Строение органических и элементоорганических соединений
Автор: Шарутин Владимир Викторович
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Физическая химия
Статья в выпуске: 3 т.11, 2019 года.
Бесплатный доступ
Методом рентгеноструктурного анализа (РСА) определено строение восьми органических и элементоорганических соединений. РСА соединений проводили на автоматическом четырехкружном дифрактометре D8 Quest Bruker (Мо Кα -излучение, λ = 0,71073 Å, графитовый монохроматор) при 293 К. Cоединение [Ph3PMe] [RuCl4(DMSO)2] (1) P -1, a = 8 ,4181(3), b = 8,9389(3), c = 11,1396(4) Å, a = 69,754(1), β = 81,913(2), g = 64,491(1) град., V = 709,75(4) Å3, Z = 1. [Ph3PC6H4CH2CN] Cl × CHCl3 (2), P 21/n, a = 9 ,846(6) Å, b = 15,782(14) Å, c = 15,111(10) Å, a = 90 , β = 91,027(18) , g = 90 град., V = 2348(3) Å3, Z = 4. Ph4SbOC6H4(NO2-4) (3), P -1, a = 11,101(6), b = 12,684(6), c = 19,359(9) Å, a = 80 , 973(17), β = 80,17(2) , g = 72,31(3) град., V = 2543(2) Å3, Z = 4. (4-BrC6H4)3Sb (4), P -1, a = 6,273(12), b = 12,83(2), c = 13,26(3) Å, a = 78,67(8), β = 84,33(9) , g = 80,81(7) град., V = 1031(3) Å3, Z = 2. Ph4PBr·H2O (5), P -1, a = 10,025(10), b = 10,676(10), c = 10,706(13) Å, a = 77,56(4), β = 71,80(4) , g = 83,26(3) град., V = 1061(2) Å3, Z = 2. [4-MeOC6H4]3Sb (6), R -3, a = 13,27(3), b = 13,27(3), c = 19,24(7) Å, a = 77,56(4), β = 90 , g = 120 град., V = 2935(20) Å3, Z = 6. [Ph3PCH2C6H4CN-4]Cl, P 21/n, a = 9,456(6), b = 14,733(9), c = 16,271(9) Å, a = 90, β = 104,83(2) , g = 90 град., V = 2191(2) Å3, Z = 4. [Ph3PCH2OH]Сl, P 21/c, a = 8,888(9), b = 17,795(19), c = 11,278(12) Å, a = 90, β = 99,52(4) , g = 90 град., V = 1759(3) Å3, Z = 4.
Строение, органическое, элементорганическое соединение, рентгеноструктурный анализ
Короткий адрес: https://sciup.org/147233133
IDR: 147233133 | УДК: 544.02+548+548.3+548.312.2+548.312.3+548.312.4+548.312.5 | DOI: 10.14529/chem190305
Текст научной статьи Строение органических и элементоорганических соединений
При появлении в Южно-Уральском государственном университете современного дифрактометра D8 Quest возможность определения кристаллических структур органических, неорганических, координационных и элементоорганических соединений неизмеримо возросла, поэтому представлялось возможным определить строение не только основных кристаллических продуктов реакций, но и минорных, иногда выделяемых из реакционной смеси в следовых количествах, а также некоторых исходных соединений.
В продолжение изучения строения производных элементов в настоящей работе впервые исследовано строение восьми неизвестных ранее соединений.
Экспериментальная часть
Рентгеноструктурный анализ кристаллов соединений 1 - 8 проводили на дифрактометре D8 Quest фирмы Bruker (Mo K α -излучение, λ 0,71073 Å, графитовый монохроматор) при 293 К. Сбор, редактирование данных и уточнение параметров элементарной ячейки, а также учет поглощения проведены по программам SMART и SAINT- Plus [1]. Все расчеты по определению и уточнению структуры выполнены по программам SHELXL/PC [2] и OLEX2 [3]. Структуры определены прямым методом и уточнены методом наименьших квадратов в анизотропном приближении для неводородных атомов. Основные кристаллографические данные и результаты уточнения структур 1 - 8 приведены в табл. 1, основные длины связей и валентные углы - в табл. 2.
Полные таблицы координат атомов, длин связей и валентных углов депонированы в Кембриджском банке структурных данных (№ 1895767 (1), № 1895761 (2), № 1812366 (3), № 1895766 (4), № 1895762 (5), № 1895768 (6), № 1895764 (7), № 1895763 (8); ; .
Обсуждение результатов
Сотрудниками лаборатории химии элементоорганических соединений Южно-Уральского государственного университета (ЮУрГУ) задепонированы в банке структурных данных Кем- бриджского университета структуры более 700 элементорганических, неорганических и органических производных [4]. Особенности строения многих комплексов переходных и непереходных металлов обсуждаются в ряде работ сотрудников ЮУрГУ [5-7] и иностранных авторов, например [8-25].
В продолжение изучения строения указанных производных, в настоящей работе были сняты и расшифрованы структуры восьми комплексных и элементоорганических соединений (рис. 1 - 8 и табл. 1, 2).
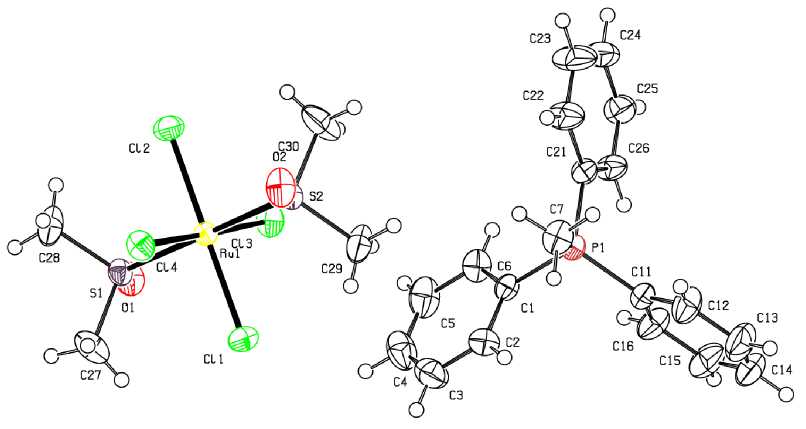
Рис. 1. Строение соединения [Ph 3 PMe] [RuCl 4 (DMSO) 2 ] (1)
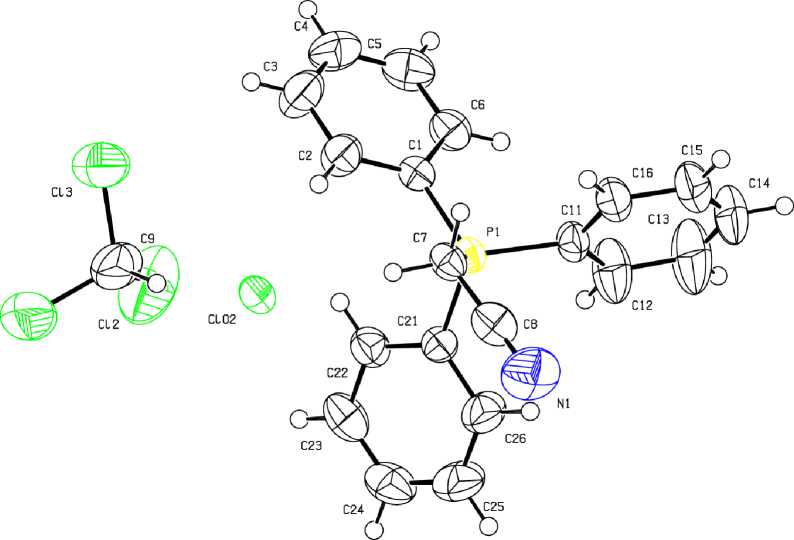
Рис. 2. Строение соединения [Ph 3 PC 6 H 4 CH 2 CN] Cl ⋅ CHCl 3 (2)
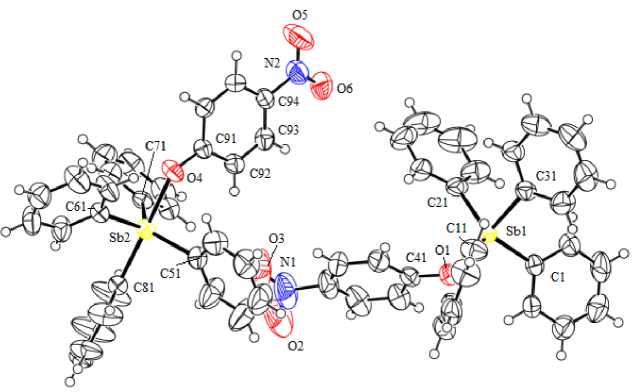
Рис. 3. Строение соединения Ph 4 SbOC 6 H 4 (NO 2 -4) (3)
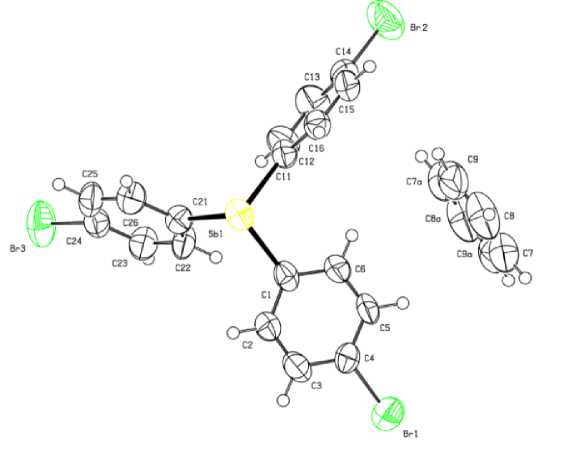
Рис. 4. Строение соединения (4-BrC 6 H 4 ) 3 Sb·С 6 H 6 (4)
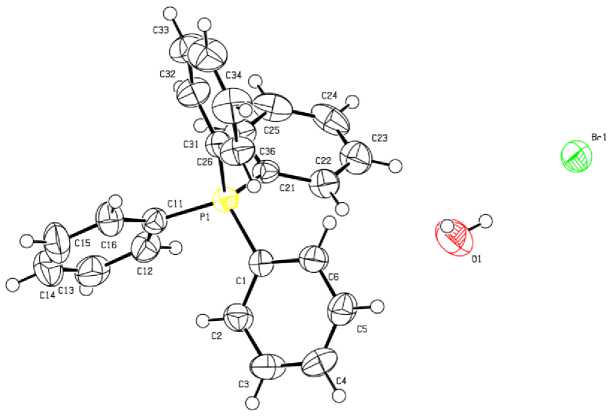
Рис. 5. Строение соединения Ph 4 PBr·H 2 O (5)
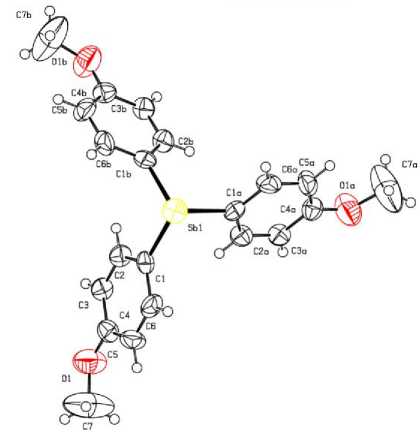
Рис. 6. Строение соединения [4-MeOC 6 H 4 ] 3 Sb (6)
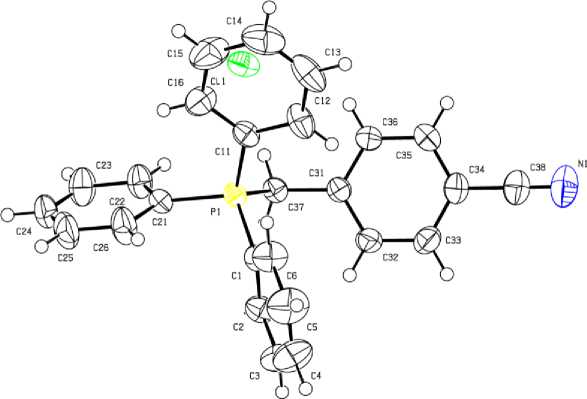
Рис. 7. Строение соединения [Ph 3 PCH 2 C 6 H 4 CN-4]Cl (7)
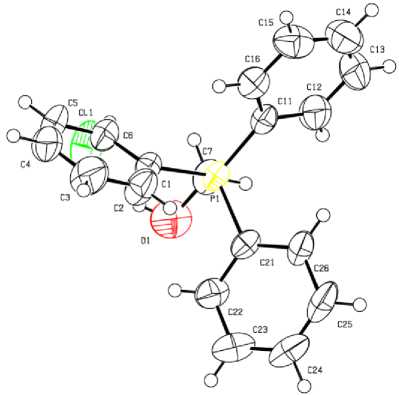
Рис. 8. Строение соединения [Ph 3 PCH 2 OH]Сl (8)

|
о х к я cd m |
ao |
о и а я и |
оо ч |
сч со х X и 5 |
ч |
5? оо ОО |
О' О' |
см ОО сч |
ч tn Os О' |
О. § |
os' |
"* |
ч |
о |
о ЭС ОС SO |
in °0, о |
см SO |
VI VI VI ■^ "^ VI VI VI ^ Г1 Ч" — 77 1 |
чО |
ч OS S — о О' и Ч 5 |
О ч о О' о |
о |
ч- ч Ч чо CS ч °. О ° II -^ |
и ^ ОС ос ос о о ч m ® 7 и 0? |
40 d |
||||
|
ч |
и Он z о |
$ |
к cd О X 5 |
с ч Ри |
S '^О os" |
£ |
£ см 3 |
S s |
£ О' см |
ч- |
ч |
О' СМг О |
ОО |
о ГЧ о" so_ о |
ЭС ч -С |
L г<> ГЧ VI VIX1 V! 7 VI VI VI чо гН Г1 7 Ч |
00 in |
ч £ Os q 7 ||_ g |
ч ч О' О' |
in |
so*" гм 00 ч 2 °г 1 |
ч 3 ? X |
1 о |
||||||
|
SO |
о м Г о |
7 |
5 X X |
pi |
ч |
Ч ГП |
ч о? |
о |
s |
о" ГМ |
X ч |
см |
a in о |
О |
о. |
X о |
х: х^ Os' 7 ч |
М СЧ Г-и VI VI VI VI VI VI ч ч ^ 7 7 । |
§ |
о" ^ II = |
ч |
so ч |
tn — tn —1 a °* 7 " |
If =7 |
5 |
||||
|
in |
5 Он о Эн о |
о |
к л X X X Н |
i |
о ч |
'О o' |
о |
£ |
S ос |
so см ГП 00 |
3 чС о |
см |
ос ГП |
ч ч" |
о. |
о" X Чг ? so ч о" |
SO in |
№ р^ °- VI VI X1 VI VI X 77 1 |
7 |
ч 00 С 7 II g |
ч ч |
О |
It |
' SO 40 Ч о °г "^ II ар |
ш CS о о |
||||
|
"* |
5 Я и |
ОО ч so |
к rd X X X н |
Рн |
Ч ч •о" |
S ос СМ |
ч сП |
£ ос |
£ ос |
о о |
гм |
ЭС О ri |
? |
о ’Т О' |
‘Ч о sOr |
-Г >0 ч MD |
42 7 ^ VI VI ^1 VI VI X 7^7 |
ш |
ГП о 00 - ч ^ Ч || = |
ч ч |
о’ о |
ч* 7 о °г °" II II ГЧ |
II Ч |
<Н ос |
|||||
|
4 |
м о Z Ей и |
X X |
Ph |
$ |
з ос MD Г1 |
3 os' сП О'" |
о" ОС |
см ОС |
СП гм" |
£ ч |
гм |
7 |
SD |
о |
о OS ? О' о |
D X) -с |
—■ ч" 0‘s vi vi vi г: -к - VI VI VI н- СЧ °-7 7 । |
ч |
ч g |
SO |
so so |
О'" 40 Ч о It |
^ г. Ч Ч- о ос in ч о Ro" ?" ар |
40 п о 4 ч г О |
|||||
|
4 |
и Z я и |
9 |
X X X S |
,x гм Ри |
3 3 ОО OS |
ос |
3 |
о о |
00 о OS |
о os |
7 ГМ |
гН О' ri |
so" as |
ч. 40 •sDr |
и XD 1 ч ■ч 43 |
^ оо" ч VI VI VI VI VI VI о 00 77 । |
ч •ОО ч |
Ч ч о Ч o'-ГП II Е |
ч |
о |
°-о' «<1 |
f - ч Ч" О' ■о ч ар |
о ОО |
||||||
|
2 о и о я и |
40 so |
X X X |
CL |
ОО |
ос 04. ОС |
SO as |
о |
QSri |
о О' |
Cs |
ЭС *п |
гм ■п |
о_ |
о X о" |
X) И 7 4D О |
VI VI VI -ы -^ ^ VI VI VI ^ m 2 77 । |
ч 5 |
7 § 7 и с |
о |
о |
м О °- о ° II II X - |
— 2 о X1 f X =5" f |
5 SO ОО |
||||||
|
s s Cd CL cd a |
cd CL О е |
х X X о X и |
Л X a CL П |
-< |
d |
X" |
n |
"о й al |
2 S |
о ^ |
1 ЬЙ CL Cl |
cd о io л ю О |
ci " CD О X |
ш о X X 3 >х | а з ё |
2Х X О о X о S |
X 5 S О-£ о |
х 5 X X п ^ |
о о о |
а V о с4 ^ о ^ X |
S V о и о = S й W CL X О X cd о |
х " о 4 О ч |
Е о X 5 X О |
|||||||
Taблица 2
Основные длины связей и валентные углы в структурах 1 - 8
|
Связь |
Длина, Å |
Угол \ |
ω , град. |
|
1 |
|||
|
Ru1 - Cl1 |
2,3535(5) |
Cl1Ru1Cl2 |
179,78(3) |
|
Ru1 - Cl4 |
2,3513(5) |
Cl4Ru1Cl1 |
91,27(2) |
|
Ru1 - Cl3 |
2,3495(5) |
Cl3Ru1Cl4 |
179,05(2) |
|
Ru1 - Cl2 |
2,3617(5) |
Cl3Ru1S1 |
92,336(18) |
|
Ru1 - S1 |
2,3520(5) |
S2Ru1Cl4 |
92,044(17) |
|
Ru1 - S2 |
2,3452(5) |
S2Ru1Cl3 |
88,144(18) |
|
P1 - C11 |
1,7968(15) |
S2Ru1S1 |
179,45(2) |
|
P1 - C1 |
1,7888(16) |
C1P1C7 |
107,89(9) |
|
P1 - C21 |
1,7852(19) |
C7P1C11 |
110,66(9) |
|
P1 - C7 |
1,7918(18) |
O1S1Ru1 |
117,76(7) |
|
S1 - O1 |
1,4729(15) |
O1S1C28 |
107,12(12) |
|
S1 - C28 |
1,775(3) |
O1S1C27 |
106,97(11) |
|
S1 - C27 |
1,778(3) |
C28S1Ru1 |
111,72(9) |
|
S2 - O2 |
1,4711(15) |
C28S1C27 |
99,30(16) |
|
S2 - C29 |
1,763(2) |
C27S1Ru1 |
112,26(9) |
|
S2 - C30 |
1,780(3) |
O2S2Ru1 |
118,08(7) |
|
2 |
|||
|
Cl1 - C9 |
1,764(5) |
C21P1C7 |
108,57(12) |
|
Cl2 - C9 |
1,732(5) |
C11P1C21 |
110,30(13) |
|
Cl3 - C9 |
1,756(4) |
C11P1C7 |
108,06(13) |
|
P1 - C21 |
1,791(3) |
C1P1C21 |
110,62(12) |
|
P1 - C11 |
1,787(3) |
C1P1C11 |
111,12(12) |
|
P1 - C1 |
1,787(3) |
C1P1C7 |
108,07(13) |
|
P1 - C7 |
1,809(3) |
C26C21P1 |
120,6(2) |
|
3 |
|||
|
Sb1 - O1 |
2,225(2) |
C1Sb1C21 |
117,68(13) |
|
Sb1 - C21 |
2,122(3) |
C1Sb1C11 |
111,39(12) |
|
Sb1 - C11 |
2,127(3) |
C21Sb1C11 |
127,95(13) |
|
Sb1 - C1 |
2,113(3) |
C31Sb1O1 |
176,92(10) |
|
Sb1 - C31 |
2,172(3) |
C21Sb1C31 |
93,84(12) |
|
Sb2 - O4 |
2,214(2) |
C1Sb1C31 |
97,82(13) |
|
Sb2 - C71 |
2,122(3) |
C1Sb1O1 |
81,79(10) |
|
Sb2 - C81 |
2,171(3) |
C61Sb2C71 |
115,57(12) |
|
Sb2 - C61 |
2,121(3) |
C51Sb2C61 |
114,59(12) |
|
Sb2 - C51 |
2,121(3) |
C51Sb2C71 |
127,12(12) |
|
O1 - C41 |
1,314(4) |
C81Sb2O4 |
178,13(10) |
|
O2 - N1 |
1,216(6) |
C71Sb2O4 |
83,93(10) |
|
O4 - C91 |
1,307(4) |
C71Sb2C81 |
94,56(11) |
|
O5 - N2 |
1,230(4) |
C61Sb2O4 |
82,59(10) |
|
4 |
|||
|
Sb1 - C1 |
2,098(8) |
C1Sb1C11 |
95,6(3) |
|
Sb1 - C11 |
2,132(8) |
C1Sb1C21 |
97,6(3) |
|
Sb1 - C21 |
2,132(8) |
C21Sb1C11 |
98,0(3) |
|
Br1 - C4 |
1,872(8) |
C3C4Br1 |
119,2(6) |
|
Br2 - C14 |
1,878(8) |
C5C4Br1 |
120,5(6) |
|
Br3 - C24 |
1,875(8) |
C13C14Br2 |
120,6(7) |
|
Преобразования симметрии: 1 1-x, 2-y, 1-z |
|||
|
5 |
|||
|
P1 - C11 |
1,786(4) |
C11P1C1 |
107,59(17) |
|
P1 - C1 |
1,793(4) |
C1P1C21 |
109,47(16) |
Окончание табл. 2
|
Связь |
Длина, Å |
Угол |
ω , град. |
|
P1 - C21 |
1,797(4) |
C1P1C31 |
111,94(17) |
|
P1 - C31 |
1,793(4) |
C31P1C21 |
109,02(17) |
|
6 |
|||
|
Sb1 - C1 1 |
2,197(15) |
C1 1 Sb1C1 |
94,9(6) |
|
Sb1 - C1 |
2,197(16) |
C1Sb1C1 2 |
94,9(5) |
|
Sb1 - C1 2 |
2,197(14) |
C1 1 Sb1C1 2 |
94,9(5) |
|
Преобразования симметрии: 1 2-y, 1+x-y, +z; 2 1+y-x, 2-x, +z |
|||
|
7 |
|||
|
P1 - C21 |
1,7894(16) |
C37P1C21 |
108,31(6) |
|
P1 - C1 |
1,7867(17) |
C11P1C21 |
109,92(6) |
|
P1 - C37 |
1,8071(16) |
C11P1C1 |
108,91(7) |
|
P1 - C11 |
1,7916(15) |
C11P1C37 |
111,98(6) |
|
8 |
|||
|
P1 - C1 |
1,786(4) |
C1P1C21 |
108,37(17) |
|
P1 - C21 |
1,800(4) |
C1P1C7 |
111,41(19) |
|
P1 - C11 |
1,782(4) |
C11P1C1 |
110,58(17) |
|
P1 - C7 |
1,834(4) |
C11P1C21 |
109,72(17) |
Выводы
В настоящей работе методом РСА расшифровано строение восьми органических и элементоорганических соединений.
Список литературы Строение органических и элементоорганических соединений
- Bruker. SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. Bruker AXS Inc., Madison, Wisconsin, USA, 1998.
- Bruker. SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures From Diffraction Data. Bruker AXS Inc., Madison, Wisconsin, USA, 1998.
- OLEX2: a Complete Structure Solution, Refinement and Analysis Program / O.V. Dolomanov, L.J. Bourhis, R.J. Gildea et al. // J. Appl. Cryst. - 2009. - V. 42. - P. 339-341. DOI: 10.1107/S0021889808042726
- Cambridge Crystallographic Data Center. 2019 (deposit@ccdc.cam.ac.uk; http://www.ccdc.cam.ac.uk).
- Шарутина, О.К. Молекулярные структуры органических соединений сурьмы (V) / О.К. Шарутина, В.В. Шарутин. - Челябинск: Издательский центр ЮУрГУ, 2012. - 395 с.
- Interaction of Pentaphenylantimony with Carboranedicarboxylic Acid / V.V. Sharutin, O.K. Sharutina, Y.O. Gubanova et al. // J. Organomet. Chem. - 2015. - V. 798. - P. 41-45.
- Synthesis and Structure of bic(tetraphenyl-l5-stibanyl)-1,7-carborane-1,7-dicarboxylate / V.V. Sharutin, O.K. Sharutina, Y.O. Gubanova et al. // Mendeleev Commun. - 2018. - V. 28. - P. 621-622.
- Rawashdeh-Omary, M.A. Oligomerization of Au(CN)2- and Ag(CN)2-ions in solution via ground-state aurophilic and argentophilic bonding / M.A. Rawashdeh-Omary, M.A. Omary, H.H. Patterson // J. Am. Chem. Soc. - 2000. - V. 122. - P. 10371-10380.
- DOI: 10.1021/ja001545w
- Luminescence thermochromism in dicyanoargentate (I) ions doped in alkali halide crystals / M.A. Rawashdeh-Omary, M.A. Omary, G.E. Shankle et. al. // J. Phys. Chem. B. - 2000. - V. 104. - P. 6143-6151.
- DOI: 10.1021/jp000563x
- Assefaa, Z. Hydrothermal syntheses, structural, Raman, and luminescence studies of Cm[M(CN)2]3 × 3H2O and Pr[M(CN)2]3 × 3H2O (M = Ag, Au) 2. Hetero-bimetallic coordination polymers consisting of trans-plutonium and transition metal elements / Z. Assefaa, R.G. Haireb, R.E. Sykorac // Journal of Solid State Chemistry. - 2008. - V. 181. - P. 382-391.
- DOI: 10.1016/j.jssc.2007.11.036
- Tunable photoluminescence of closed-shell heterobimetallic Au-Ag dicyanide layered systems / J.C.F. Colis, Ch.Larochelle, E.J. Ferna'ndez et. al. // J. Phys. Chem. B. - 2005. - V. 109. - P. 4317-4323.
- DOI: 10.1021/jp045868g
- Hydrothermal synthesis, structural, Raman, and luminescence studies of Am[M(CN)2]3×3H2O and Nd[M(CN)2]3 × 3H2O (M=Ag, Au): Bimetallic coordination polymers containing both trans-plutonium and transition metal elements / Z. Assefaa, K. Kalachnikova, R.G. Hairec et al. // Journal of Solid State Chemistry. - 2007. - V. 180. - P. 3121-3129.
- DOI: 10.1016/j.jssc.2007.08.032
- Roberts, R.J. Color-tunable and white-light luminescence in lanthanide-dicyanoaurate coordination polymers / R.J. Roberts, D. Le, D.B. Leznoff // Inorg. Chem. - 2017. - V. 56, iss. 14. - P. 7948-7959.
- DOI: 10.1021/acs.inorgchem.7b00735
- Wheatley, P.J. The Crystal and Molecular Structure of Aspirin / P.J. Wheatley // J. Am. Chem. Soc. - 1964. - P. 6036-6048.
- DOI: 10.1039/JR9640006036
- Carbodicarbenes: Unexpected π-Accepting Ability during Reactivity with Small Molecules / W.-C. Chen, W.-C. Shih, T. Jurca et al. // J. Am. Chem. Soc. - 2017. - V. 139. - P. 12830-12836.
- DOI: 10.1021/jacs.7b08031
- The Chemistry of Heteroarylphosphorus Compounds, Part 16. An X-Ray Structural Study of (2-Thienyl)bis(2,2′-biphenylylene)phosphorane. A Comparison with Related Methyl and Aryl bis(2,2′-biphenylylene)-spirophosphoranes / D.W. Allen, L.A. March, I.W. Nowell, J.C. Tebby // Z. Naturforsch. B. Chem. Sci. - 1983. - Bd. 38. - P. 465-469.
- DOI: 10.1515/znb-1983-0413
- Form Formation of a Dicyanotriorganophosphorane from the Reaction of Triphenylphosphane with Phenylselenocyanate / N.A. Barnes, S.M. Godfrey, R.T.A. Halton et al. // Angew. Chem. Int. Ed. - 2006. - V. 45. - P. 1272-1275.
- DOI: 10.1002/anie.200503335
- 5-Organyl-5-phosphaspiro[4.4]nonanes: a contribution to the structural chemistry of spirocyclictetraalkylphosphonium salts and pentaalkylphosphoranes / U. Monkowius, N.W. Mitzel, A. Schier, H. Schmidbaur // J. Am. Chem. Soc. - 2002. - V. 124. - P. 6126-6132.
- DOI: 10.1021/ja012041g
- Diphosphanylketenimines: new reagents for the synthesis of unique phosphorus heterocycles / J. Ruiz, F.Marquínez, V. Rieraet al. // Chem.-Eur. J. - 2002. - V. 8. - P. 3872-3878.
- DOI: 10.1002/1521-3765(20020902)8:17
- Muller, G. Crystal and Molecular Structure of P(C6H5)5·0.5 THF / G. Muller, U.J. Bildmann // Z. Naturforsch. B. Chem. Sci. - 2004. - Bd. 59, № 11-12. - P. 1411-1414.
- DOI: 10.1515/znb-2004-11-1207
- Day, R.O. Molecular structure of the methyl and phenyl derivatives of bis(2,2'-biphenylylene)phosphorene / R.O. Day, S. Husebye, R.R. Holmes // Inorg. Chem. - 1980. - V. 19. - P. 3616-3622.
- DOI: 10.1021/ic50214a011
- A Facile Access to 1λ5, 3λ5-Benzodiphospholes / H.J. Bestmann, H.P. Oechsner, C. Egerer-Sieber et. al. // Angew. Chem. Int. Ed. - 1995. - V. 34. - P. 2017-2020.
- DOI: 10.1002/anie.199520171
- Hazell, A. Mono-, di- and poly-nuclear transition-metal complexes of a bis(tridentate) ligand: towards p-phenylenediamine-bridged co-ordination polymers / A. Hazell, C.J. McKenzie, L.P. Nielsen // J. Chem. Soc., Dalton Trans. - 1998. - P. 1751-1756.
- DOI: 10.1039/A800602D
- Palladium complexes with pyrimidine-functionalized n-heterocyclic carbene ligands: synthesis, structure and catalytic activity / D. Meyer, M.A. Taige, A. Zeller et al. // Organometallics. - 2009. - V. 28, № 7. - P. 2142-2149.
- DOI: 10.1021/om8009238
- On the electronic impact of abnormal C4-bonding in N-heterocyclic carbene complexes / M. Heckenroth, A. Neels, M.G. Garnier et al. // Chem. Eur. J. - 2009. - V. 15, № 37. - P. 9375-9386.
- DOI: 10.1002/chem.200900249


