatment of brachial plexus injuries. IntOrthop. 1985;9(1):29-36. doi: 10.1007/BF00267034
- Kaiser R, Waldauf P, Haninec P. Types and severity of operated supraclavicular brachial plexus injuries caused by traffic accidents. Acta Neurochir (Wien). 2012;154(7):1293-1297. doi: 10.1007/s00701-012-1291-7
- MacDonald BK, Cockerell OC, Sander JW, Shorvon SD. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain. 2000;123(Pt 4):665-676. doi: 10.1093/brain/123.4.665
- Rasulic L, Simic V, Savic A, et al. Management of brachial plexus missile injuries. Acta Clin Croat. 2018;57(3):487-496. doi: 10.20471/acc.2018.57.03.12
- Flores LP. Estudo epidemiológico das lesoes traumáticas de plexo braquial em adultos [Epidemiological study of the traumatic brachial plexus injuries in adults]. Arq Neuropsiquiatr. 2006;64(1):88-94. (In Portuguese) doi: 10.1590/ s0004-282x2006000100018
- Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16(5):E5. doi: 10.3171/ foc.2004.16.5.6
- Siqueira MG, Malessy MJA. Traumatic brachial plexus lesions: clinical and surgical aspects. In: Treatment of peripheral nerve lesions. Prism Books; 2011:93-110.
- Terzis JK, Papakonstantinou KC. The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg. 2000;106(5):1097-1122; quiz 1123-4. doi: 10.1097/00006534-200010000-00022
- Bertelli JA, Ghizoni MF. Results and current approach for Brachial Plexus reconstruction. J Brachial Plex Peripher NerveInj. 2011;6(1):2. doi: 10.1186/1749-7221-6-2
- Millesi H. Update on the treatment of adult brachial plexus injuries. In: Brachial plexus injuries. Martin Dunitz Ltd.; 2001:77-90.
- Siquiera MG, Martins R, Heisse O, Socolovsky M (2010) Lesiones traumaticas del plexobraquial e adultos: Parte 1. Mecanismos de lesión, métodosdiagnósticos e indicaciones del tratamientoquirúrgico. In: Neurocirugía, AspectosClínicosyQuirurgícos, 1st Ed. Corpus, 2010: Ch. 95.
- Krishnan KG, Pinzer T, Reber F, Schackert G. Endoscopic exploration of the brachial plexus: technique and topographic anatomy--a study in fresh human cadavers. Neurosurgery. 2004;54(2):401-408; discussion 408-409. doi: 10.1227/01. neu.0000103423.08860.a9
- Braga-Silva J, Gehlen D, Kuyven CR. Endoscopic exploration of a brachial plexus injury. J Reconstr Microsurg. 2006;22(7):539-541. doi: 10.1055/s-2006-951320
- Сагдиев Р.Х., Дыдыкин С.С., Шапкин А.Г. и др. Эффективность невролиза плечевого сплетения под видеоэндоскопической ассистенцией при лечении брахиоплексопатий у взрослых. Гений ортопедии. 2023;29(1):7-11. doi: 10.18019/1028-4427-2023-29-1-7-11. EDN: AGNBKO.
- Mantovani G, Liverneaux P, Garcia JC Jr, et al. Endoscopic exploration and repair of brachial plexus with telerobotic manipulation: a cadaver trial. J Neurosurg. 2011;115(3):659-664. doi: 10.3171/2011.3.JNS10931
- Lafosse T, Le Hanneur M, Lafosse L. All-Endoscopic Brachial Plexus Complete Neurolysis for Idiopathic Neurogenic Thoracic Outlet Syndrome: A Prospective Case Series. Arthroscopy. 2017;33(8):1449-1457. doi: 10.1016/j.arthro.2017.01.050
- Le Hanneur M, Colas M, Serane-Fresnel J, et al. Endoscopic brachial plexus neurolysis in the management of infraclavicular nerve injuries due to glenohumeral dislocation. Injury. 2020;51(11):2592-2600. doi: 10.1016/j. injury.2020.08.005
- Беляк Е.А., Лазко Ф.Л., Призов А.П. и др. Эндоскопический невролиз плечевого сплетения у пациента с повреждением вращательной манжеты плеча и посттравматической плексопатией (случай из практики). Georgian Med News. 2021;(316-317):30-35.
- Shin, A.Y., Pulos, N. (eds) Operative Brachial Plexus Surgery. Springer, Cham.; 2021:639. doi: 10.1007/978-3-030-69517-0
- Шевелев И.Н. Травматические поражения плечевого сплетения (клиника, диагностика, микрохирургия). М.; 2005:52-55.
- Lusskin R, Campbell JB, Thompson WA. Post-traumatic lesions of the brachial plexus: treatment by transclavicular exploration and neurolysis or autograft reconstruction. J Bone Joint Surg Am. 1973;55(6):1159-76.
- Nagai H. Tunnel-endoscopy. Arthroscopy. 1980;(5):1-4.
- Monsivais JJ, Narakas AO, Turkof E, Sun Y. The endoscopic diagnosis and possible treatment of nerve root avulsions in the management of brachial plexus injuries. J Hand Surg Br. 1994;19(5):547-549. doi: 10.1016/0266-7681(94)90111-2
- Abdellaoui A, Atwan M, Reid F, Wilson P. Endoscopic assisted transaxillary first rib resection. Interact Cardiovasc Thorac Surg. 2007;6(5):644-646. doi: 10.1510/icvts.2007.151423
- Суфианов А.А., Сагдиев Р.Х., Беляк Е.А., Лазко Ф.Л., Суфианов Р.А. Способ расширения рёберно-ключично-го пространства при эндоскопической ревизии плечевого сплетения. Патент РФ на изобретение № 2794823С1. 25.04.2023. Бюл. № 12. Доступно по: https://new.fips.ru/registers-doc-view/fips_servlet?DB=RUPAT&rn=7174&Doc Number=2794823&TypeFile=html. Ссылка активна на 26.07.2023.


