Studies on the changes, occurring at the initial stage of the interaction of btadiene rubber and its vulkanized produkt with nitric acid
Автор: Dimov Milen, Taneva Ira, Dimov Ivan
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Статья в выпуске: 3 т.12, 2019 года.
Бесплатный доступ
The purpose of this work was to study the chemical changes, taking place during the initial stage of the interaction of butadiene rubber and its vulcanized product with nitric acid by means of the differential IR spectroscopy. Thus some mechanistic knowledge, associated with the complex nature of this process could be obtained.
Rubber chunks, coal, oxidative destruction, butadiene rubber
Короткий адрес: https://sciup.org/146281198
IDR: 146281198 | УДК: 678.47:665.652.8:546.175-323 | DOI: 10.17516/1999
Текст научной статьи Studies on the changes, occurring at the initial stage of the interaction of btadiene rubber and its vulkanized produkt with nitric acid
the film remained well fastened between the two frames. Then, the film was detached using water. Film thickness was about 30 μm and it was calculated by the formula: m=λr2.ρ.d, where “m” is the precisely pleasured amount of rubber necessary for the preparation of the film; “r” is the radius of the glass Petri dish (cm); ρ is the relative weight of rubber = 0.93 g/cm3.
Preparation of sulfur vulcanizate films based on butadiene rubber.
Interaction of butadiene rubber films and sulfur vulcanizate based on it with nitric acid. A 100 cm3 beaker equipped with thermometer and covering glass lid containing 50 cm3, 65% HNO 3 is placed in a thermostat. After reaching the reaction temperature (20 ºC and 60 ºC), the sample is placed in it (butadiene rubber film or vulcanizate) for interaction period from 30 s to 450 min (7 h and 30 min). At the end of the period the film is taken out of the acid, washed with distilled water and dried to constant weight. Then the IR spectrum is recorded. The experiments were carried out with sample of the corresponding type (BR or vulcanizate).
Spectral analysis of the films obtained.
After the reaction period in the nitric acid, only absorption bands corresponding to the new functional groups appear in the spectra of the oxidized samples. Using the light absorption, an attempt was made to perform semi-quantitative analysis of the processes taking place under these interaction conditions using the logarithm of the ratio А=lgI0/І of the incident and absorbed light. The method of base line was used. The spectra were interpreted on the basis of literary sources [8-12].
Results and discussion
The interest of some authors is focused on studies on polymers stability, particularly butadiene rubber, in aggressive media while the aim of others is to utilize shreds of protector vulcanizates [1-4, 6]. Using IR spectroscopy, parallel processes of nitration, oxidation, structuring and destruction were proved to occur. The changes taking place in the rubber as a result from its interaction with nitric acid and oxides were observed by the appearance of absorption bands corresponding to new functional groups (NO 2 , OH, C=O) and by the decreased light absorption of the existing double bonds. By this experimental method, even semi-quantitative changes in the spectra cannot be read due to overlapping of the absorption bands corresponding to the new functional groups and these if the existing ones (1660 – 1605 cm-1 – double bonds and ONO2, ONO groups; 860 – 835 cm-1 – double bonds and ONO2, ONO groups, etc.). Ermakova et al. [6] carried out qualitative studies on the cis-trans isomerization of the double bonds in butadiene rubber under the influence of nitrogen dioxide and observed increased absorption at 965 cm-1 which overlaps the one of the initial double bond. To avoid this disadvantage, we aimed to study the initial stage of the interaction of 1,4 – cis – butadiene rubber with nitric acid by differential IR spectroscopy (Table 1).
It can be seen from the studies carried out that as a result of the interaction between a film (30 μm) of non-filled sulfur vulcanizate and nitric acid at 20 and 60 ºC, in the first 30 s of the contact at both temperatures, absorption bands of significantly higher intensity appeared at 1540 cm-1(NO2), 965 cm-1 (trans structures) and 1080 cm-1(ОН) beside the absorption bands corresponding to the ONO 2 groups. A short time later appeared also the band at 1700 cm-1(C=O). All the bands increased their intensities with time except for the one at 965 cm-1 the intensity of which decreased.
Table 1. Absorption bands of a sample of the initial butadiene rubber.
|
Frequency (γ, сm-1) |
type of vibration. |
Frequency (γ, сm-1) |
type of vibration |
|
3070 |
γаs СНβ = СН 2 |
1400 |
δ CH(1,2) |
|
3020 – 3006 |
γ СНβ = СН 2 |
1300 |
δ β-CH 2 - |
|
2050 – 2915 |
γаs СН 2 |
1275 |
γ-C-C- |
|
2850 |
γs СН 2 |
1245 |
γ-CН 2 - |
|
1665 |
γs=(1,4-cis) |
990 |
δpl.СН(1,2) |
|
1645 |
γs=С(1,2) |
965 |
δpl.СН(1,4-trans) |
|
1460 |
δ CH 2 |
910 |
δ CH(1,2) |
|
730 |
δ СН(1,4-cis) |
A
time (min)
Fig. 1
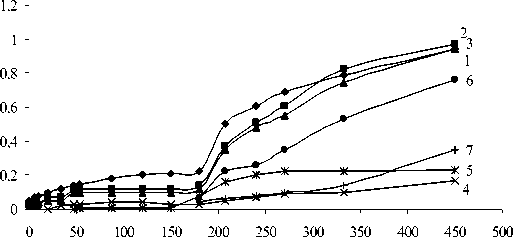
It can be seen from Fig. 1 that a process of formation of nitrate groups takes place at low temperature of interaction (20 ºC) up to 35th minute (curves 1, 2, 3). This fact was considered enough to assume that, under these conditions, electrophilic incorporation of nitric acid to rubber double bonds occurs by ionic mechanism [12]. With the formation of nitrogen dioxide, it begins joining to the double bonds to form nitro- and nitrite groups and, simultaneously, isomerization of the cis- structures to transstructures takes place (curves 4, 5).
It is well known that nitrogen oxide has two reaction centers and so it forms nitro-compound when it bonds to nitrogen and nitrites when it bonds to oxygen.
From this moment on, the reaction proceeds by a mixed ion-radical mechanism [12]. The oxidation processes begin much later with the formation of OH and C=O groups after 180 min (curves 6, 7). Until the end of the interaction after 450 min, the amounts of nitrate and hydroxyl groups increases (curves 1, 2, 3, 6) while the degree of isomerization and nitration did not change significantly. It was assumed, therefore, that the ionic mechanism of bonding the nitric acid prevails at low temperatures (20 ºC) and the amount of nitrogen incorporated is in the form of nitrate groups. The increase of sample weight with the interaction duration is due to predominantly to the accumulation of nitrate and hydroxyl groups.
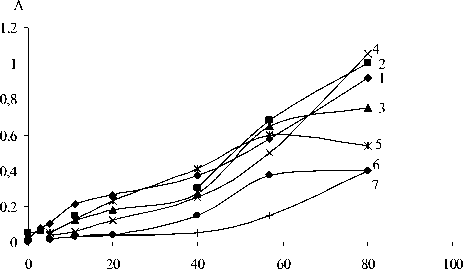
At higher temperature (60 ºC), all the processes discussed above intensify at the same sequence (Fig. 2).
The nitro-group forms with 1 min delay after the nitrate one, i.e. the reaction proceeds by ionic mechanism and after a minute it is transformed into mixed ion-radical mechanism. Obviously, the trans-double bonds started breaking after 60 min. The initial weight increase stopped which indicates for destructive processes.
Unlike butadiene rubber, the sulfur vulcanizate based on it reacted with nitric acid by mixed ion-radical mechanism from the beginning [12]. During the very first 30 s at temperature of 20 ºC, the absorption band characteristic for the nitro-group appeared with small intensity. Simultaneously, the absorption band characteristic for the ONO 2 group was registered with much higher intensity. After 5 min, the intensities of the two bands were almost equal but until the end of the experiment the second band (ONO 2 ) remained with higher intensity (Fig. 3).
At the same moment (5 min), intense isomerization of cis-structures into trans-structures began (curve 5). After 10 min, the intensity of the band corresponding to the trans- double bond
time (min)
Fig. 2
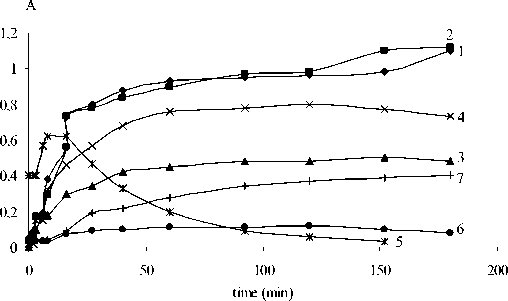
Fig. 3
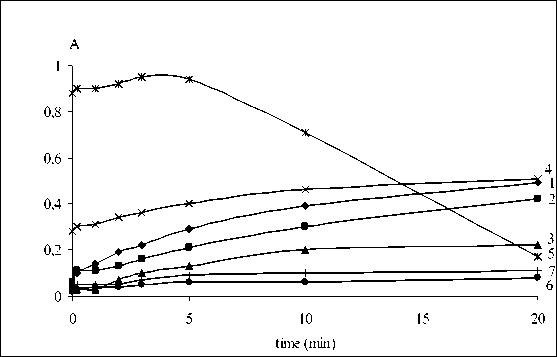
Fig. 4
strongly decreased while that indicating for incorporation of nitric acid and nitrogen oxide increased (curves 1-4). The process of oxidation expressed by the amount of OH and C=O groups was suppressed. The insignificant slope of curves 1-4, 6 and 7 after 60 min of interaction was probably due to densification of the vulcanizate film as a result of the cross-linking processes taking place. When the interaction was carried out at 60 ºC, the most intensive processes were these of cis-trans isomerization of the double bond and nitration (Fig. 4, curves 4, 5). Intense breaking of the double bond began after the 5th minute of contact accompanied by abrupt decrease of sample weight as a result of the exuberant destructive processes which lead to destruction of the film itself after 20 min.
The formation of nitrogen oxide during the first seconds of the interaction between the sulfur vulcanizate and the nitric acid was probably due to a process during which the nitric acid is reduced to release nitrogen oxide.
As can be seen from Fig. 1-4, the resistance of rubber in nitric acid medium was significantly better than that of the sulfur vulcanizate. Under all the reaction conditions employed, the vulcanizate films were destroyed for much shorter period of interaction.
Supposedly, oxidation processes take place in the rubber component by the roll milling and vulcanization which, together with the sulfur bridges formed, worsen the resistance to aggressive reagents.
Conclusions
-
1. The initial stage of the interaction between 1,4-cis-butadiene rubberand sulfur vulcanizate based on it with nitic acid was studied by differential IR spectroscopy.
-
2. It was proved that cis, trans isomerization of the double bonds occurs during the process of interaction between the rubber and vulcanizate with nitric acid under the influence of the nitrogen oxide released by the nitric acid.
-
3. The resistance of rubber was proved to be better than that of the vulcanizate in nitric acid medium.
Список литературы Studies on the changes, occurring at the initial stage of the interaction of btadiene rubber and its vulkanized produkt with nitric acid
- Авторско свидетелство, № 20483, Бoлгария.
- Генов Г., Младенов Ив., Юбелейна научна сесия на ВХТИ. -Бургас, 4-6.10.1974, Болгария.
- Dimov М., Stoeva S., Tsaikova S: Interaction of Nitric Acid with Rubber Chunks Derived From Waste Tires. Oxidation Communications, 2008. 31, 4, 931-941.
- Me Myrry H.L., Thornton V., Analyt. Chem., 1959, 24, 318.
- Салимов М.А. Азербаджанский химический журнал, 1960, 3, 45-53.
- Ермакова И.И., Долгополски Б.А., Кропачева Е.Н., ДАН СССР, 1961, 141(6), 1363-1365.
- Малышев А.И., Помагайбо А.С. Анализ резин, Москва: Химия, 1977, 192 с.
- Тулторский И.А., Дюлмаева Т.Н., Методическое руководство. Ч. 6 II. ИК-спектроскопии. Институт тонкой хим. техн. Москва, 1975.
- Дайер Дж. Приложения абсорционной спектроскопии органических соединений. Москва: Химия, 1970.
- Спасов С., Арнаудов М. Приложение на спектроскопията в органичната химия. София, Наука и изкуство, 1978, Болгария.
- Силверстейн Р., Басслер Г., Моррил Т. Спектроскопическая индентификация органических соединений. Москва: Мир, 1977
- Tsaikova S., Stoeva S., Georgiev T., Tsaikov P. Interaction of 1,4-cis-polybutadiene with Nitric Acid. Oxidation Communications, 2005. 28, 2, 341-351.


