Структурные перестройки генов NTRK: характеристика, методы детекции и таргетная терапия онкологических заболеваний
Автор: Кечин Андрей Андреевич, Андриянова Анна Игоревна, Филипенко Максим Леонидович
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Обзоры
Статья в выпуске: 6 т.20, 2021 года.
Бесплатный доступ
Введение. В ноябре 2018 г. FDA одобрило применение ларотректениба для лечения распространенных солидных опухолей, содержащих слияния с генами NTRK других генов, в августе 2019 г. - энтректиниб. Цель исследования - представить современные данные о структуре и функциях генов NTRK, частоте встречаемости перестроек с их участием, последствиях от их возникновения на клеточном уровне, методах детекции таких перестроек, а также о таргетных препаратах, применяемых при наличии химерных генов NTRK. Материал и методы. Поиск статей осуществлялся в базах данных PubMed NCBI, Web of Science, Scopus. Результаты. Продукты генов NTRK являются рецепторами к нейротрофинам, и их высокая экспрессия в норме наблюдается только в узком диапазоне типов тканей. Внутрихромосомные или межхромосомные перестройки приводят к значительному повышению уровня экспрессии химерного гена благодаря попаданию под сильный промотор гена-партнера. Высокая транскрипционная активность такого гена наряду с постоянным включением киназной активности белкового продукта приводят к включению метаболических путей, отвечающих за уход клетки от апоптоза и нарушение регуляции клеточного цикла. Встречаемость химерных генов NTRK варьирует между различными типами опухолей, с наиболее высокой (до 90 %) - при редко встречающихся онкологических заболеваниях (секреторная карцинома молочной железы, секреторная карцинома слюнных желез, врождённая мезобластическая нефрома, детская фибросаркома). Новые таргетные препараты (ларотректениб и энтректиниб) имеют высокую эффективность подавления роста опухоли, несущей перестройки NTRK, вне зависимости от типа опухоли. В связи с этим актуальными представляются внедрение новых высокоточных методов детекции химерных генов NTRK, а также исследование механизмов развития резистентности с предположением способов ее преодоления. Заключение. Перестройки генов NTRK встречаются достаточно часто при различных видах онкологии и являются эффективной мишенью для современных таргетных препаратов.
Ntrk, химерные гены, таргетная терапия, метаболические пути, trk-ингибиторы, диагностика
Короткий адрес: https://sciup.org/140261342
IDR: 140261342 | УДК: 616-006-08:575.113 | DOI: 10.21294/1814-4861-2021-20-6-120-133
Текст научной статьи Структурные перестройки генов NTRK: характеристика, методы детекции и таргетная терапия онкологических заболеваний
stRuctuRal ReaRRaNgeMeNts oF NTRK geNes: cHaRacteRistics, MetHods oF detectioN aNd taRgeted tHeRapY FoR caNceR
a.a. Kechin1,2, a.i. andriyanova2, M.l. Filipenko1,2
Institute of Chemical Biology and Fundamental Medicine, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia 1
-
8, Lavrentiev av., Novosibirsk, 630090, Russia 1
Novosibirsk State University, Novosibirsk, Russia 2
-
2, Pirogova st., Novosibirsk, 630090, Russia 2
Background. The first-generation TRK inhibitors, larotrectinib and entrectinib, were approved by the U.S. Food and Drug Administration (FDA) for the treatment of advanced solid tumors harboring NTRK gene fusions in November 2018 and in August 2019, respectively. the purpose of the study was to present up-to-date data on the structure and functions of NTRK genes, the frequency of occurrence of rearrangements with their participation, the consequences of their occurrence at the cellular level, methods of detecting such rearrangements, as well as targeted drugs used in the presence of chimeric NTRK genes. Material and Methods. A systemic literature search was conducted in PubMed NCBI, Web of Science, Scopus databases. Results. The products of NTRK genes are receptors for neurotrophins, and their high expression is normally observed only in a narrow range of tissue types. Intrachromosomal or interchromosomal rearrangements lead to a significant increase in the level of expression of the chimeric gene regulated by the strong promoter of the partner gene. The high transcriptional activity of such a gene, along with the constant activation of the kinase activity of the protein product, leads to the activation of metabolic pathways responsible for cell escape from apoptosis and disruption of the regulation of the cell cycle. The occurrence of chimeric NTRK genes varies between different types of tumors, with the highest (up to 90 %) in rare cancers (secretory carcinoma of the breast, secretory carcinoma of the salivary glands, congenital mesoblastic nephroma, children’s fibrosarcoma). Larotrectinib and entrectinib are highly effective targeted drugs in suppressing the growth of a tumor carrying NTRK rearrangements, regardless of the type of tumor. In this regard, the introduction of new high-precision methods for the detection of chimeric NTRK genes, as well as the study of the mechanisms of the development of resistance with the assumption of ways to overcome it, seems relevant. conclusion. Rearrangements of NTRK genes are quite common in various types of oncology and are an effective target for modern targeted drugs.
NTRK рецепторы. Открытие и строение
Продуктами генов NTRK (neurotrophin tropomyosin receptor kinase) являются тирозинкиназные рецепторы к нейротрофинам (TRK): TRKA (ген NTRK1), TRKB (NTRK2) и TRKC (NTRK3). TRK-семество было так названо в честь онкогена, выделенного в 1982 г. из опухоли толстой кишки [1]. Этот онкоген представлял собой последовательность семи из восьми экзонов немышечного тропомиозина, присоединённую к 3’-фрагменту гена NTRK1, содержащего экзоны, кодирующие трансмембранный и цитоплазматический домены. В 1989 г. была получена кДНК протоонкогена NTRK1 и описан его продукт, TRKA, как белок, состоящий из 790 аминокислот. TRKA представляет собой гликопротеин с молекулярной массой 140 кДа, который изначально образуется в виде предшественника с молекулярной массой 110 кДа и затем подвергается дальнейшему гликозилированию по мере транспортирования к плазматической мембране [2, 3]. В 1991 г. было доказано, что TRKA экс- прессируется в нервной системе и фосфорилируется при связывании с нейротрофином – фактором роста нейронов (NGF – nerve growth factor) [4, 5]. Гены NTRK2 и NTRK3 обладают высокой гомологией с геном NTRK1, и сравнение их последовательностей с другими трансмембранными тирозинкиназами показало, что они составляют новую отдельную группу тирозинкиназных рецепторов, ассоциированных с поверхностью клетки [6].
В норме TRK участвуют в развитии и функционировании нервных тканей: дифференцировке, пролиферации, формировании синапсов, аксонов и дендритов, мембранном транспорте [7]. TRK-рецепторы имеют внеклеточный, трансмембранный и внутриклеточный регионы, последний из которых содержит тирозинкиназный домен. Внеклеточный домен включает в себя два кластера, богатых цистеином (C1 и C2), между которыми расположены три 24-аминокислотных повтора, обогащенных лейцином (LRR – leucine rich repeat). Между C2 и трансмембранным доменом находятся
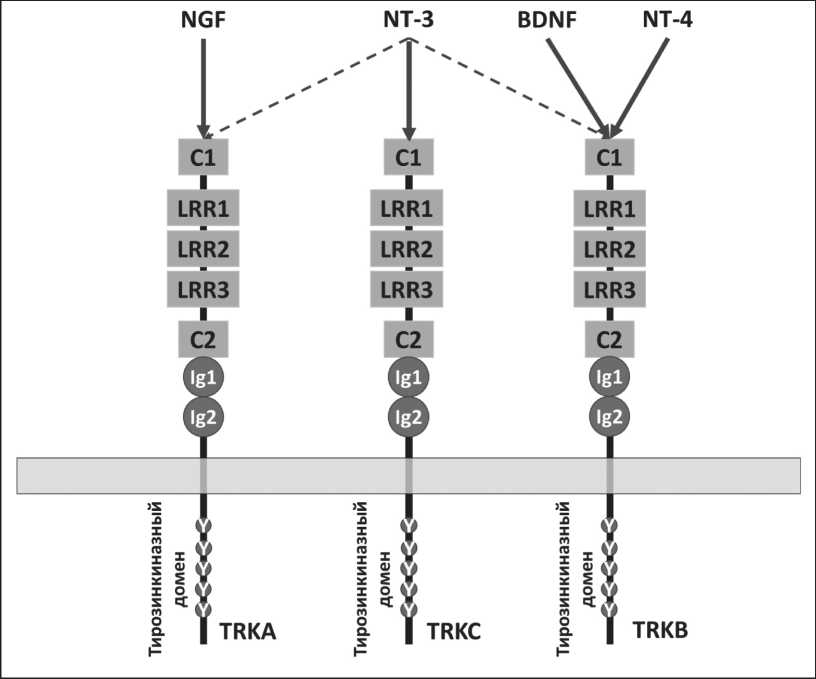
Рис. 1. Структура TRK-рецепторов и взаимодействующие с ними нейро-трофины
Fig. 1. The structure of TRK receptors and the neurotrophins interacting with them
два иммуноглобулин-подобных домена (Ig1 и Ig2) (рис. 1).
Основная функция TRK-рецепторов осуществляется путем связывания со специфическими лигандами – нейротрофинами: NGF, нейротрофическим фактором мозга (BDNF – brain-derived neurotrophic factor), нейротрофином-3 (NT-3) и нейротрофином-4 (NT-4). NGF связывается исключительно с TRKA [8], BDNF и NT-4 – с TRKB [9], NT-3 способен связываться со всеми TRK-рецепторами, но аффинность связывания с TRKC наибольшая [10, 11]. Нейротрофины связываются с рецепторами в виде димеров, из-за чего возможно взаимодействие одновременно с двумя рецепторами [12]. При этом вторым рецептором может выступать не только TRK, но и p75-рецептор к нейротрофинам (p75 NTR ), регулируя тем самым количество нейротрофина, необходимого для передачи сигнала [13].
Метаболические пути, активируемые TRK-рецепторами
Основные работы, описывающие молекулярные механизмы проведения сигнала через TRK, были выполнены на клетках феохромоцитомы надпочечников крыс (PC12), которые экспрессировали TRKA и p75 и использовались в качестве модельной клеточной линии для симпатических нейронов [14]. В отличие от первичных нейронов, эти клетки продолжают делиться, и у них отсутствует специализация частей клетки на аксон и дендриты. Поэтому первые работы описывали молекулярные механизмы проведения сигнала через TRK, базируясь на NGF и TRKA. Результаты этих работ были экстраполированы на TRKB и TRKC. После изучения механизмов передачи сигналов от TRK-рецепторов как в первичных нейронах, так и за пределами нервной системы стало ясно, что многие пути являются общими для активации разных TRK-рецепторов, в то время как некоторые запускаются TRK-специфично или тканеспецифично.
При связывании нейротрофинов с TRK-рецепторами происходит димеризация рецепторов и аутофосфорилирование по остаткам тирозина, находящимся внутри клетки [15]. В частности, фосфорилируются Y676, Y680 и Y681 в TRKA и соответствующие аминокислотные остатки в TRKB и TRKC, которые расположены в активационной петле киназного домена. Фосфорилирование этих остатков тирозина необходимо для полной активации киназы [16]. Дополнительное фосфорилирование Y496 и Y791 TRKA и соответствующих аминокислотных остатков TRKB и TRKC усиливает внутриклеточный сигнал. Фосфорилированный Y496 привлекает трансформирующий белок SHC и субстрат рецептора фактора роста фибробластов 2 (FRS2 – fibroblast growth factor receptor substrate 2), в то время как фосфорилированный Y791 взаимодействует с фосфолипазой фосфоинозитида Cγ (PLCγ – phosphoinositide phospholipase Cγ) (рис. 2) [17]. Этими белками активируются другие внутриклеточные эффекторы, в том числе участники сигнальных путей митоген-активируемой протеинкиназы (MAPK – mitogen-activated proteinkinase), фосфатидилинозитол-3 киназы (PI3K – phosphatidylinositol 3-kinase) и протеинкиназы C (PKC). Каждый активированный таким образом сигнальный путь регулирует транскрипцию генов, участвующих в дифференцировке
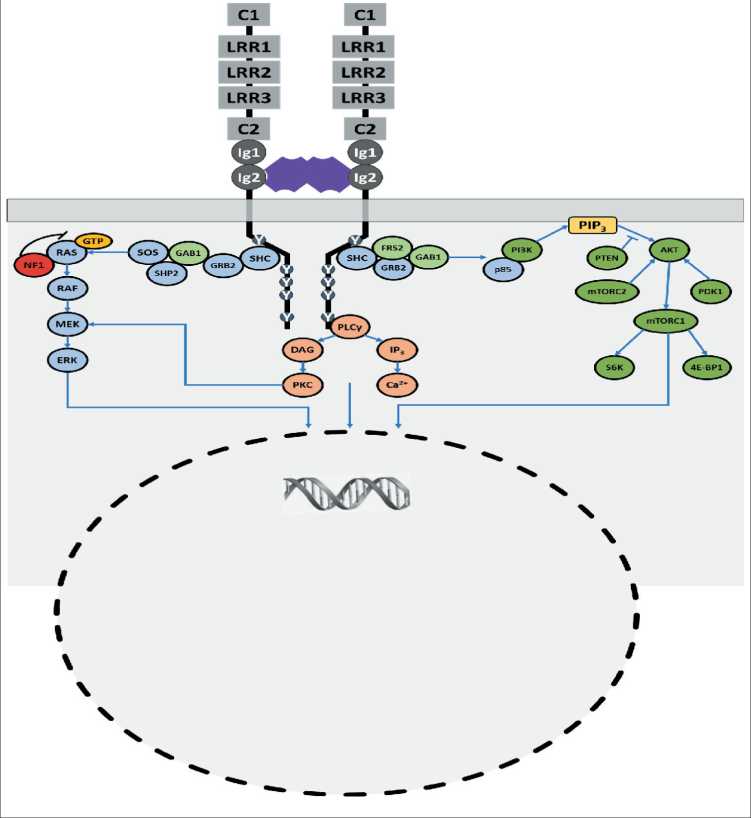
Рис. 2. Метаболические пути, в которых участвуют TRK-рецепторы Fig. 2. Metabolic pathways involving TRK receptors
и выживании нейронов, в том числе регулируются гены, кодирующие транскрипционные факторы-белки, связывающиеся с элементами ответа на циклический АМФ (CREB – cyclic AMP response element-binding protein) [14].
NTRK-химерные гены.Наиболее частые гены-партнеры
Онкогенная активация TRK-рецепторов происходит в результате формирования химерных NTRK-генов. В результате внутрихромосомной или межхромосомной перестройки образуется химерный ген, содержащий на 3'-конце последовательность киназного домена NTRK, присоединённую к 5'-последовательности гена-партнёра. Продукт такого гена – химерный белок – обладает свойством лиганд-независимой активации киназного домена [15, 18]. В химерных генах различных киназ последовательность гена-партнёра часто содержит олигомеризационные домены: супер-спирализованные домены, цинковые пальцы или WD-повторы (мотивы из ~40 а.о., заканчивающихся триптофаном и аспартатом), которые требуются для димеризации и активации киназы в некоторых случаях. Поэтому преобладающая часть генов-партнёров NTRK содержит в своём составе такие домены (например, TPM3, LMNA, PAN3, TFG содержат в своём составе суперспирализованные домены, IRF2BP2 и TRAF2 – цинковые пальцы, RFWD2, STRN, EML4 – WD-повторы). Но также известны гены-партнёры с другим механизмом димеризации, либо не содержащие соответствующих доменов (ETV6, BCR, CD74), либо имеющие неизвестный механизм димеризации (AFAP1, LYN). Одним из альтернативных механизмов является замещение автоингибирующего домена, находящегося во внеклеточной области TRK-рецептора, последовательностью гена-партнёра.
Также в результате иммуногистохимического анализа было показано, что ген-партнёр может отвечать за расположение химерного белка в клетке, причем это относится не только к химерным TRK-рецепторам, но и к другим киназам (например, ALK) [19]. В опухолях с перестройками NTRK1 - LMNA при иммуногистохимическом окрашивании проявлялся характерный паттерн окрашивания ядерной мембраны, что связано с биологической ролью гена LMNA , кодирующего ламин A/C – структурный компонент внутренней ядерной мембраны. Среди всех перестроек NTRK чаще других встречаются ETV6 - NTRK3 и TPM3 - NTRK1 [20].
Встречаемость NTRK-химерных генов в разных типах опухолей
По частоте возникновения перестроек NTRK злокачественные опухоли можно отнести к двум основным категориям:
– редко встречающиеся типы опухолей с частотой перестроек NTRK более 90 %, например, секреторная карцинома молочной железы, секреторная карцинома слюнных желез, врождённая мезобластическая нефрома, детская фибросаркома [21–25];
– более распространённые типы рака с низкой частотой перестроек NTRK (до 25 %), к которым относятся рак молочной железы, колоректальный рак, рак лёгкого, меланома, папиллярный рак щитовидной железы, рак поджелудочной железы, гастроинтестинальные стромальные опухоли, глиомы у детей и др. [21].
Секреторная карцинома молочной железы – редкий подтип инфильтративной карциномы протоков, в более чем 90 % случаев содержащий химерный ген ETV6 - NTRK3 [26–28]. В 2010 г. описан так называемый аналог секреторной карциномы молочной железы (MASC – mammary analogue secretory carcinoma) – секреторная карцинома слюнной железы, также содержащая перестройки ETV6 - NTRK3 в более чем 90 % случаев [29]. Сравнение геномов показало, что секреторная карцинома молочной железы генетически ближе к секреторной карциноме слюнной железы, чем к другим типам опухолей молочной железы [26]. Недавно обнаружены подтипы секреторной карциномы слюнной железы, содержащие перестройки ETV6 - RET [30]. Также были описаны случаи секреторной карциномы щитовидной железы (также известной как аналог секреторной карциномы щитовидной железы) и кожи, содержащие перестройки ETV6 - NTRK3 [31].
Врождённая мезобластическая нефрома – редкий тип опухолей почек, в основном возникает в младенчестве или у маленьких детей, почти в каждом случае причиной являются перестройки ETV6 - NTRK3 , которые обычно сопровождаются трисомией по 11 хромосоме [31, 32]. Но встречаются опухоли этого типа и с другими NTRK -перестройками [33]. Детская фибросаркома – редкий тип саркомы мягких тканей, не относящийся к рабдомиосаркомам, возникающий в течение первых лет жизни. Показано, что частота перестроек ETV6 - NTRK3 в этом типе опухолей колеблется от 70 до 91 % [34]. При этом также были описаны перестройки LMNA - NTRK1 и EML4 - NTRK3 , встречающиеся намного реже [33, 35, 36].
При анализе 112 случаев глиом у детей было выявлено 8 (7 %) опухолей, содержащих перестройки генов NTRK , из которых 4 были обнаружены у 10 детей моложе 3 лет с глиомами, развившимися не из стволовых клеток мозга (40 %) [37]. При изучении глиом, возникших у взрослых, перестройки
NTRK были обнаружены в 2 % случаев (8 из 390), при этом 75 % перестроек составляли химерные гены NTRK2 [38].
Перестройки NTRK у детей с папиллярным раком щитовидной железы обнаруживаются в 26 % случаев [39], у взрослых частота встречаемости перестроек NTRK при раке щитовидной железы составляет менее 10 % [20].
В распространённых типах злокачественных новообразований (рак молочной железы, рак лёгкого, колоректальный рак, меланома, рак поджелудочной железы, опухоли головы и шеи, почечные карциномы, саркомы мягких тканей и не желудочно-кишечного происхождения, гастроинтестинальные стромальные опухоли) частота перестроек NTRK варьирует от 0,1 до 2 % [22].
В гематологических опухолях NTRK -перестройки встречаются достаточно редко, в основном при остром лимфобластном лейкозе (ОЛЛ) и остром миелоидном лейкозе (ОМЛ). В исследовании 2018 г. проведено секвенирование ДНК и РНК из гематологических опухолей 7311 пациентов, из которых у 8 (0,1 %) обнаружены перестройки NTRK [21]. Три случая составляли опухоли, развившиеся из гистиоцитов ( LMNA - NTRK1 , TFG - NTRK1 ) и дендритных клеток ( TPR - NTRK1 ) с частотой перестроек 3,8 % (3 из 78), 1 случай ОЛЛ ( ETV6 - NTRK3 ; 1 из 659; 0,15 %), 2 случая ОМЛ ( ETV6 - NTRK2 , ETV6 - NTRK3 ; 2 из 1201; 0,17 %).
Метаболические путихимерных TRK-белков
Через химерные TRK-белки запускаются те же молекулярные механизмы проведения сигнала, что и через TRK-рецепторы без перестроек, однако возможна тканеспецифичность. Например, химерный белок гена ETV6 - NTRK3 , выявляемый почти во всех случаях MASC, чаще всего передает сигнал через Ras-ERK, но при экспрессии в Ba/F3-клетках (лимфоциты мыши) – преимущественно через PLCγ-путь [23]. В клеточных линиях рака лёгкого и колоректального рака с перестройками NTRK1 преобладающей является передача сигнала через SHC–RAS–MAPK, но была описана и активация PI3K и STAT3 путей [40]. Преобладание определенных путей активации также зависит не только от типа ткани, но и от локализации химерного белка, которая частично определяется геном-партнёром.
Таргетные препараты ларотректиниб и энтректиниб
Ларотректиниб и энтректиниб –TRK-инги-биторы первого поколения, действующие на TRKA-, TRKB- и TRKC-рецепторы [15, 18]. Для ингибирования TRKA/B/C половина максимальной ингибирующей концентрации (IC50) ларотректини-ба составляет 5–11 нМ, энтректиниба – 1–5 нМ [22, 41]. Данные препараты отличаются по специфичности действия на киназные рецепторы. Ларотрек- тиниб действует TRK-селективно, специфичность связывания с TRK-рецепторами более чем в 100 раз выше по сравнению с другими 229 киназными рецепторами и более чем в 1000 раз выше по сравнению с другими 80 мишенями, не входящими в группу киназных рецепторов [40]. Энтректиниб – мультикиназный ингибитор, проявляющий активность в отношении ALK (IC50=1,6 нМ), ROS1 (IC50=0,2 нМ) и TRKA/B/C [42, 43]. Ларотректиниб эффективно замедляет рост клеточных линий или ксенографтов, содержащих перестройки TPM3-NTRK1, MPRIP-NTRK1, TRIM24-NTRK2 или ETV6-NTRK3, что связано с ингибированием RAF-MEK-ERK или PI3K-AKT сигнальных путей [44]. Сходным образом энтректиниб ингибирует рост клеточных линий и ксенографтов, содержащих LMNA-NTRK1 или EVT6-NTRK3 [43].
Имеются другие мультикиназные ингибиторы, одобренные к применению против других перестроек: кабозантиниб, кризотиниб, нинтеданиб, понати-ниб, а также препараты, находящиеся на различных стадиях клинических исследований: алтиратиниб, форетиниб, лестауртиниб, мерестиниб, MGCD516, PLX7486, DS-6051b и TSR-011 [15]. Активность этих мультикиназных ингибиторов варьирует по отношению к TRKA/B/C. Причём некоторые из них имеют менее выраженный эффект по сравнению с ларотректинибом и энтректинибом, а также клинический эффект части этих ингибиторов в отношении TRK-рецепторов изучен недостаточно.
Клинические исследованияингибиторов химерных TRK-рецепторов
В настоящее время преимущественно применяется стратегия испытания противоопухолевых препаратов по типу «корзины» (basket trial), в рамках которой принимают участие пациенты, у которых имеются определённые генетические маркеры, независимо от типа рака. Больные получают препарат, нацеленный на специфические мутации или биомаркеры, найденные в опухолях. Такая стратегия позволяет проводить более быстрое тестирование.
Для оценки эффективности ларотректиниба при терапии опухолей, содержащих перестройки NTRK, проведены следующие клинические испытания: испытания I фазы среди взрослых (NCT02122913), фаза I/II испытаний среди детей (SCOUT, NCT02637687) и испытания II фазы по типу «корзины» среди взрослых и подростков (NAVIGATE, NCT02576431) [22]. По результатам лечения 55 пациентов разных возрастов и с разными опухолями опубликованы данные, согласно которым доля больных с объективным ответом (ORR – objective response rate) составила 75 % (CI95: 61–85 %), с полным ответом на терапию (CRR – complete response rate) – 13 %. Вскоре набор данных был расширен: полный список включал 159 пациентов, в возрасте от 1 мес до 84 лет с различными злокаче- ственными новообразованиями, содержащими TRK [45]. Чаще всего это были пациенты с секреторной карциномой, детской фибросаркомой (18 %), раком щитовидной железы (16 %), слюнных желез (13 %) и легкого (8 %). Большая часть перестроек включала химерные гены NTRK1 (40 %) или NTRK3 (55 %), обнаруженные с помощью молекулярного профилирования опухолей. Результирующий ORR составил 79 % (CI95: 72–85 %), ответ не зависел от типа опухоли, возраста, типа гена NTRK и гена-партнёра. Полный ответ наблюдался у 16 % пациентов, частичный – у 63 %. Медиана времени до ответа составила 1,8 мес, средняя продолжительность жизни до прогрессирования (PFS – median progression-free survival) – 28,3 мес, медиана общей выживаемости (OS – overall survival ) – 44,4 мес [45]. У 5 % пациентов (6/122) обнаружены метастазы в мозг, ORR на ларотректиниб составил 60 %. У двух пациентов с метастазами в мозг при аденокарциноме лёгкого и раке молочной железы применение ларотректиниба привело к полному ответу [46]. В ноябре 2018 г. FDA одобрило применение ларотректиниба для лечения взрослых и педиатрических злокачественных опухолей при наличии в них перестроек генов NTRK.
При клинических испытаниях энтректиниба STARTRK-2, STARTRK-1 и ALKA-372-001 получены следующие данные: терапию получали 54 пациента с солидными опухолями, содержащими перестройки NTRK ( NTRK1 – 41 %, NTRK3 – 57 %), ORR составил 57 % (CI 95 : 43–71 %), полный ответ наблюдался у 7 % пациентов [47]. Ответ был одинаковый вне зависимости от типа опухоли или гена NTRK с перестройкой. Медиана продолжительности ответа составила 10 мес, медиана PFS – 11 мес, OS – 21 мес. В этих испытаниях принимали участие взрослые, средний возраст составил 58 лет (21–83 года). Причём у 12/54 пациентов с метастазами в центральную нервную систему (ЦНС). В этих случаях ORR составил 55 % (CI 95 : 23–83 %), полный и частичный ответы наблюдались в 27 % случаев, средняя продолжительность времени до прогрессирования после терапии – 14,3 мес.
В 2019 г. представлены результаты клинического испытания энтректиниба у детей [48]. В STARTRK-NG получили терапию 7 пациентов, среди них трое с глиомой, один с меланомой, один с эмбриональной опухолью ЦНС, двое с врождённой фибросаркомой. Большая часть перестроек включала NTRK3 (n=4). У 6 пациентов опухоль уменьшилась более чем в половину, наибольшая продолжительность положительного ответа составила 15 мес.
В начале 2019 г. FDA на основании результатов клинических исследований ALKA-372-001, STARTRK-1 и STARTRK-2 предоставило право на приоритетное рассмотрение энтректиниба для лечения взрослых и детей с солидными NTRK-зависимыми опухолями, при наличии прогрес- сирования после предшествующей терапии или в качестве первой линии терапии при отсутствии существующих стандартных методов лечения, а также для лечения больных с метастатическим ROS1-положительным немелкоклеточным раком легкого.
Для гематологических опухолей клинические испытания ларотректиниба и энтректиниба пока не проведены. На клеточных линиях (HMC-1, HMC-1.2), а также на мышах, которым ксенотран-сплантировали клетки линии HMC-1, было показано, что применение энтректиниба уменьшает активацию TRKA [49]. Также в in vitro и in vivo моделях было показано, что при ингибировании TRKA восстанавливается чувствительность клеток к ингибированию рецептора KIT [49].
На клеточных линиях IMS-M2 и M0-91, выделенных от пациентов с ОМЛ и содержащих перестройки ETV6 - NTRK3 , после применения энтректиниба в наномолярных концентрациях (0,47 и 0,65 нМ соответственно) наблюдалось ингибирование пролиферации, а также вступление клеток в апоптоз [50].
При применении ларотректиниба и кризоти-ниба на in vivo модели ETV6 - NTRK3 ОЛЛ было зафиксировано TRK-ингибирование с полным подавлением пролиферации опухолевых клеток, причём ларотректиниб был примерно в 20 раз эффективнее, что является предпосылкой для проверки больных с ОЛЛ на наличие ETV6 - NTRK3 перестроек и клинических испытаний TRK-ингибиторов для гематологических опухолей, содержащих эти типы перестроек [51].
Выявлено значительное ингибирующее действие ларотректиниба на клеточные линии, полученные от пациентов с гематологическими опухолями и перестройками NTRK , вне зависимости того, какой тип сигнальных путей был активирован перестройкой [52]. В рамках этого исследования по результатам ксенографтной модели пациент с хронической лимфоцитарной лейкемией, у которого развилась ОМЛ с перестройками ETV6 – NTRK2 , ETV6 - MECOM и ещё семью другими мутациями, принимал ларотректиниб. В результате было достигнуто ингибирование клонов, содержащих перестройки ETV6 – NTRK2 [52], что подчёркивает важность дальнейших исследований эффективности ларотректиниба против гематологических опухолей, содержащих NTRK -перестройки, и проведения клинических испытаний.
Резистентность
У некоторых пациентов развивается устойчивость к таргетным препаратам, подразделяющаяся на устойчивость в целевом белке (on-target) и вне целевого белка (off-target). Устойчивость в целевом белке связана с мутациями, возникающими в киназном домене TRK в ответ на таргетную терапию.
Эти мутации приводят к заменам аминокислот, которые стерически затрудняют связывание рецептора с ингибитором, изменяют конформацию киназного домена или аффинность к АТФ [53]. Такие изменения паралогичны мутациям, возникающим в киназном домене ALK и ROS1 -химерных генов при таргетной терапии ALK - или ROS1 -содержащих опухолях лёгкого [54, 55]. Большая часть из таких мутаций киназных доменов возникает в генах NTRK1 и NTRK3 , и, хотя частоту приобретения такой устойчивости ещё предстоит определить, маловероятно, что эти события редкие [44].
Устойчивость вне целевого белка связана с возникновением мутаций в других тирозинкиназных рецепторах или включением новых сигнальных путей в ответ на TRK-ингибирование. Например, у некоторых пациентов с NTRK -позитивными опухолями в ответ на TRK-ингибирование возникли V600E BRAF -мутации, KRAS -мутации или произошла амплификация гена MET [56]. Было показано, что применение комбинированной терапии позволяет бороться с развившейся резистентностью. Например, пациент с перестройками NTRK в опухоли, в клетках которой появилась MET -амплификация после применения TRK-ингибиторов первого поколения, продолжил отвечать на терапию при одновременном применении TRK- и MET-ингибиторов [56].
TRK-ингибиторы следующего поколения
TRK-ингибиторы следующего поколения позволяют преодолевать устойчивость в целевом белке, развившуюся после применения TRK-ингибиторов первого поколения, воздействуя на дикий тип TRKA/B/C. Основные ингибиторы, селитректиниб (также известный как LOXO-195) и репотректиниб (TPX-0005), находятся на стадии клинических исследований. По сравнению с TRK-ингибиторами первого поколения они обладают повышенной ингибирующей активностью в отношении TRKA/B/C. Ингибирующая активность этих препаратов была исследована в экспериментах in vitro и in vivo на моделях, которые содержали аминокислотные замены в киназном домене TRK, что приводило к резистентности к препаратам первого поколения. Значения IC 50 для селитректиниба и репотректиниба оказались в 10–100 раз ниже значений для ларотректиниба и энтректиниба, а для некоторых мутаций препараты следующего поколения были единственными активными агентами [53, 57].
В клинических испытаниях селитректиниба (фазы I/II) принял участие 31 пациент, которым проводили лечение TRK-ингибиторами первого поколения, со средней продолжительностью терапии 11 мес (2–30 мес), а затем против возникших мутаций в целевом белке применялся селитректиниб. При мутациях в киназном домене ORR составил 45 % (9/20), ни у одного пациента с устойчивостью, возникшей за счёт активации других сигнальных путей, не было получено ответа на терапию, так же как у больных с неизвестными механизмами устойчивости [58].
Для пациента с MASC, содержащей перестройку ETV6 - NTRK3 и мутацию G623E, после лечения энтректинибом был подтверждён частичный ответ после применения репотректиниба [59]. У пациентки с метастазирующей холангиокарциномой, содержащей перестройку LMNA - NTRK1 , возникли мутации G595R и F589L после лечения ларотректи-нибом в течение 14 мес. После 6 мес применения репотректиниба произошло уменьшение размеров опухоли на 50 % [57]. В настоящий момент проходит клиническое испытание (TRIDENT-1) репотректиниба для пациентов с солидными опухолями, содержащими ROS1-, NTRK- или ALK -перестройки.
Выявление химерных генов NTRK
Существует несколько методов диагностики перестроек NTRK : иммуногистохимический; флуоресцентная гибридизация in situ (FISH – fluorescence in situ hybridization); полимеразная цепная реакция с обратной транскрипцией (RT-PCR – reverse transcriptase polymerase chain reaction); секвенирование следующего поколения (NGS – next generation sequencing). Рассмотрим преимущества и недостатки каждого.
Иммуногистохимия позволяет быстро и относительно недорого установить уровень экспрессии целевого гена в опухолевых клетках. В каждом анализе возможна проверка единственной мишени, и для его проведения требуется исследование двух срезов образца. Применение этого метода для выявления химерного белка базируется на предположении, что хромосомная перестройка повышает экспрессию такого гена в опухолевых клетках по сравнению с наблюдаемой в нормальных клетках той же ткани. Была показана эффективность иммуногистохимического анализа для диагностики перестроек ALK у пациентов с немелкоклеточным раком лёгкого и эквивалентность этого метода методу FISH [18, 60].
Для обнаружения перестроек NTRK рекомендуется проводить иммуногистохимический анализ опухолей с низкой частотой NTRK -перестроек, чтобы отобрать образцы для последующего молекулярного тестирования. В таком анализе используются антитела, распознающие одновременно все три TRK белка (так называемые pan-TRK антитела). Связываясь с С-концевым эпитопом тирозинкиназного домена, pan-TRK антитела способны выявлять перестройку в гене NTRK независимо от типа гена-партнёра.
В двух исследованиях была показана эффективность использования моноклонального pan-TRK антитела кролика для диагностики перестроек NTRK [19, 61]. Антитело распознаёт участок рядом с С-концом TRK-белков, присутствующий во всех TRK-химерных белках. J.F. Hechtman et al. исследовали тремя различными методами 23 образца, содержащих перестройки NTRK, найденные по результатам NGS образцов ДНК [19]. Последующее исследование РНК-образцов подтвердило 21 из 23 положительных результатов, из которых 20 оказались положительными при pan-TRK иммуногистохимическом анализе. Также был проведён иммуногистохимический анализ 20 образцов, не содержащих перестроек NTRK, и во всех случаях был получен отрицательный результат. Таким образом, чувствительность метода составила 95 %, специфичность – 100 %.
E.R. Rudzinski et al. исследовали эффективность pan-TRK антитела EPR17341 и моноклонального антитела кролика к TRKA (EP1058Y) на 30 образцах детских мезенхимальных опухолей, содержащих перестройки NTRK , и 48 образцах без перестроек [61]. Чувствительность метода составила 97 % (положительными оказались 29 из 30 образцов, для которых была характерна цитоплазматическая и/или ядерная локализация белка), специфичность 98 % (в одном случае из 48 наблюдался слабый цитоплазматический паттерн). Но нельзя экстраполировать эти данные на все типы тканей, так как для разных тканей характерен свой тип экспрессии TRK-белков без перестроек. В параллельном анализе с антителом к TRKA-химерному белку подтвердились 26 из рассмотренных 26 случаев, содержащих перестройки NTRK , включая не только опухоли с перестройками NTRK1 , но и NTRK2 , и NTRK3 . Для этих образцов была выявлена цитоплазматическая и/или ядерная локализация белка. Но специфичность связывания такого антитела всего 63 %: из 49 отрицательных контрольных образцов в 14 случаях наблюдалась цитоплазматическая локализация.
В исследовании pan-TRK антитела EPR17341 на выборке из 11502 зафиксированных формалином образцов чувствительность составила 75 % (21/28 образцов): 45 % опухолей, содержащих NTRK3 -перестройки, были негативными по результатам иммуногистохимического окрашивания [20]. Специфичность связывания составила 96 % (3942 из 4108).
В другом исследовании того же антитела на выборке из 3574 образцов была обнаружена зависимость чувствительности и специфичности связывания pan-TRK антитела от типа опухоли [62]. По результатам иммуногистохимического анализа 288 образцов были отмечены как экспрессирующие TRK, 139 из них оказались пригодными для дальнейшего тестирования. 12 из 139 содержали перестройки NTRK , у оставшихся 127 количество TRK-белка было характерно и для предшественника опухолевых клеток или же являлось следствием включения других опухоль-ассоциированных механизмов.
Таким образом, иммуногистохимический анализ является достаточно надёжным методом начальной диагностики для некоторых типов рака, например колоректального рака. При этом повышенная экспрессия гена NTRK в нейроэндокринных опухолях в 50 % случаев не связана с перестройками в генах NTRK . Следует обращать внимание на чувствительность теста для каждого конкретного образца, так как изменение этого параметра существенно сказывается на количестве выявленных перестроек, которые и так являются довольно редким событием.
Для FISH-диагностики образцов, выделенных из парафиновых блоков, используются двуцветные FISH-зонды, которые позволяют обнаружить перестройку с помощью анализа «распадающимися» зондами (break-apart probes) или зондами «слияния» (fusion probe) [63]. Зонды «слияния» в клетках дикого типа гибридизуются в отдаленных друг от друга участках (например, на разных хромосомах), помечая потенциальные гены-партнеры химерного гена. В клетках, содержащих химерный ген, оба сигнала будут расположены близко. «Распадающиеся» зонды, наоборот, в клетках дикого типа гибридизуются рядом, а в клетках с перестройкой – отдаленно друг от друга. Зонды «слияния» можно использовать, если для данного типа опухолей характерны транслокации между двумя определёнными генами. Если же имеется несколько потенциальных генов-партнёров и не все из них охарактеризованы, более подходящим методом диагностики являются «распадающиеся» зонды, при котором оба зонда гибридизуются с локусами гена-протоонкогена. При этом на чувствительность и специфичность «распадающихся» зондов влияют несколько факторов: как далеко друг от друга будут располагаться зонды, если гены-партнёры внутрихромосомные, порог, определяющий наличие перестройки. Например, в гене NTRK1 часто возникают внутрихромосомные перестройки, что повышает вероятность ложноотрицательных результатов при делеции внутри хромосомы. До появления многоцветного метода требовались отдельные анализы для выяснения статуса каждого из NTRK -гена FISH-методом.
Для диагностики перестроек NTRK применяются как «распадающиеся» зонды, так и зонды «слияния». В частности, для подтверждения гистологического диагноза врождённой мезо-бластической нефромы, секреторных карцином молочных и слюнных желез и фибросаркомы у детей используется FISH-анализ на наличие перестройки ETV6-NTRK3 [33]. Так как данная перестройка преобладает среди всех остальных в этих типах опухолей, то такие анализы основаны на распадающихся зондах, гибридизующихся на ETV6. Но в этих опухолях встречаются и другие перестройки, поэтому такой метод не может считаться универсальным, и в случае отрицательного результата требуются дальнейшие тесты для постановки диагноза.
Существует 2 основных подхода для диагностики NTRK -перестроек с помощью c полимеразной цепной реакции с обратной транскрипцией (RT-PCR), когда в анализируемом типе опухоли большая часть перестроек возникает между определённым 5’ геном-партнёром и определённым NTRK -геном, и экзоны гена-партнёра, участвующие в образовании химерного гена, повторяются во многих случаях, то используют следующий алгоритм дизайна праймеров: прямой праймер подбирается исходя из последовательности 5'-конца гена-партнёра перед точкой разрыва, а обратный – по последовательности NTRK -экзона после точки разрыва.
Праймеры должны быть специфичны к экзонам, фланкирующим предполагаемую точку разрыва, чтобы обеспечить минимальный размер ампликонов, так как РНК, выделяемая из парафиновых гистологических блоков, в значительной степени фрагментирована. Была показана эффективная диагностика таким способом перестроек ETV6 - NTRK3 для постановки диагноза в случае врождённой ме-зобластической нефромы, секреторных карцином молочных желез и и фибросаркомы у детей [32, 34, 64, 65].
При таком подходе необходима оптимизация методики на этапе выделения ткани, фиксации и выделения РНК из ткани, а также для оценки качества выделяемой РНК желательно использовать внутренний контроль. К недостаткам такого подхода относится и то, что он не может охватить все гены-партнёры во всех точках разрывов.
Другой подход основан на исследовании 5' и 3' экспрессии ампликонов NTRK -транскриптов. Две пары праймеров гибридизуются на кДНК транскрипта на участке, соответствующем гену NTRK до предполагаемой точки разрыва (на 5' конце) и после точки разрыва (на 3' конце). Если в выбранной ткани в норме наблюдается низкий уровень экспрессии гена (3' фрагмента), то после перестройки он может попасть под сильный промотор 5' фрагмента гена-партнёра, и с помощью RT-PCR будет заметна разница в количестве получаемых продуктов. Такой метод был использован для выявления NTRK1 -перестроек при папиллярном раке щитовидной железы: сравнивалось соотношение между коротким 3' RT-PCR- ампликоном NTRK1 -гена после предполагаемой точки разрыва и более протяжённым амплифицированным участком до точки разрыва [66].
Массовое параллельное секвенирование позволяет одновременно определить многие соматические мутации, что необходимо в условиях постоянного увеличения мишеней для таргет-ной терапии и иммунотерапии. В основе NGS-диагностики лежит анализ последовательности ДНК или РНК, при этом это может быть полногеномная последовательность экзом, транскриптом
Последовательность неизвестного гена-партнера Unknown partnergene sequence

Универсальные адаптеры пришиваются к каждому из концов всех фрагментов
Universal adapters are attached to each end of all pieces

Добавляются один NTRK-специфичный праймер и один праймер комплементарный универсальному адаптеру на 5-конце

One NTRK-specific primer anil one pr imer complementary to the universal adapter at the 5 -end are added
Амплификация Amplification
Рис. 3. «Якорная» мультиплексная ПЦР
Fig. 3. Anchored multiplex PCR или панель генов, необходимая для постановки диагноза, выбора терапии и прогноза [67]. Хотя таким способом можно получить полногеномную последовательность и, теоретически, обнаружить все возможные перестройки, чувствительность метода для полногеномной последовательности ниже по сравнению с чувствительностью для панели генов. Также полногеномное секвенирование по сравнению с панелью генов требует больших вычислительных и аналитических ресурсов.
Покрытие ДНК-панели генов достигается двумя способами. Первый основан на ПЦР-амплификации нужных фрагментов и требует меньше ткани, но при этом для успешного обнаружения перестройки должны быть известны все гены-партнёры и точное положение точек разрыва. Во втором подходе используются зонды, с помощью которых захватывается нужная последовательность. Таким гибридизационным способом можно обнаружить новые гены-партнёры, но для этого требуется больше исходного материала. Перестройка внутри интронного района с повторяющимися элементами является существенным препятствием для применения этого подхода. И для соответствующих интронных районов генов NTRK2 и NTRK3 может происходить снижение чувствительности гибридизационного ДНК-NGS-метода [18, 67].
В качестве альтернативы ДНК-NGS-метода можно использовать РНК-NGS-метод. Основное преимущество РНК-NGS-метода заключается в том, что в зрелой мРНК отсутствуют интронные участки, что позволяет создавать более точную систему анализа на основе ПЦР-амплификации фрагментов с перестройкой или их гибридизации с зондом, а также облегчает биоинформационный анализ. Один из вариантов ПЦР-амплификации фрагментов – «якорная» мультиплексная ПЦР (AMP – anchored multiplex PCR) (рис. 3) [68]. При этом происходит присоединение к концам двуцепочечной кДНК универсальных адаптеров, для одного из которых на последующем этапе добавляется комплементарный праймер.
Текущие клинические рекомендациипо выявлению NTRK химерных генов
В зависимости от частоты NTRK -перестроек и уровня экспрессии TRK-белков рекомендуется использовать следующий алгоритм (рис. 4):
– при высоком уровне экспрессии TRK-белков и низкой частоте NTRK -перестроек (гастроинтестинальная стромальная опухоль, саркома мягких тканей, нейроэндокринные опухоли) следует проводить NGS-тестирование (включая NTRK -перестройки);
– при высокой встречаемости NTRK -перестроек (фибросаркома у детей, секреторная карцинома слюнных желез, секреторная карцинома молочной железы, врождённая мезобластическая нефрома) или проводят FISH и в случае отрицательного результата исследуют образец с помощью NGS-тестирования, или сразу анализируют при помощи NGS;
-
– при низком уровне экспрессии и низкой частоте NTRK -перестроек (рак лёгкого, рак молочной железы, рак щитовидной железы, глиома, колоректальный рак, меланома, холангиокарцинома);
– опухоли, не прошедшие молекулярного тестирования, исследуют с помощью pan-TRK иммуногистохимического метода. Если по его результатам подтверждается высокая экспрессия


Высокая частота экспрессии TRK; низкая встречаемость химерных NTRK-генов
High frequency of TRK expression; low occurrence of chimeric NTRK genes
-
• Гастроинтестинальная стромальная опухоль (Gastrointestinal stromal tumor)
-
• Саркома мягких тканей (Soft tissue sarcoma)
Нейроэндокринные опухоли (Neuroendocrine tumors)


Опухоли, для которых проводится молекулярное тестирование Tumors for which molecular testing is performed -

Низкая частота экспрессии ТНК; низкая встречаемость химерных NTRK-генов Low frequency of ТНК expression; low occurrence of chimeric NTRK genes
Рак легкого (Lung cancer)
Глиома (Glioma)
Хол ангиокарцинома (Cholangiocarcinoma) Рак щитовидной железы (Thyroid cancer)
Колоректальный рак (Colorectal cancer) Рак молочной железы (Breast cancer) Меланома (Melanoma)
Рак поджелудочной железы (Prostate cancer)
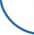
Высокая встречаемость химерных NTRK-генов
High frequency of chimeric NTRK genes
Детская фибросаркома (Fibrosarcoma in children) Секреторный рак слюнных желез (Secretory cancer of the salivary glands)
Секреторный рак молочной железы (Secretory breast cancer) Врожденная мезобластическая нефрома (Congenital mesoblastic nephroma)

молекулярного тестирования
Tumors without molecular
Исследование pan-TRK антителами Pan-TRK antibody таргетное тестирование(без NTRK) Previously conducted targeted testing
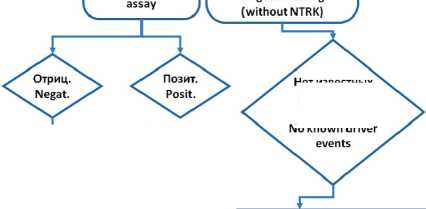
Mo known driver
Подтвердить альтернативным методом Confirm with an alternative method
Нет известных драйверных событий
Расширенное NGS-тестирование, включая на наличие химерных NTRK-генов Extended NGS testing, including for chimeric NTRK genes
Рис. 4. Рекомендуемый алгоритм выявления перестроек NTRK . Адаптировано из [18]NTRK2, and NTRK3 genes (NTRK genes Fig. 4. Recommended algorithm for detecting NTRK rearrangements. Adapted from [18]NTRK2, and NTRK3 genes (NTRK genes
TRK-белка, то проводят дополнительные тесты для подтверждения диагноза. Если опухоль не содержит NTRK -перестроек по результатам иммуногистохимического исследования, то проводят NGS-тестирование на наличие различных мутаций, в том числе NTRK -перестроек.
Список литературы Структурные перестройки генов NTRK: характеристика, методы детекции и таргетная терапия онкологических заболеваний
- Pulciani S., Santos E., Lauver A.V., Long L.K., Aaronson S.A., Barbacid M. Oncogenes in solid human tumours. Nature. 1982 Dec 9; 300(5892): 539–42. doi: 10.1038/300539a0.
- Barbacid M. The TRK family of neurotrophin receptors. J Neurobiol. 1994 Nov; 25(11): 1386–403. doi: 10.1002/neu.480251107.
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human TRK protooncogene. Mol Cell Biol. 1989 Jan; 9(1): 24–33. doi: 10.1128/mcb.9.1.24-33.
- Klein R., Jing S.Q., Nanduri V., O’Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5; 65(1): 189–97. doi: 10.1016/0092-8674(91)90419-y.
- Kaplan D.R., Hempstead B.L., Martin-Zanca D., Chao M.V., Parada L.F. The TRK proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991; 252(5005): 554–8. doi: 10.1126/science.1850549.
- Huang E.J., Reichardt L.F. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003; 72: 609–42. doi: 10.1146/annurev.biochem.72.121801.161629.
- Amatu A., Sartore-Bianchi A., Bencardino K., Pizzutilo E.G., Tosi F., Siena S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann Oncol. 2019 Nov 1; 30. doi: 10.1093/annonc/mdz383.
- Kaplan D.R., Martin-Zanca D., Parada L.F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991 Mar 14; 350(6314): 158–60. doi: 10.1038/350158a0.
- Soppet D., Escandon E., Maragos J., Middlemas D.S., Reid S.W., Blair J., Burton L.E., Stanton B.R., Kaplan D.R., Hunter T., Nikolics K., Parada L.F. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991 May 31; 65(5): 895–903. doi: 10.1016/0092-8674(91)90396-g.
- Naito Y., Lee A.K., Takahashi H. Emerging roles of the neurotrophin receptor TrkC in synapse organization. Neurosci Res. 2017; 116: 10–17. doi: 10.1016/j.neures.2016.09.009.
- Ip N.Y., Stitt T.N., Tapley P., Klein R., Glass D.J., Fandl J., Greene L.A., Barbacid M., Yancopoulos G.D. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993 Feb; 10(2): 137–49. doi: 10.1016/0896-6273(93)90306-c.
- Heymach Jr J.V., Shooter E.M. The biosynthesis of neurotrophin heterodimers by transfected mammalian cells. J Biol Chem. 1995 May 19; 270(20): 12297–304. doi: 10.1074/jbc.270.20.12297.
- Mahadeo D., Kaplan L., Chao M.V., Hempstead B.L. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J Biol Chem. 1994 Mar 4; 269(9): 6884–91.
- Deinhardt K., Chao M.V. Trk receptors. Handbook of Experimental Pharmacology. 2014; P. 103–19. doi.org/10.1007/978-3-642-45106-5_5.
- Cocco E., Scaltriti M., Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018 Dec; 15(12): 731–47. doi: 10.1038/s41571-018-0113-0.
- Cunningham M.E., Greene L.A. A function-structure model for NGF-activated TRK. EMBO J. 1998; 17(24): 7282–93. doi: 10.1093/emboj/17.24.7282.
- Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006; 361(1473): 1545–64. doi: 10.1098/rstb.2006.1894.
- Hsiao S.J., Zehir A., Sireci A.N., Aisner D.L. Detection of Tumor NTRK Gene Fusions to Identify Patients Who May Benefit from Tyrosine Kinase (TRK) Inhibitor Therapy. J Mol Diagn. 2019; 21(4): 553–71. doi: 10.1016/j.jmoldx.2019.03.008.
- Hechtman J.F., Benayed R., Hyman D.M., Drilon A., Zehir A., Frosina D., Arcila M.E., Dogan S., Klimstra D.S., Ladanyi M., Jungbluth A.A. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am J Surg Pathol. 2017 Nov; 41(11): 1547–51. doi: 10.1097/PAS.0000000000000911.
- Gatalica Z., Xiu J., Swensen J., Vranic S. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol. 2019 Jan; 32(1): 147–53. doi: 10.1038/s41379-018-0118-3.
- Amatu A., Sartore-Bianchi A., Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016 Mar 18; 1(2). doi: 10.1136/esmoopen-2015-000023.
- Drilon A., Laetsch T.W., Kummar S., DuBois S.G., Lassen U.N., Demetri G.D., Nathenson M., Doebele R.C., Farago A.F., Pappo A.S., Turpin B., Dowlati A., Brose M.S., Mascarenhas L., Federman N., Berlin J., El-Deiry W.S., Baik C., Deeken J., Boni V., Nagasubramanian R., Taylor M., Rudzinski E.R., Meric-Bernstam F., Sohal D.P.S., Ma P.C., Raez L.E., Hechtman J.F., Benayed R., Ladanyi M., Tuch B.B., Ebata K., Cruickshank S., Ku N.C., Cox M.C., Hawkins D.S., Hong D.S., Hyman D.M. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018 Feb 22; 378(8): 731–9. doi: 10.1056/NEJMoa1714448.
- Drilon A., Li G., Dogan S., Gounder M., Shen R., Arcila M., Wang L., Hyman D.M., Hechtman J., Wei G., Cam N.R., Christiansen J., Luo D., Maneval E.C., Bauer T., Patel M., Liu S.V., Ou S.H., Farago A., Shaw A., Shoemaker R.F., Lim J., Hornby Z., Multani P., Ladanyi M., Berger M., Katabi N., Ghossein R., Ho A.L. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol. 2016; 27(5): 920–6. doi: 10.1093/annonc/mdw042.
- Halalsheh H., McCarville M.B., Neel M., Reynolds M., Cox M.C., Pappo A.S. Dramatic bone remodeling following larotrectinib administration for bone metastasis in a patient with TRK fusion congenital mesoblastic nephroma. Pediatr Blood Cancer. 2018 Oct; 65(10). doi: 10.1002/pbc.27271.
- Davis J.L., Lockwood C.M., Albert C.M., Tsuchiya K., Hawkins D.S., Rudzinski E.R. Infantile NTRK-associated Mesenchymal Tumors. Pediatr Dev Pathol. 2018 Jan-Feb; 21(1): 68–78. doi: 10.1177/1093526617712639.
- Krings G., Joseph N.M., Bean G.R., Solomon D., Onodera C., Talevich E., Yeh I., Grenert J.P., Hosfield E., Crawford E.D., Jordan R.C., van Zante A., Zaloudek C., Shin S.J., Chen Y.Y. Genomic profiling of breast secretory carcinomas reveals distinct genetics from other breast cancers and similarity to mammary analog secretory carcinomas. Mod Pathol. 2017 Aug; 30(8): 1086–99. doi: 10.1038/modpathol.2017.32.
- Laé M., Fréneaux P., Sastre-Garau X., Chouchane O., Sigal-Zafrani B., Vincent-Salomon A. Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum. Mod Pathol. 2009 Feb; 22(2): 291–8. doi: 10.1038/modpathol.2008.184.
- Vasudev P., Onuma K. Secretory breast carcinoma: unique, triplenegative carcinoma with a favorable prognosis and characteristic molecular expression. Arch Pathol Lab Med. 2011; 135(12): 1606–10. doi: 10.5858/arpa.2010-0351-RS.
- Skálová A., Vanecek T., Sima R., Laco J., Weinreb I., Perez-Ordonez B., Starek I., Geierova M., Simpson R.H., Passador-Santos F., Ryska A., Leivo I., Kinkor Z., Michal M. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010; 34(5): 599–608. doi: 10.1097/PAS.0b013e3181d9efcc.
- Skalova A., Vanecek T., Martinek P., Weinreb I., Stevens T.M., Simpson R.H.W., Hyrcza M., Rupp N.J., Baneckova M., Michal M. Jr., Slouka D., Svoboda T., Metelkova A., Etebarian A., Pavelka J., Potts S.J., Christiansen J., Steiner P., Michal M. Molecular Profiling of Mammary Analog Secretory Carcinoma Revealed a Subset of Tumors Harboring a Novel ETV6-RET Translocation: Report of 10 Cases. Am J Surg Pathol. 2018 Feb; 42(2): 234–46. doi: 10.1097/PAS.0000000000000972.
- Dogan S., Wang L., Ptashkin R.N., Dawson R.R., Shah J.P., Sherman E.J., Tuttle R. M., Fagin J.A., Klimstra D.S., Katabi N., Ghossein R.A. Mammary analog secretory carcinoma of the thyroid gland: A primary thyroid adenocarcinoma harboring ETV6-NTRK3 fusion. Mod Pathol. 2016; 29: 985–95. https://doi.org/10.1038/modpathol.2016.115
- Knezevich S.R., Garnett M.J., Pysher T.J., Beckwith J.B., Grundy P.E., Sorensen P.H. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998 Nov 15; 58(22): 5046–8.
- Church A.J., Calicchio M.L., Nardi V., Skalova A., Pinto A., Dillon D.A., Gomez-Fernandez C.R., Manoj N., Haimes J.D., Stahl J.A., Dela Cruz F.S., Tannenbaum-Dvir S., Glade-Bender J.L., Kung A.L., DuBois S.G., Kozakewich H.P., Janeway K.A., Perez-Atayde A.R., Harris M.H. Recurrent EML4-NTRK3 fusions in infantile fibrosarcoma and congenital mesoblastic nephroma suggest a revised testing strategy. Mod Pathol. 2018 Mar; 31(3): 463–73. doi: 10.1038/modpathol.2017.127.
- Sheng W.Q., Hisaoka M., Okamoto S., Tanaka A., Meis-Kindblom J.M., Kindblom L.G., Ishida T., Nojima T., Hashimoto H. Congenitalinfantile fibrosarcoma. A clinicopathologic study of 10 cases and molecular detection of the ETV6-NTRK3 fusion transcripts using paraffin-embedded tissues. Am J Clin Pathol. 2001; 115(3): 348–55. doi: 10.1309/3H24-E7T7-V37G-AKKQ.
- Wong V., Pavlick D., Brennan T., Yelensky R., Crawford J., Ross J.S., Miller V.A., Malicki D., Stephens P.J., Ali S.M., Ahn H. Evaluation of a Congenital Infantile Fibrosarcoma by Comprehensive Genomic Profiling Reveals an LMNA-NTRK1 Gene Fusion Responsive to Crizotinib. J Natl Cancer Inst. 2015 Nov 12; 108(1). doi: 10.1093/jnci/djv307.
- Tannenbaum-Dvir S., Glade Bender J.L., Church A.J., Janeway K.A., Harris M.H., Mansukhani M.M., Nagy P.L., Andrews S.J., Murty V.V., Kadenhe-Chiweshe A., Connolly E.P., Kung A.L., Dela Cruz F.S. Characterization of a novel fusion gene EML4-NTRK3 in a case of recurrent congenital fibrosarcoma. Cold Spring Harb Mol Case Stud. 2015 Oct; 1(1). doi: 10.1101/mcs.a000471.
- Wu G., Diaz A.K., Paugh B.S., Rankin S.L., Ju B., Li Y., Zhu X., Qu C., Chen X., Zhang J., Easton J., Edmonson M., Ma X., Lu C., Nagahawatte P., Hedlund E., Rusch M., Pounds S., Lin T., Onar-Thomas A., Huether R., Kriwacki R., Parker M., Gupta P., Becksfort J., Wei L., Mulder H.L., Boggs K., Vadodaria B., Yergeau D., Russell J.C., Ochoa K., Fulton R.S., Fulton L.L., Jones C., Boop F.A., Broniscer A., Wetmore C., Gajjar A., Ding L., Mardis E.R., Wilson R.K., Taylor M.R., Downing J.R., Ellison D.W., Zhang J., Baker S.J. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014 May; 46(5): 444–50. doi: 10.1038/ng.2938.
- Ferguson S.D., Zhou S., Huse J.T., de Groot J.F., Xiu J., Subramaniam D.S., Mehta S., Gatalica Z., Swensen J., Sanai N., Spetzler D., Heimberger A.B. Targetable Gene Fusions Associate With the IDH Wild-Type Astrocytic Lineage in Adult Gliomas. J Neuropathol Exp Neurol. 2018; 77(6): 437–42. doi: 10.1093/jnen/nly022.
- Prasad M.L., Vyas M., Horne M.J., Virk R.K., Morotti R., Liu Z., Tallini G., Nikiforova M.N., Christison-Lagay E.R., Udelsman R., Dinauer C.A., Nikiforov Y.E. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016; 122(7): 1097–107. doi: 10.1002/cncr.29887.
- Vaishnavi A., Capelletti M., Le A.T., Kako S., Butaney M., Ercan D., Mahale S., Davies K.D., Aisner D.L., Pilling A.B., Berge E.M., Kim J., Sasaki H., Park S., Kryukov G., Garraway L.A., Hammerman P.S., Haas J., Andrews S.W., Lipson D., Stephens P.J., Miller V.A., Varella-Garcia M., Jänne P.A., Doebele R.C. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013 Nov; 19(11): 1469–72. doi: 10.1038/nm.3352.
- Ardini E., Menichincheri M., Banfi P., Bosotti R., De Ponti C., Pulci R., Ballinari D., Ciomei M., Texido G., Degrassi A., Avanzi N., Amboldi N., Saccardo M.B., Casero D., Orsini P., Bandiera T., Mologni L., Anderson D., Wei G., Harris J., Vernier J.M., Li G., Felder E., Donati D., Isacchi A., Pesenti E., Magnaghi P., Galvani A. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Mol Cancer Ther. 2016 Apr; 15(4): 628–39. doi: 10.1158/1535-7163.MCT-15-0758.
- Drilon A., Siena S., Ou S.I., Patel M., Ahn M.J., Lee J., Bauer T.M., Farago A.F., Wheler J.J., Liu S.V., Doebele R., Giannetta L., Cerea G., Marrapese G., Schirru M., Amatu A., Bencardino K., Palmeri L., Sartore-Bianchi A., Vanzulli A., Cresta S., Damian S., Duca M., Ardini E., Li G., Christiansen J., Kowalski K., Johnson A.D., Patel R., Luo D., Chow-Maneval E., Hornby Z., Multani P.S., Shaw A.T., De Braud F.G. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017 Apr; 7(4): 400–9. doi: 10.1158/2159-8290.CD-16-1237.
- Menichincheri M., Ardini E., Magnaghi P., Avanzi N., Banfi P., Bossi R., Buffa L., Canevari G., Ceriani L., Colombo M., Corti L., Donati D., Fasolini M., Felder E., Fiorelli C., Fiorentini F., Galvani A., Isacchi A., Borgia A.L., Marchionni C., Nesi M., Orrenius C., Panzeri A., Pesenti E., Rusconi L., Saccardo M.B., Vanotti E., Perrone E., Orsini P. Discovery of Entrectinib: A New 3-Aminoindazole As a Potent Anaplastic Lymphoma Kinase (ALK), c-ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) inhibitor. J Med Chem. 2016 Apr 14; 59(7): 3392–408. doi: 10.1021/acs.jmedchem.6b00064.
- Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019 Nov 1; 30. doi: 10.1093/annonc/mdz282.
- Hong D.S., DuBois S.G., Kummar S., Farago A.F., Albert C.M., Rohrberg K.S., van Tilburg C.M., Nagasubramanian R., Berlin J.D., Federman N., Mascarenhas L., Geoerger B., Dowlati A., Pappo A.S., Bielack S., Doz F., McDermott R., Patel J.D., Schilder R.J., Tahara M., Pfister S.M., Witt O., Ladanyi M., Rudzinski E.R., Nanda S., Childs B.H., Laetsch T.W., Hyman D.M., Drilon A. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020 Apr; 21(4): 531–40. doi: 10.1016/S1470-2045(19)30856-3.
- Rosen E.Y., Schram A.M., Young R.J., Schreyer M.W., Hechtman J.F., Shu C.A., Ku N.C., Hyman D.M., Drilon A. Larotrectinib Demonstrates CNS Efficacy in TRK Fusion-Positive Solid Tumors. JCO Precis Oncol. 2019 May 16; 3. doi: 10.1200/PO.19.00009.
- Doebele R.C., Drilon A., Paz-Ares L., Siena S., Shaw A.T., Farago A.F., Blakely C.M., Seto T., Cho B.C., Tosi D., Besse B., Chawla S.P., Bazhenova L., Krauss J.C., Chae Y.K., Barve M., Garrido-Laguna I., Liu S.V., Conkling P., John T., Fakih M., Sigal D., Loong H.H., Buchschacher G.L. Jr., Garrido P., Nieva J., Steuer C., Overbeck T.R., Bowles D.W., Fox E., Riehl T., Chow-Maneval E., Simmons B., Cui N., Johnson A., Eng S., Wilson T.R., Demetri G.D.; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020 Feb; 21(2): 271–82. doi: 10.1016/S1470-2045(19)30691-6.
- Robinson G.W., Gajjar A.J., Gauvain K.M., Basu E.M., Macy M.E., Maese L.D., Sabnis A.J., Foster J.H., Shusterman S., Yoon J., Weiss B.D., Abdelbaki M., Farid-Kapadia M., Meneses-Lorente G., Cardenas A., Hutchinson K., Bergthold G., Maneval E.C., Fox E., Desai A.V. Phase 1/1B trial to assess the activity of entrectinib in children and adolescents with recurrent or refractory solid tumors including central nervous system (CNS) tumors. J Clin Oncol. 2019 May 20; 37(15): 10009. doi: 10.1200/JCO.2019.37.15_suppl.10009.
- Yang M., Pan Z., Huang K., Büsche G., Feuerhake F., Chaturvedi A., Nie D., Heuser M., Thol F., von Neuhoff N., Ganser A., Li Z. Activation of TRKA receptor elicits mastocytosis in mice and is involved in the development of resistance to KIT-targeted therapy. Oncotarget. 2017 May 19; 8(43): 73871–83. doi: 10.18632/oncotarget.18027.
- Smith K.M., Fagan P.C., Pomari E., Germano G., Frasson C., Walsh C., Silverman I., Bonvini P., Li G. Antitumor activity of entrectinib, a pan-TRK, ROS1, and ALK inhibitor, in ETV6-NTRK3-positive acute myeloid leukemia. Mol Cancer Ther. 2018; 17(2): 455–63. doi: 10.1158/1535-7163.MCT-17-0419.
- Roberts K.G., Janke L.J., Zhao Y., Seth A., Ma J., Finkelstein D., Smith S., Ebata K., Tuch B.B., Hunger S.P., Mullighan C.G. ETV6-NTRK3 induces aggressive acute lymphoblastic leukemia highly sensitive to selective TRK inhibition. Blood. 2018; 132(8): 861–5. doi: 10.1182/blood-2018-05-849554.
- Taylor J., Pavlick D., Yoshimi A., Marcelus C., Chung S.S., Hechtman J.F., Benayed R., Cocco E., Durham B.H., Bitner L., Inoue D., Chung Y.R., Mullaney K., Watts J.M., Diamond E.L., Albacker L.A., Mughal T.I., Ebata K., Tuch B.B., Ku N., Scaltriti M., Roshal M., Arcila M., Ali S., Hyman D.M., Park J.H., Abdel-Wahab O. Oncogenic TRK fusions are amenable to inhibition in hematologic malignancies. J Clin Invest. 2018; 128(9): 3819–25. doi: 10.1172/JCI120787.
- Drilon A., Nagasubramanian R., Blake J.F., Ku N., Tuch B.B., Ebata K., Smith S., Lauriault V., Kolakowski G.R., Brandhuber B.J., Larsen P.D., Bouhana K.S., Winski S.L., Hamor R., Wu W.I., Parker A., Morales T.H., Sullivan F.X., DeWolf W.E., Wollenberg L.A., Gordon P.R., Douglas-Lindsay D.N., Scaltriti M., Benayed R., Raj S., Hanusch B., Schram A.M., Jonsson P., Berger M.F., Hechtman J.F., Taylor B.S., Andrews S., Rothenberg S.M., Hyman D.M. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov. 2017 Sep; 7(9): 963–72. doi: 10.1158/2159-8290.
- Gainor J.F., Dardaei L., Yoda S., Friboulet L., Leshchiner I., Katayama R., Dagogo-Jack I., Gadgeel S., Schultz K., Singh M., Chin E., Parks M., Lee D., DiCecca R.H., Lockerman E., Huynh T., Logan J., Ritterhouse L.L., Le L.P., Muniappan A., Digumarthy S., Channick C., Keyes C., Getz G., Dias-Santagata D., Heist R.S., Lennerz J., Sequist L.V., Benes C.H., Iafrate A.J., Mino-Kenudson M., Engelman J.A., Shaw A.T. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016 Oct; 6(10): 1118–33. doi: 10.1158/2159-8290.
- Awad M.M., Katayama R., McTigue M., Liu W., Deng Y.L., Brooun A., Friboulet L., Huang D., Falk M.D., Timofeevski S., Wilner K.D., Lockerman E.L., Khan T.M., Mahmood S., Gainor J.F., Digumarthy S.R., Stone J.R., Mino-Kenudson M., Christensen J.G., Iafrate A.J., Engelman J.A., Shaw A.T. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013 Jun 20; 368(25): 2395–401. doi: 10.1056/NEJMoa1215530.
- Cocco E., Schram A.M., Kulick A., Misale S., Won H.H., Yaeger R., Razavi P., Ptashkin R., Hechtman J.F., Toska E., Cownie J., Somwar R., Shifman S., Mattar M., Selçuklu S.D., Samoila A., Guzman S., Tuch B.B., Ebata K., de Stanchina E., Nagy R.J., Lanman R.B., Houck-Loomis B., Patel J.A., Berger M.F., Ladanyi M., Hyman D.M., Drilon A., Scaltriti M. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med. 2019 Sep; 25(9): 1422–7. doi: 10.1038/s41591-019-0542-z.
- Drilon A., Zhai D., Deng W., Zhang X., Lee D., Rogers E., Whitten J., Huang Z., Graber A., Liu J., Stopatschinskaja S., Cui J.J., Kim D.-W., Cho B.C., Doebele R.C., Ou S.-H.I., Lee J., Shaw A.T. Abstract 442: Repotrectinib, a next generation TRK inhibitor, overcomes TRK resistance mutations including solvent front, gatekeeper and compound mutations. Cancer Res. 2019; 442. doi: 10.1158/1538-7445.AM2019-442.
- Hyman D., Kummar S., Farago A., Geoerger B., Mau-Sorensen M., Taylor M., Garralda E., Nagasubramanian R., Natheson M., Song L., Capra M., Jorgensen M., Ho A., Shukla N., Smith S., Huang X., Tuch B., Ku N., Laetsch T.W., Drilon A., Hong D. Abstract CT127: Phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi). Cancer Res. 2019 Jul; doi: 10.1158/1538-7445.
- Drilon A., Ou S.I., Cho B.C., Kim D.W., Lee J., Lin J.J., Zhu V.W., Ahn M.J., Camidge D.R., Nguyen J., Zhai D., Deng W., Huang Z., Rogers E., Liu J., Whitten J., Lim J.K., Stopatschinskaja S., Hyman D.M., Doebele R.C., Cui J.J., Shaw A.T. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent-Front Mutations. Cancer Discov. 2018; 8(10): 1227–36. doi: 10.1158/2159-8290.CD-18-0484.
- Lindeman N.I., Cagle P.T., Aisner D.L., Arcila M.E., Beasley M.B., Bernicker E.H., Colasacco C., Dacic S., Hirsch F.R., Kerr K., Kwiatkowski D.J., Ladanyi M., Nowak J.A., Sholl L., Temple-Smolkin R., Solomon B., Souter L.H., Thunnissen E., Tsao M.S., Ventura C.B., Wynes M.W., Yatabe Y. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018 Mar; 142(3): 321–46. doi: 10.5858/arpa.2017-0388-CP.
- Rudzinski E.R., Lockwood C.M., Stohr B.A., Vargas S.O., Sheridan R., Black J.O., Rajaram V., Laetsch T.W., Davis J.L. Pan-Trk Immunohistochemistry Identifies NTRK Rearrangements in Pediatric Mesenchymal Tumors. Am J Surg Pathol. 2018 Jul; 42(7): 927–35. doi: 10.1097/PAS.0000000000001062.
- Feng J., Ebata K., Hansen F., Kivi L., Kriegshauser C., Morosini D., Shen T.-S., Sireci A.N., Thorne-Nuzzo P., Tuch B. TRK wild-type and fusion protein expression in solid tumors: Characterization by immunohistochemistry and in situ hybridization. Ann Oncol. 2018 Sep; 29(Suppl 6). 80P.
- Cheng L., Zhang S., Wang L., MacLennan G.T., Davidson D.D. Fluorescence in situ hybridization in surgical pathology: principles and applications. J Pathol Clin Res. 2017 Feb 23; 3(2):73–99. doi: 10.1002/cjp2.64.
- Bourgeois J.M., Knezevich S.R., Mathers J.A., Sorensen P.H. Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol. 2000 Jul; 24(7): 937–46. doi: 10.1097/00000478-200007000-00005.
- Tognon C., Knezevich S.R., Huntsman D., Roskelley C.D., Melnyk N., Mathers J.A., Becker L., Carneiro F., MacPherson N., Horsman D., Poremba C., Sorensen P.H. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002 Nov; 2(5): 367–76. doi: 10.1016/s1535-6108(02)00180-0.
- Brzeziańska E., Karbownik M., Migdalska-Sek M., Pastuszak-Lewandoska D., Włoch J., Lewiński A. Molecular analysis of the RET and NTRK1 gene rearrangements in papillary thyroid carcinoma in the Polish population. Mutat Res. 2006 Jul 25; 599(1–2):26–35. doi: 10.1016/j.mrfmmm.2005.12.013.
- Sheikine Y., Kuo F.C., Lindeman N.I. Clinical and Technical Aspects of Genomic Diagnostics for Precision Oncology. J Clin Oncol. 2017 Mar 20; 35(9): 929–33. doi: 10.1200/JCO.2016.70.7539.
- Zheng Z., Liebers M., Zhelyazkova B., Cao Y., Panditi D., Lynch K.D., Chen J., Robinson H.E., Shim H.S., Chmielecki J., Pao W., Engelman J.A., Iafrate A.J., Le L.P. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014 Dec; 20(12): 1479–84. doi: 10.1038/nm.3729.


