Study of the tolerance of ten accessions of carrot ( Daucus carota L.) to salinity
Автор: Kahouli Basma, Borgi Zied, Hannachi Chrif
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.10, 2014 года.
Бесплатный доступ
The present work has focused on the assessment of the tolerance of ten accessions of carrot (L 1, L 2, L 3, F 4, E 5, R 7, R 8, L 10, G 11 and S 12) to salt stress, grown in the region of Sidi Bouzid. The tests were carried out under plastic greenhouse (during 5 months). The results obtained show that the salt stress reduced the parameters of growth and production. However, a difference in the response to salt stress was recorded between the accessions studied. A reduction in yield of up to 70 % with the concentration 3 g / l of NaCl, for different accessions studied is recorded. However, the accession L 1 who has given the longer roots and dry matter yield and the largest root shows the most tolerant accessions unlike L 10, G 11 and S 12 which are the most sensitive.
Carrot, accessions, salt stress, dry matter
Короткий адрес: https://sciup.org/14323886
IDR: 14323886
Текст научной статьи Study of the tolerance of ten accessions of carrot ( Daucus carota L.) to salinity
Several environmental constraints are limiting for the growth and development of the plant. Salinity and drought are considered as two major factors affecting agriculture in arid and semi arid areas. Salinity affects approximately 5% of cultivated land in the world (Munns et al ., 1999).
The carrot is a glycophyte plant. Therefore the salinity may be one of major factors that affect the yield in irrigated areas, including the arid and semi-arid areas, which are characterized by high evaporation of water from soil and irregular rainfall and inadequate (Munns et al., 2006). The culture of the carrot need of contributions of water relatively important, while irrigation water used in Tunisia are often loaded with salt. Indeed, the water from the dams has a load salt of 2 to 3 g/l, and the wells as 4 to 7 g/l (Hachicha and Braudeau, 1998), as a result of overuse or intrusion of brackish water or seawater. On the other hand, trying to combat the drought by irrigation, added to the soil a constraint called secondary salinisation. In this context, improving plant tolerance to salinity is an attractive proposition for farmers. In fact, the carrot is considered sensitive (Berstain and Ayers, 1953 Gibberd et al, 2002) or moderately sensitive (Maas and Hoffman, 1977; Matsubara and Tasaka, 1988. Mangal et al., 1989) to salinity. When the conductivity electrical (CE) is 1.5 ds/m, the yield decline of 35% and can reach 50% reduction to a CE of the order of 2.5 dS/m (Unlukara et al., 2011). Indeed, salinity reduces the growth and productivity of the culture due to the decrease in the osmotic potential in soil and increase in the concentration of Na+ and Cl-, which then reaches a toxic level for the plant (Chartzoulakis and Klapaki 2000). The imbalance of the ionic balance induced by salt affects directly and/or indirectly many physiological and metabolic processes reflected inhibiting plant growth (Munns, 2002).
The objective of our work is to study, in ten accessions of carrot grown in the region of Sidi Bouzid, the effect of salinity on plant growth and root yield. And to subsequently identify which could be irrigated with brackish water unless his performance is significantly decreased.
MATERIALS AND METHODS
The plant material used consists of ten accessions of cultivated carrot, their seeds were collected in the region of Sidi Bouzid from seven areas: Lessouda (3 accessions: L 1 , L 2 and L 3 ) Faid (1 accession: F 4 ) El Ogla (1 accession: E 5 ) Regueb (2 accessions: R 7 and R 8 ), Lahweze (1 accession: L 10 ), Garet Hdid (1 accession: G 11 ) and Souk Jadid (1 accession: S 12 ). The experiment was performed under plastic greenhouse of the Regional Agricultural Research Center. The seeds of ten accessions of carrot studied are sown in the soil (30 January). Seedlings were irrigated with tap water (salinity 1.7) for two months. When seedlings have reached the required dimensions (two leaves), they are subjected to salt stress by adding different doses of sodium chloride in tap water (3, 6 and 9 g/l NaCl). These concentrations are close to the salinity of irrigation water in Tunisia. Throughout the trial period irrigation is four times/week. The test was conducted in a completely randomized block device (BAC), with three blocks (replicates). For each repetition, there are 25 plants. The total number of plants was 3000. After five months of culture, the measures interested height and diameter of the main stem which are respectively determined by graduated ruler and a vernier caliper. Plants are harvested and separated into aerial part and root part. The roots are rinsed four or five times with distilled water, and then carefully blotted with filter paper water. These bodies are quickly put in paper bags aluminum previously tared, and then are weighed. MS is determined using a precision balance after spending 48 hours in an oven at 70 ° C.
Production was characterized by the weight, length, diameter and the root diameter. For each parameter measured, the averages of the various treatments and their ecartypes are determined. To release the effects of saline treatment, the results are submitted to analysis of variance using statistical software "SPSS 13.00". The comparison of the means was performed by the Student-Newman-Keuls test threshold probability of 5%.
RESULTS
Height growth of plants
The results presented in Figure 1, show that without NaCl the plants of carrot aged five months have a length of the main stem varying from 20 (accession L 10 ) to 25 cm (accession L 1 ). But, in the presence of NaCl, the main stem of the plants of ten accessions shortens depending on the concentration. For example, the concentration 9 g/l caused a reduction in the length ranging from 30% (accession R 7 ) to 54 % (accession L 10 ) compared to control plants. These results are consistent with those of Abou El- Magd et al ., (2008) and Cemek et al ., (2011). When the diameter of the stem varies from 4,4 (accession R 7 ) to 5,3 mm (accession L 1 ), without salt stress (Fig. 2). However, under salt stress, it decreases with the concentration of NaCl (3 to 9 g/l). The concentration 9 g/l caused a reduction ranging from 26% (accessions R 7 and L 2 ) to 47% (accession L 10 ) relative to the corresponding control plants.
Our results remind of those of Kusvuran et al. (2007) and Ibn Maaouia (2011) which showed that the growth of stems decreases when the salinity exceeds 4 g/l. This depressive effect can have two causes non- exclusive. First, difficulties of water supply and nutriments and second the toxicity of ions accumulated in excess in the plant (Xiong and Zhu, 2002). According to Zhu (2001), reducing growth of the air parts is an adaptive capacity necessary for the survival of plants exposed to abiotic stress. Indeed, the delay of development allows the plant to accumulate energy and resources to combat stress before the imbalance between the inside and outside the organism increases up to a threshold where damage is irreversible. In fact, reducing growth can result from the increase of abscisic acid concentration in the air part or a reduction in cytokinin concentration (Itai, 1999).
Yield and fruit quality
The total yield of the ten accessions studied was significantly reduced by salinity (NaCl) due to a significant decrease in average fruit weight, these results are also observed by De Pascale and Barbieri (2000); Fabre et al ., (2011) and Unlukara et al ., (2011). In fact, these reductions were even greater than the salt concentration was high (Table 1). For example, the presence of the highest concentration (9 g/l NaCl) in irrigation water procreated a reduction of the yield per plant in two accessions R 7 and L 1 of 66 and 71% respectively compared to the control. Three accessions G 11 , S 12 and L 10 were more sensitive to salt accusing respective reductions of 84, 85 and 90% compared to control plants. Indeed, Maas (1986) reported that root yield declined 14% for each increase unit of salinity beyond the threshold of 1.0 dS/m. Matsubara and Tasaka (1988) noted a reduction of 50% of the fresh weight root of carrot with
NaCl concentrations ranging from 68 to 102 mM, same Mangal et al ., (1989) reported the same reduction with a salinity of 78 mM.
Similarly, the length of the root decreases with increasing salinity confirming the study of Unlukara et al ., (2011) on carrot. The results, presented in Table 1, suggest that without salt stress, the length of the root varies according to accession. Indeed, both accessions L 2 and G 11 have the longest root respectively comprised between 14,9 and 14,8 cm. While both accessions L 10 and S 12 gave the shortest root, respectively comprised between 13 and 13,2 cm. But, once the plants are stressed, the length of the root decreases with the increase of the concentration of NaCl. For example, with the highest concentration (9 g/l), in both S 12 and E 5 accessions, reducing of the length represent respectively 12 and 34% compared to control plants. In other words, even in the presence of the highest concentration (9 g/l), carrot can give a root, however, the latter does not exceed 12 cm for ten accessions studied. As well as the salinity has further reduced the growth of the aerial parts of carrot compared to the roots, this is in agree with the results of the work of Ibn Maaouia (2011).
The diameter of the root, which is another qualitative character, decreased depending on the concentration of NaCl and confirming the results of other authors (Öztürk, 1997; Unlukara et al., 2011.). In fact, in absence of salt, two accessions L2 and E 5 present respectively the highest diameter 16,8 and 16,9 mm. On the other hand, the two accessions G11 and S12 present the lowest diameter which is in around 13,3 mm. However, in the presence of salt in the culture medium, the diameter of the root has decreased according the concentration of NaCl. The highest falls are noted in plants stressed by 9 g/l of NaCl, they are (compared with control plants) of -37 (accession L1) to 52% (accession L10). However, irrigation water containing salt caused a reduction in the diameter of the heart of the root according to Table 1, plants grown in the presence of 6 or 9 g/l did not differ to each other. While, the plants grown in the presence of 3 g /l have the lowest diameter of 2,5 (accession S12) to 3,9 mm (accession E5 ). The decrease in yield is correlated with the decrease in diameter which means that carrot yield reductions were the result of the decrease in diameter, this is consistent with the work of Unlukara et al., (2011).
Dry matter yield
About producing dry matter, plants grown in absence of salt stress gave a total whole-plant dry matter yield between 1,5 (accession L10) and 3,4 g/plant (accession L2) (fig. 3). But under salt stress conditions, the whole-plant dry matter yield decreases as the concentration of NaCl. Indeed, in the presence of the highest concentration (9 g/l), the yield of the ten accessions is included between 0,3 g/plant (accessions L10, G11 and S12) and 0,7 g/plant (accessions L1, E 5 and R7). The reduction in dry matter of the whole plant, subject to an increasing concentration of NaCl (3 to 9 g/l), also concerned the root part as the aerial part. In addition, the lowest dry matter yield of aerial parts (leaves and stems) and roots of ten accessions is obtained in the presence of the highest concentration of NaCl (9 g/l). Indeed, accession R7 is less sensitive to salt treatment accusing reductions compared to control of 38% for the aerial part and 78% for roots. ON the other hand, accession L10 shows the most sensitive since the rate of reduction is 85% for the aerial part and 89% for the roots. These results resemble those obtained by Kaya and Higgs (2002) on cucumber; Graifenberg et al., (1996) on fennel and Rode and Nothnagel (2012) on carrot. Salt affects more producing root biomass than the aerial parts, because usually it is the absorption bodies how show greater sensitivity to salt stress as photosynthetic organs (Brugnoli and Bjorkman, 1992) stress. Furthermore, reduced dimensions of the plant (length and diameter of the stem) and biomass (root and aerial part) is a result of a reduction in the number of cells or chlorophyll content, or both causing a decrease in the synthesis of dry matter (Kaya and Higgs, 2002; Hannachi, 2008).
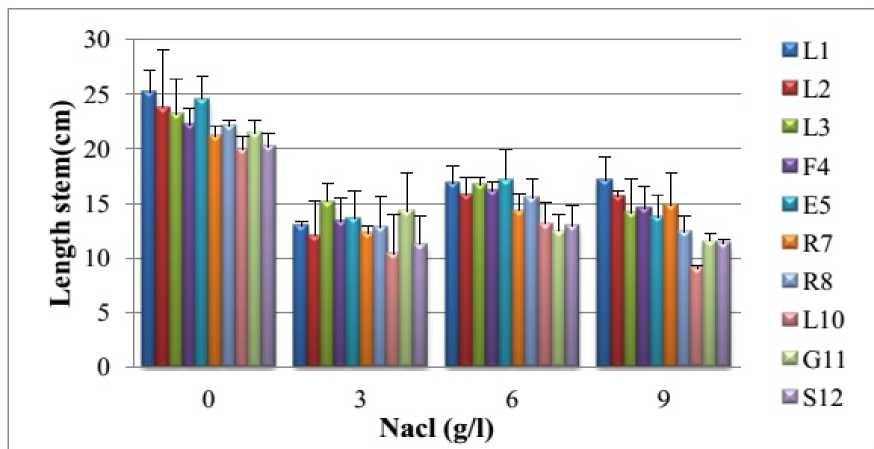
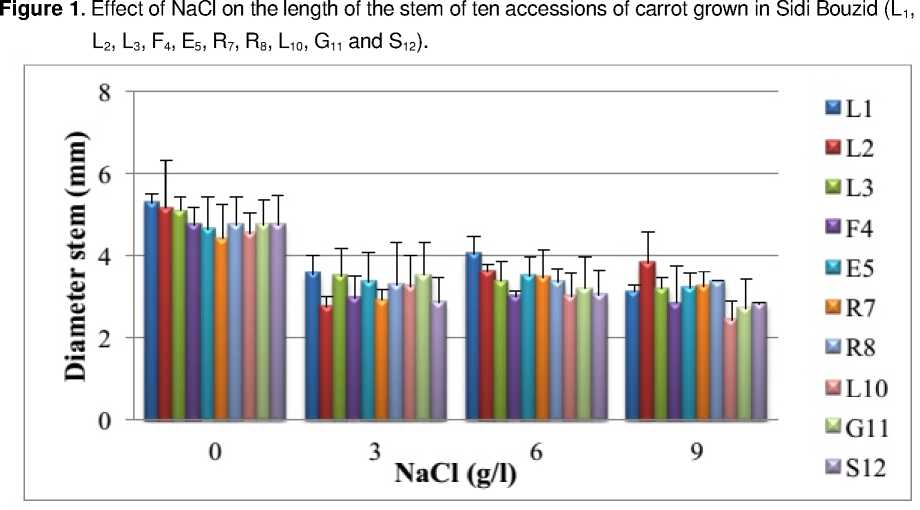
Figure 2. Effect of NaCl on the diameter of the stem of ten accessions of carrot grown in Sidi Bouzid (L 1 , L 2 , L 3 , F 4 , E 5 , R 7 , R 8 , L 10 , G 11 and S 12 ).
Table 1 Treatment effects of sodium chloride on the average weight, length, diameter and the diameter of the heart of the root
|
Accessions /NaCl (g/1) |
Average weight (g) |
Length (cm) |
Diameter (mm) |
Diameter heart (mm) |
|
L1 |
||||
|
0 |
16,29a |
13,66a |
16,59a |
6,54a |
|
3 |
4,69b |
11,20a |
11,32b |
3,77b |
|
6 |
5,54b |
13,16a |
11,16b |
3,91b |
|
9 |
4,66b |
11,59a |
10,46b |
3,91b |
|
L2 |
||||
|
0 |
17,31a |
14,88a |
16,77a |
5,97a |
|
3 |
2,84b |
9,72b |
9,62b |
2,69b |
|
6 |
3,77b |
12,22ab |
9,20b |
3,14b |
|
9 |
3,33b |
11,28ab |
9,92b |
3,57b |
|
L3 |
||||
|
0 |
16,97a |
14,73a |
16,49a |
5,90a |
|
3 |
3,92b |
11,10b |
10,42b |
3,16b |
|
6 |
4,50b |
12,25ab |
10,18b |
3,63b |
|
9 |
3,56b |
10,83b |
9,62b |
3,18b |
|
F4 |
||||
|
0 |
13,53a |
13,65a |
15,35a |
5,93a |
|
3 |
3,98b |
9,25b |
10,69b |
3,29b |
|
6 |
4,10b |
11,22ab |
10,36b |
3,46b |
|
9 |
3,40b |
11,08ab |
9,34b |
3,43b |
|
E5 |
||||
|
0 |
18,48a |
13,81a |
16,98a |
6,56a |
|
3 |
4,43b |
11,09a |
11,22b |
3,92ab |
|
6 |
6,58b |
12,33a |
12,23b |
4,19ab |
|
9 |
3,68b |
12,15a |
9,23b |
3,20b |
|
R7 |
||||
|
0 |
13,29a |
14,00a |
15,48a |
5,87a |
|
3 |
3,48b |
8,77b |
10,90b |
3,04b |
|
6 |
5,00b |
11,65ab |
10,42b |
3,73b |
|
9 |
4,57b |
11,15ab |
9,13b |
3,55b |
|
R8 |
||||
|
0 |
14,13a |
14,73a |
16,57a |
6,98a |
|
3 |
4,18b |
9,10b |
11,94b |
3,70b |
|
6 |
4,36b |
11,52b |
10,37b |
3,69b |
|
9 |
2,52b |
10,58b |
9,10b |
2,66b |
|
L10 |
||||
|
0 |
12,61a |
13,01a |
15,21a |
5,22a |
|
3 |
3,58b |
10,56a |
10,03b |
3,24a |
|
6 |
3,00b |
11,03a |
8,78b |
3,03a |
|
9 |
1,30b |
10,62a |
7,31b |
2,69a |
|
G11 |
||||
|
0 |
10,44a |
14,77a |
13,29a |
4,68a |
|
3 |
3,87b |
11,05ab |
9,99b |
3,20ab |
|
6 |
2,22b |
9,51b |
8,41b |
2,72b |
|
9 |
1,64b |
11,89ab |
7,67b |
2,60b |
|
S12 |
||||
|
0 |
10,23a |
13,17a |
13,27a |
4,70a |
|
3 |
2,36b |
10,39b |
8,29b |
2,54b |
|
6 |
3,36b |
10,55b |
9,47b |
3,31b |
|
9 |
1,57b |
8,63b |
7,48b |
2,63b |
Means followed by the same letter are not significantly different at 5% level according to Student-Newman-Keuls test.
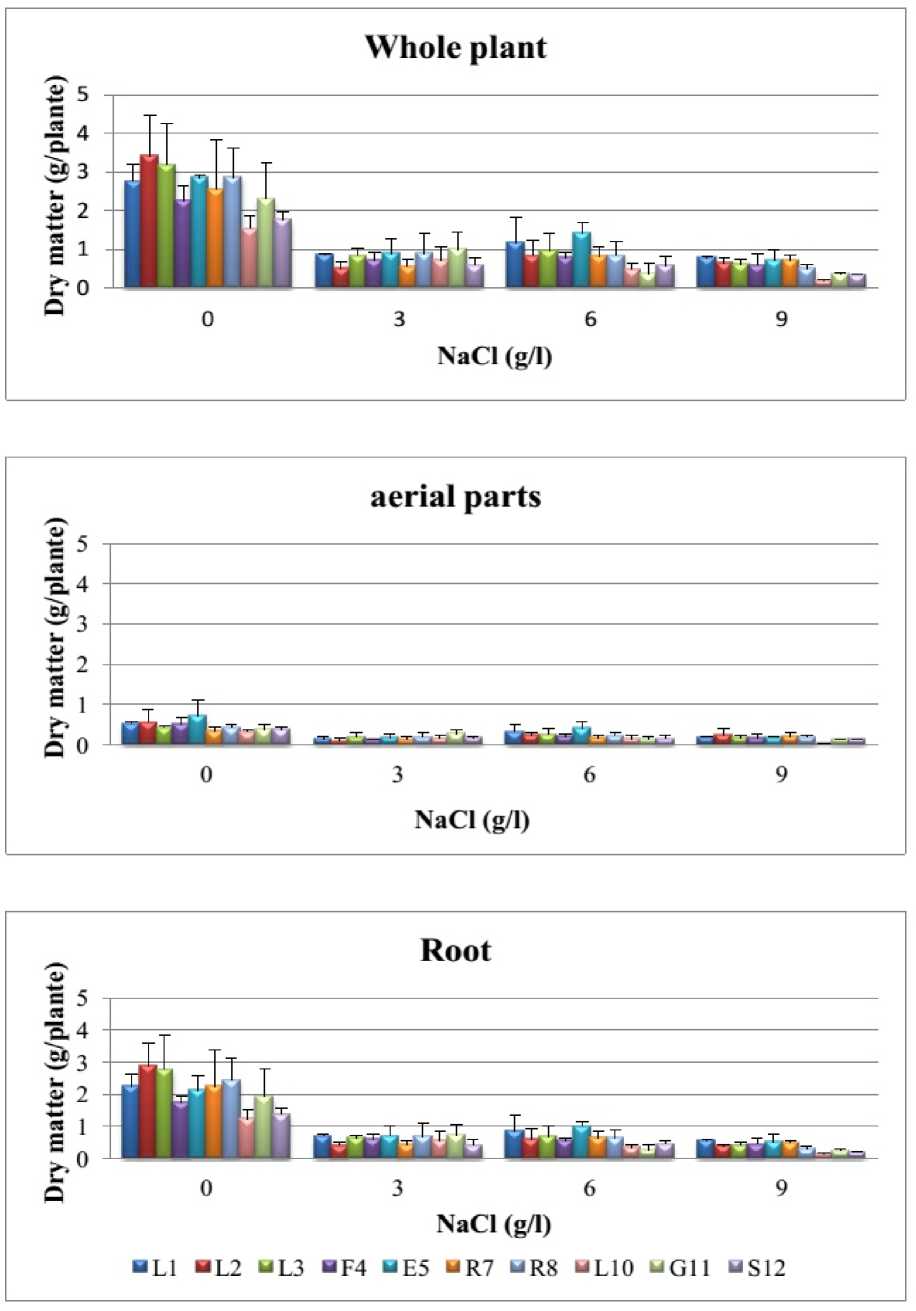
Figure 3 . Effect of NaCl on producing dry matter of the whole plant and its two parts air and root in the ten accessions of carrot grown in the region of Sidi Bouzid (L 1 , L 2 , L 3 , F 4 , E5, R7, R8, L10, G11 and S 12 ).
CONCLUSION
Список литературы Study of the tolerance of ten accessions of carrot ( Daucus carota L.) to salinity
- Abou El-Magd, M.M., Zaki, M.F. and Abou-Hussein, S.D. (2008) Effect of organic manure and different levels of saline irrigation water on growth, green yield and chemical content of sweet Fennel. Austarlian Journal of Basic and Applied Sciences, 2(1): 90-98
- Allah, C.R. (1991) Grown tomato under saline conditions. In: Allah CR, ed. Plant Salinity. Research, New Chellenges, 201-212
- Berstein, L. and Ayers, A.D. (1953) Salt tolerance of five varieties of carrots. Journal of American Society of Horticultural Science, 61, 360-366
- Brügnoli, E. and Björkman, O. (1992) Growth of cotton under continuous salinity stress: influence on allocation pattern, stomatal and non-stomatal components of photosynthesis and dissipation of excess light energy, Planta, 187, 335-347
- Cemek, B., Unlukara, A., Karaman, S. and Gokalp, Z. (2011) Effects of evapotranspiration and soil salinity on some growth parameters and yield of lettuce (Lactuca sativa var. Crispa). Zemdirbyste=Agriculture, 98(2), 139-148
- Chartzoulakis, K. and Klapaki, G. (2000) Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Scientia Horticulturae, 86, 247-260
- De Pascale, S. and Barbieri, G. (2000) Yield and quality of carrot as affected by soil salinity from long-term irrigation with saline water. Proc. 3rd IS on Irrigation Hort. Crops Eds. Ferreira & Jones. Acta Hort., 537, 621-628
- Fabre, R., Duval, M. et Jeannequin, B. (2011) Influence de la salinité sur la qualité gustative et le rendement de tomates greffées cultivées hors-sol sous serre chauffée dans le sud de la France. Cah. Agric., 20, 266-273
- Gibberd, M.R., Turner, N.C. and Storey, R. (2002) Influence of saline irrigation on growth, ion accumulation and partitioning, and leaf gas exchange of carrot (Daucus carota L.). Annals of Botany, 90, 715-724
- Graifenberg, A., Botrini, L., Giustiniani, L. and Lipucci Di Paola, M. (1996) Salinity affects growth, yield and elemental concentrations of fennel. HortScience, 31 (7), 1131-1134
- Hachicha, M. et Braudeau, E. (1998) Irrigation et salinisation en Tunisie, Sols de Tunisie, 18, 3-11
- Ibn Maaouia, S.H., Denden, M., Dridi, B.M. et Ben Mansour, S.G. (2011) Caractéristiques de la production en fruits chez trois variétés de piment (Capsicum annuum L.) sous stress salin. Tropicultura, 29(2), 75-81
- Itai, C. (1999) Role of phytohormones in plant responses to stresses. In: Lerner H.R. (ed). Plant response to environmental stresses, from phytohormones to genome reorganization. Marcel Dekker Inc., Basel, NY, USA, pp. 287-301
- Kaya, C. and Higgs, D. (2002) Calcium nitrate as a remedy for salt-stressed cucumber plants. Journal of Plant Nutrition, 25 (4), 861-871
- Kusvuran, S., Yasar, F., Ellialtioglu, S. and Abak, K. (2007) Utilizing Some of Screening Methots in Order to Determine of Tolerance of Salt Stress in the Melon (Cucumis melo L.). Research Journal of Agriculture and Biological Sciences, 3(1), 40-45
- Levigneron, A., Lopez, F., Vansuyt, G., Berthomieu, P., Fourcroy, P. et Casse -Delbart, F. (1995) Les plantes face au stress salin, Cahiers Agricultures, 4, 263-273
- Maas, E.V. (1986) Salt tolerance of plants. Appl. Agric. Res., 1, 12-26
- Mangal, J.L., Lal, S. and Hooda, P.S. (1989) Salt tolerance in carrot seed crop. Haryana Agricultural University Journal of Research, 19, 9-18
- Matsubara, S. and Tasaka, Y. (1988) Studies of salt tolerance of vegetables. II. Sand culture. Scientific reports of Faculty of Agriculture. Okayama University, 72, 9-18
- Munns, R. (2002) Comparative physiology of salt and water stress. Plant Cell Environment, 25, 239-250
- Munns, R., Cramer, G.R and Ball, M.C. (1999) Interactions between rising CO2, soil salinity and plant growth. In: Luo Y, Mooney HA, eds. Carbon dioxide and environmental stress. London: Academic Press
- Munns, R., James, R.A. and Lauchli, A. (2006) Approaches to increasing the salt tolerance of wheat and other cereals. Journal of Experimental Botany, 57, 1025-1043
- Munns, R. and Passioura, J.B. (1984) Hydraulic resistance of plants. III. Effects of NaCl in barley and lupin. Aust. J. Plant Physiol, 11(5), 351-359
- Ozturk, A. (1997) Sulama suyu tuzluluğu ve taban suyu değisiminin havuc bitkisinin bazı ozellikleri uzerine etkileri. Tarım Bilimleri Dergisi, 3(1), 54-58
- Rode, A., and Nothnagel, T. (2012) Developing methods to evaluate salt stress tolerance in carrot cultivars. Proc. Vth Balkan Symp. on Vegetables and Potatoes. Eds.: A. Balliu and N. Gruda. Acta Hort. 960, ISHS, 393-400
- Unlukara, A., Cemek, B., Kesmez, D. and Ozturk, A. (2011) Carrot (Daucus carota L.): yield and quality under saline conditions. Andalu. J. Sci., 26(1), 51-56
- Xiong, L. and Zhu, J.K. (2002) Salt torerance, The Arabidopsis Book, American Society of Plant Biologists, 1-22
- Zhu, J.K. (2001) Plant salt tolerance. Trends in Plant Science, 6, 66-71


