Study on the effect of paraquat dichloride’s (PD) acute toxicity on Anabas testudineus (Bloch, 1792)
Автор: Mandal Ganga, Mandal Sayan, Mandal Basudev
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.20, 2024 года.
Бесплатный доступ
A commonly used herbicide in agriculture, paraquat dichloride (PD) has caused a great deal of concern due to its high toxicity and potential impact on the environment. The present study aims to study the effect of paraquat dichloride’s (PD) acute toxicity, behavioural and morphological changes on Anabas testudineus. The species Anabas testudineus , also known as the climbing Perch. Fish were treated with five different doses of PD concentrations in a Fishery Science lab to estimate the LC50 value. The probit analysis method was used to calculate the LC50 value for PD exposure. Fish exposed to (PD) exhibited behavioral abnormalities such as altered nervous behavior, elevated stress response, and respiratory distress. When exposed fish were examined morphologically, several abnormalities were found, such as Sclerosis in the head and tail region, Mucous layer on the whole body, Blood from gills, Redness in eyes, Belly swelling, Red color appearing in the head and tail region, pelvic fin, and anal fin destroyed. The finding of the study shows, Anabas testudineus exposed to PD had an LC50 value of 116.94 mgL-1, which implies the level of toxicity concentration. These results indicate that exposure to (PD) influences the behavior and external morphology of Anabas testudineus . This study emphasizes how PD affects freshwater fish, specifically Anabas testudineus , in an acute toxicological way. In addition, observed morphological and behavioral changes highlight the significance of tracking and controlling the use of PD in agricultural practices to minimize any potential adverse environmental impacts and protect the aquatic ecosystem.
Anabas testudineus, herbicide, lc50, morphology, toxicity
Короткий адрес: https://sciup.org/143182813
IDR: 143182813
Текст научной статьи Study on the effect of paraquat dichloride’s (PD) acute toxicity on Anabas testudineus (Bloch, 1792)
The population has grown enormously in recent years, and rising agricultural productivity has made it possible to maintain this huge population. Use a variety of agrochemicals, such as insecticides, fertilizers, herbicides, and pesticides, that have either direct or indirect toxicological effects on the environment from the application site in order to increase agricultural productivity. Chemical analysis can determine the amount of environmentally toxic components, but it cannot determine the impacts of these components on aquatic organisms; therefore, an experiment involving mortality or bioassay is essential for assessing these components' effects (Bagheri, 2007). As a result of their intimate connection to the aquatic environment, fish rapidly exhibit measurable changes in response to physical and chemical changes in their surroundings (Adhikari et al. 2004).
Paraquat dichloride, also known as , -dimethyl- ,4, 4B Pyridinium chloride, is a non-selective contact herbicide that is used to eliminate vegetative pests. It is used to control aquatic plants and terrestrial weeds in various countries and has been discovered in several water sources around the world (Filizadeh, 2002; Ye et.al., 2002; Ismail et al., 20 ). More than 00 crops, including corn, rice, soya, wheat, potatoes, apples, oranges, bananas, beverages, coffee, tea, cocoa, cotton, oils, palm, sugarcane rubber, etc. have been treated using paraquat both before and after planting to control weeds (Pic, 20 7). since paraquat herbicides are inexpensive and efficiently eliminate weeds quickly, almost all farmers use them. As soon as paraquat comes into contact with soil, it dissolves and dissociates quickly in an aqueous solution, is quickly absorbed, mixes with the soil particles, and is leached to the aquatic field by surface leaching, and then accumulates in aquatic biota. When paraquat falls into water, it breaks down into 4-carboxy- -methyl Pyridium ion and methylamine through biochemical and photochemical processes (Kearney et al., 985; Eisler, 990). According to Martin-Rubi et al. (2007), Sureda et al. (2006), and Banaee et al. (20 3)a,b, it accumulates in aquatic organisms during the degradation period, particularly in fish. Auto-oxidation and photochemical disintegration of paraquat produce many products, including hydrogen peroxide, superoxide radical, oxygen, and hydroxyl radical, which are highly important in causing oxidative stress, cellular toxicity, and pathological changes in animal and plant cells.
It's crucial to test the toxicity levels of aquatic pollution for the lowest possible concentrations that the environment can tolerate. Therefore, it's significant to figure out the acute toxic LC 50 value to evaluate the safety level of any chemicals for fish. According to Richmonds and Dutta ( 992), the best way to identify toxic pollution is through behavioral changes. The crucial elements in critical biological monitoring are the morphological and behavioral alterations in the toxicity test. According to Nowak ( 992), Lapido et al. (20 ), Dutta et al. ( 994), Senapati et al. (20 3), and other researchers, a number of literature and works have been reported on various fish species against insecticide, herbicide, and heavy metal, which can alter behaviour, morphology, biochemistry, and histopathological changes. Pandey et al. (20 ) investigated the acute toxicity and behavioral changes in Channa punctatus (Bloch) caused by the organophosphate pesticide profenofos. This study aims to evaluate the 96-hour LC 50 value, behavioural, morphological, and temperature effects on freshwater climbing perch Anabas testudineus (Bloch, 792) during exposure in order to fill the knowledge gap regarding the ecological impact of this herbicide. Anabas testudineus was chosen as the model for this experiment due to its distinctive characteristics, which include its ability to adapt to laboratory conditions with comfort, its yearround availability, its high tolerance, hardness, ability to breathe air, and its ability to live in wet areas of Indian paddy fields.
MATERIALS AND METHODS
Study site and G-map.
They are shown on Figure .
Species collection:
Test Organisms:
For this toxicity test, mature, healthy adult Anabas testudineus with a mean weight of 55 ± 20.76 g and a mean length of 6 ± 0.99cm were used. Fishes for the experiment were collected from East and West Midnapore of West Bengal. This fish was chosen due to its throughout-the-year availability, hardness, high-stress tolerance, and lab adaptability.
Acclimation of the test organisms:
After they were collected from the market, the fish were maintained in a static environment for two weeks in cement tanks constructed in the Department of Fishery Sciences' outdoor culture unit at Vidyasagar University. This allowed the fish to easily acclimatize to their new environment. Before fish were released, the top of the cement tank was covered with a net to prevent fish escape. The cement tank was thoroughly cleaned and filled with dechlorinated tap water. The water was kept at the necessary temperature, alkalinity, PH, DO, and oxygenation level during the acclimatization phase using an air pump followed by APHA (20 2). To prevent dermal infection, the fish were also sanitized with 0. % KmNO 4 solution and were fed a commercial food palate twice a day. The feeding schedule was interrupted one day before the toxicity test.
Test chemical:
The herbicide applied in the study was commercial paraquat dichloride preparations under the trade name Rider 24% SL(W/W), which was purchased from the local market in the Medinipur, west Bengal, India. It was manufactured by M/s ADVANCE AGROLIFE PVT LTD, E-39, RIICO Industrial Area, Bagru (Ext.) Jaipur, Rajasthan-303007, under registration number CIR-04688/20 3. This herbicide was liquid and had a deep green colour.
Preparation of test chemical:
-
V C =V 2 C 2
The chemical was prepared using this formula.
where V is the volume of stock used (ml), C is the initial concentration of the stock solution (gL- ), C 2 is the desired concentration (gL- ), and V 2 is the volume of water needed for dilution (ml).
The following formulae were used to prepare the paraquat range finding test: 00 mgL- , 200 mgL- , 400 mgL- , and 800 mgL- .
In the same way, volume and concentration were prepared using the following methods for the paraquat acute toxicity test: 50 mgL- , 00 mgL- , 200 mgL- , and 250 mgL- .
The determination of the physio-chemical parameter of the test water:
Before the acute toxicity test, the test water's physiochemical parameters were measured using multifunction water testing kits (Transchem). According to the APHA (20 2), these parameters, which include dissolved oxygen (D.O.), PH, water temperature, alkalinity, and BOD, were measured.
Experimental design:
A non-renewal, 96-hour short-term static bioassay was performed utilizing Anabas testudineus. A total of 60 adult, healthy Anabas test subjects were obtained and distributed into 6 treatment groups, control group, and 5 concentration groups of paraquat. The test aquariums were cleaned, filled with 40L of dechlorinated tap water, aerated, and maintained in the same conditions as the acclimatization period before the toxicity test.
Range finding test:
Range finding tests, also known as preliminary tests, were conducted frequently with a wide range of paraquat concentrations. To reach a 00 % death to no effect result, the maximum concentration to minimum concentration was used to begin this test. The rangefinding test helped to determine the acute toxicity concentration and was performed every 24 hours, 48 hours, 72 hours, and 96 hours.
Definite Test:
Acute toxicity test for 24 hours, 48 hours, 72 hours, and 96 hours, 60 adults with healthy Anabas testudineus were exposed to control, 50 mgL- , 00 mgL- , 50 mgL- , 200 mgL- , and 250 mgL- . Feeding was discontinued and the water was unchanged during the test. Every two hours, mortality was observed, and during this period, morphological and behavioural changes were also observed. Fish were regarded as dead when their mouths and opercula stopped moving in response to mechanical stimuli. Fish that were dead were removed instantly, and LC 50 values were calculated based on their mortalities.
Statistical Analysis:
Probit analysis (Finny, 97 ) was used for calculating the mortality results and obtaining the LC50 value of Anabas testudineus at different levels of paraquat dichloride. Microsoft Excel 202 edition, windows analysis of variance (ANOVA) was utilized to perform a significant analysis between the test and control treatments with a confidence level of 95 %.
RESULTS
Physio-chemical parameter of the test water:
DO 6.92 ± 0.83mgL- , pH-7.0-7.5, the temperature in winter and summer are 4.4 ± .85 °C and 30.8 ± 2. 3 °C.
Acute toxicity test:
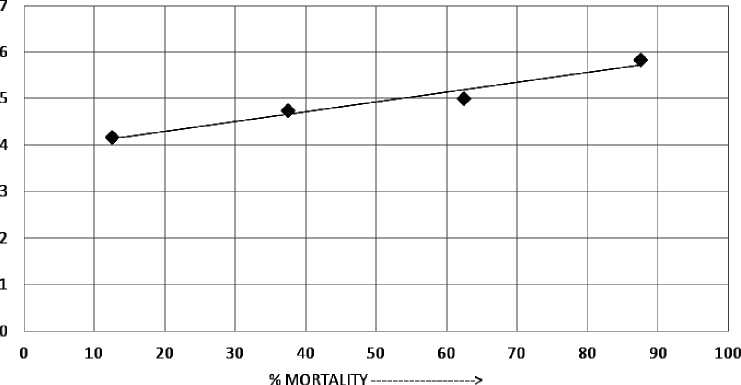
According to Table 's acute toxicity testing findings, no deaths occurred during the control treatment; however, as the concentration improved and the exposure duration increased, the death rate progressively increased. The test reveals mortality rates of 20 % at 50 mgL- , 50 % at 50 mgL- , and 00 % at 250 mgL- after 96 hours. 6.94 mgL- is the LC 50 value calculated by this test using probit analysis (Finney, 972). Fish mortality increased as PD concentration increased, corresponding to a linear relationship between probit mortality and PD concentration, indicating a positive correlation (r2=0.9596) (Figure 2).
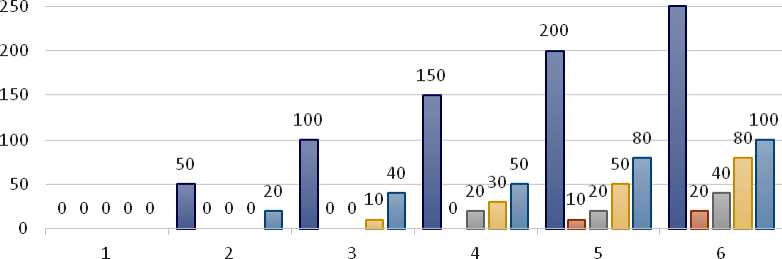
Fish mortality is provided in the following chart for various concentrations and exposure durations. Fish mortality increased with exposure time and concentration, but it was not observed at the control unit or 0h. With 250 mgL- , 20 % mortality happens at 24 hours, and at 96 hours, 00 % mortality increases. There was no mortality at 24 hours for 50 mgL- , but at 96 hours, there was 20 % mortality (Figure 3). These findings demonstrate a proportionate relationship between Anabas testudineus mortality and PD concentration and time.
Two tables (3,4) describe behavioral changes related to swimming or movements, while Table 4 describes behavioural changes related to respiratory distress.
Table 3 and Figure 3 presents the swimming and movement behavioural changes that were investigated in five different concentrations as well as control treatments in the PD-exposed Anabas testudineus. Fish in the control treatment exhibited normal swimming behaviour for the duration of the exposure, but increased abnormal swimming activity was observed at low and high concentrations. First fish displayed altered swimming, frequent bottom-to-surface movements, a state of motionlessness, swirling and sluggish movements, sudden darts, aggression, increased surface activity, and loss of reflex after ingesting treatment of 50 mgL- and 200 mgL- for 24 hours. Fish exhibited strong behaviours in response to 72-hour concentrations of 200 mgL- , 250 mgL- , and 96-hour concentrations of 50 mgL- and 200 mgL- of PD. At 250 mgL- , however, all these behaviours showed slowly at 96 hours, and the fish sank to the bottom, became sluggish and their operculums opened wide before they died.
Table 4 and Figure 4 shows the behavioural changes related to respiratory distress at five different PD treatments as well as control units. Fish in the control treatment did not exhibit respiratory distress behaviour Fish first displayed air gulping, opercula movement, mucus secretion, snout extension, and air bubble production at 00 mgL- and 50 mgL- throughout a 24-hour exposure. Fish exposed to 50–250 mgL- of pd for 72 hours and 00– 50 mgL- for 96 hours displayed these excessive behaviours. However, during the 96-hour exposure period, 200 mgL- and 250 mg/L- mucous secretion resulted in the entire body being covered in excessive mucus, but the opercula moved slowly, the fish gulped air very slowly, and the fish died.
Table 5 and Figure 5 illustrates the morphological changes in Anabas testudineus caused by varying concentrations of paraquat dichloride during periods of exposure. In this investigation, fish in the control treatments did not exhibit any morphological changes. Fish with different morphological changes were first observed after a 24-hour exposure to the highest concentration of 250 mgL- . The body was covered in layers of mucus, and the fish were slippery. Sclerosis primarily affects the head and tail region of the fish, with red color in these areas as well and the eyeballs have become red in color. 200 mgL- and 250 mgL- of PD fish exposed for 72–96 hours displayed these morphological changes excessively, and the fish's body muscles were straight without a curve after death.
Temperature effects on Paraquat toxicity:
Figure 6 showed that temperature has an impact on the toxicity of paraquat dichloride; at 50 mgL- in the winter ( 4.4 ± .85 °C), the percentage of deaths increases to 7 compared to 20 in the summer (30.8 ±
-
2. 3 °C). In 96 hours of exposure to 200 mgL- , 7
fish died in the winter ( 4.4 ± .85 °C) and 80 in the summer (30.8 ± 2. 3 °C) in the same condition at Anabas testudineus.
Figure 1. Study site. Source: MAP:
Table 1 : Percentage Mortality of A. testudineus exposed to different concentrations.
|
Sl. No. |
Conc.(mgL-1) |
No. Exposed fish |
24 |
48 |
72 |
96 |
Mortality |
|
CONTROL |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
2 |
50 |
0 |
0 |
0 |
0 |
2 |
20 |
|
3 |
00 |
0 |
0 |
0 |
3 |
40 |
|
|
4 |
50 |
0 |
0 |
2 |
2 |
50 |
|
|
5 |
200 |
0 |
3 |
3 |
80 |
||
|
6 |
250 |
0 |
2 |
2 |
4 |
2 |
00 |
Table 2: Percentage Mortality & probit value of A. testudineus exposed to different concentrations.
|
Conc.(mgL-1) |
Log dose |
% Mortality |
Adjusted percent Mortality |
Probit value |
|
50 |
.698 |
20 |
20 |
4. 6 |
|
00 |
2 |
40 |
40 |
4.75 |
|
50 |
2. 7609 |
50 |
50 |
5 |
|
200 |
2.30 03 |
80 |
80 |
5.84 |
MORTALITY PROFILE% у = 0.0212x4-3.3795
R2 = 0.9596
----Линейная (Ряд1) LC5O=116.94mg/l
-
Figure 2. Graphical presentation of LC 50 value using probit analysis.
Relationship between concentration,mortality and time
□ concentration(mg/L) □ %mortality(24h) □%mortality(48h)
□ %mortality(72h) □%mortality(96h)
-
Figure 3. Graphical presentation of the relationship between concentration of PD, time and mortality.
Table 3: Swimming changes/Movement changes.
|
Exposure Time (hr) |
Conc. (MgL-1) |
Erratic swimmin g |
FBSM |
State Of Motionless |
SSM |
Lost reflex |
SAI |
Aggression |
Sudde n darts |
Death |
|
24 |
0 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
50 |
- |
- |
- |
- |
- |
- |
- |
+ |
- |
|
|
100 |
- |
+ |
+ |
- |
- |
+ |
+ |
+ |
- |
|
|
150 |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
||
|
200 |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
250 |
+ |
++ |
++ |
++ |
++ |
++ |
++ |
++ |
+ |
|
|
48 |
0 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
50 |
- |
+ |
- |
- |
+ |
- |
- |
+ |
- |
|
|
100 |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
|
|
150 |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
200 |
+ |
++ |
+ |
+ |
+ |
++ |
+ |
++ |
+ |
|
|
250 |
+ |
+++ |
++ |
++ |
++ |
+++ |
+++ |
++ |
+ |
|
|
72 |
0 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
50 |
- |
+ |
- |
+ |
+ |
+ |
- |
+ |
- |
|
|
100 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
150 |
+ |
++ |
+ |
+ |
+ |
++ |
+ |
++ |
+ |
|
|
200 |
+++ |
+++ |
++ |
+++ |
+++ |
+++ |
+++ |
+++ |
++ |
|
|
250 |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
++ |
|
|
96 |
0 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
50 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
100 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
150 |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
++ |
|
|
200 |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
++ |
|
|
250 |
+ |
- |
++ |
++ |
++ |
- |
++ |
++ |
++ |
*FBSM= Frequent bottom to surface movements, SSM- Swirling and sluggish movements, SAI- Surface activity inceased. *-, normal; +, mild; ++, moderate; +++, strong
Table 4: Respiratory changes due to the effect of toxicity.
|
Exposure time(hr) |
Concentration (MgL-1 ) |
Air gulping |
Opercula movement |
Mucus secretion |
Snout extension |
Air bubble creation |
|
24h |
0 |
- |
- |
- |
- |
- |
|
50 |
- |
+ |
- |
- |
- |
|
|
100 |
- |
+ |
+ |
++ |
+ |
|
|
150 |
+ |
++ |
+ |
++ |
++ |
|
|
200 |
+ |
++ |
+ |
++ |
++ |
|
|
250 |
++ |
++ |
++ |
+++ |
++ |
|
|
48h |
0 |
- |
- |
- |
- |
- |
|
50 |
- |
+ |
+ |
+ |
+ |
|
|
100 |
+ |
++ |
+ |
+ |
++ |
|
|
150 |
+ |
++ |
+ |
++ |
++ |
|
|
200 |
++ |
++ |
++ |
++ |
++ |
|
|
250 |
+++ |
+++ |
++ |
+++ |
++ |
|
|
72h |
0 |
- |
- |
- |
- |
- |
|
50 |
+ |
++ |
+ |
+ |
+ |
|
|
100 |
+ |
+++ |
+ |
++ |
++ |
|
|
150 |
+++ |
+++ |
+++ |
+++ |
+++ |
|
|
200 |
+++ |
+++ |
+++ |
+++ |
+++ |
|
|
250 |
+++ |
++ |
+++ |
+++ |
+++ |
|
|
96h |
0 |
- |
- |
- |
- |
- |
|
50 |
++ |
++ |
++ |
++ |
++ |
|
|
100 |
++++ |
++ |
+++ |
+++ |
+++ |
|
|
150 |
++++ |
++ |
+++ |
+++ |
+++ |
|
|
200 |
++ |
+ |
++++ |
++ |
+ |
250 + - ++++ + +
*-, normal; +, mild; ++, moderate; +++, strong
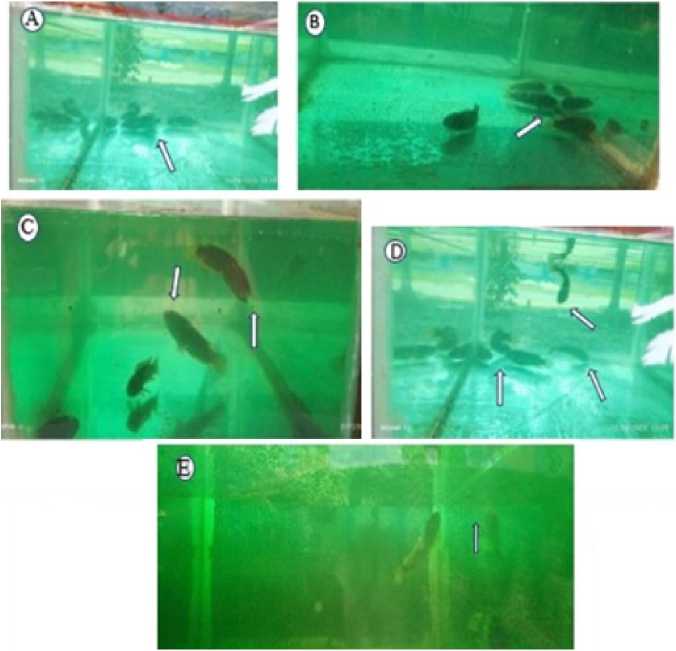
Figure 4. Behavioural changes due to the effect of toxicity. *A-Grouping and concerning the edge of the tank, B- Hitting the wall of the tank, C-Become surfacing and air-breathing, D-Hitting each other, E-Air bubble creation.
Table 5. Morphological changes due to the effect of toxicity.
|
Exposure Time (hr) |
Conc. (mgL-1) |
SHT |
MLB |
BG |
RE |
BS |
RHT |
PAD |
|
24h |
0 |
- |
- |
- |
- |
- |
- |
- |
|
50 |
- |
- |
- |
- |
- |
- |
- |
|
|
00 |
- |
- |
- |
- |
- |
- |
- |
|
|
50 |
- |
- |
- |
- |
- |
- |
- |
|
|
200 |
- |
+ |
- |
- |
- |
- |
- |
|
|
250 |
+ |
+ |
+ |
+ |
- |
+ |
- |
|
|
48h |
0 |
- |
- |
- |
- |
- |
- |
- |
|
50 |
- |
- |
- |
- |
- |
- |
- |
|
|
00 |
- |
- |
- |
- |
- |
- |
- |
|
|
50 |
- |
+ |
- |
- |
- |
- |
- |
|
|
200 |
- |
+ |
- |
+ |
- |
+ |
- |
|
|
250 |
+ |
++ |
+ |
+ |
- |
+ |
+ |
|
|
72h |
0 |
- |
- |
- |
- |
- |
- |
- |
|
50 |
- |
+ |
- |
- |
- |
- |
- |
|
|
00 |
- |
+ |
- |
- |
- |
- |
- |
|
|
50 |
- |
+ |
- |
+ |
- |
- |
- |
|
|
200 |
+ |
+ |
+ |
+ |
- |
+ |
+ |
|
|
250 |
+ |
++ |
+ |
++ |
- |
+ |
+ |
|
|
96h |
0 |
- |
- |
- |
- |
- |
- |
- |
|
50 |
- |
+ |
- |
- |
- |
- |
- |
|
|
00 |
- |
+ |
- |
+ |
- |
- |
- |
|
|
50 |
+ |
++ |
+ |
+ |
- |
+ |
- |
|
|
200 |
++ |
+++ |
+ |
+ |
- |
+ |
+ |
|
|
250 |
++ |
+++ |
++ |
++ |
+ |
++ |
++ |
*-, normal; +, mild; ++, moderate; +++, strong
SHT-Scolerosis in head and tail region, MLB- Mucous layer on whole body, BG- Blood from gills, RE-Redness in eyes, BS- Belly swollen, RHT- Red color appear in head and tail region, PAD- pelvic fin and anal fin destruction.
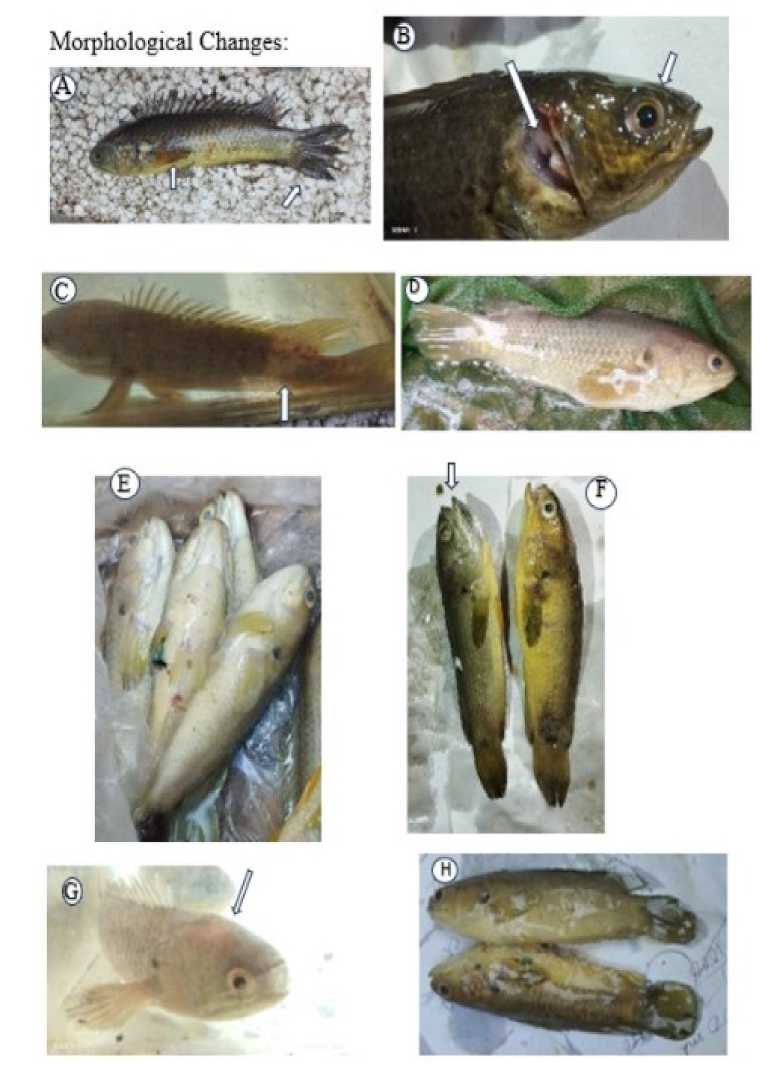
Figure 5. Morphological changes due to the effect of toxicity.
*A-The anal fin and pectoral fin are destroyed, B-Gill covered with excessive mucous and eye become red, C-sclerosis in the tail region and red color appeared, D- Red in the body and the whole body covered with a mucous layer, E-excessive mucous on body and belly slightly swollen, F-widely opened mouth, G-sclerosis in the head region, H-Thick layer of mucous in the whole body.
Mortality related to Temperature
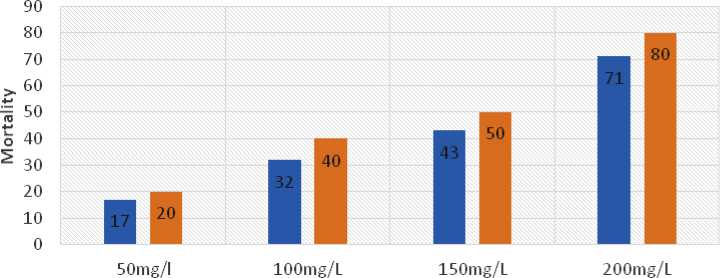
Concentration of PD
■ winter(14.4+1.8 50C) ■ Summ er( 30.8+2.130C.)
Figure 6. Temperature effects on mortality of the sample.
DISCUSSION
Fish and other vertebrates are examples of nontarget organisms that may be toxically impacted by various environmental pollutants or their metabolites. As acute or chronic exposure, several xenobiotic or toxicant concentration toxicity tests represent a threat to survival (HESIS and LOHP, 986; OECD, 20 2). A widely utilized non-contact nitrogen-base herbicide that can accumulate and biomagnify, paraquat dichloride is used to control weeds both terrestrially and aquatically. Test organisms can encounter a variety of effects during exposure, such as behavioural changes, morphological changes, hematological changes, changes in histopathology, and disruption of numerous significant organs.
Fish are in direct and constant contact with the aquatic environment, where exposure to contaminants occurs over the entire body surface, resulting in them being ideal monitors for behavioural assays of various toxic chemical exposures. According to Little and Brewer (200 ), behaviour represents a special viewpoint that connects an organism's physiology and ecology and the environment in which it lives. An organism can adapt its behaviour to both internal and external stimuli to best meet the challenge of surviving in an environment that is constantly changing. On the other hand, behaviour also results from adjustments made to environmental circumstances. Consequently, behaviour can be understood as a selective response that is in a state of constant adaptation due to direct interaction with environmental physical, chemical, social, and physiological factors. The most sensitive warning sign for possible harmful effects was a behaviour change (Farah et al., 2004). In the current study, swimming or movement changes and respiratory behavioural changes were observed for up to 96 hours during the acute toxicity study of paraquat on Anabas testudineus.
Swimming ability is a behaviour metric that is used to assess the physiological condition of aquatic life and to measure the impact and presence of pollutants (Ballesteros et al., 2009; Cailleaud et al., 20 ; Almeida et al., 20 2). The common characteristics of the control fish's behaviour are their ability to swim actively, move their operculum normally, and move freely inside the tank without colliding with the walls or other fish. In the current investigation, fish exposed to Paraquat for ninety-six hours at five different concentrations displayed changes in their typical behaviour patterns as erratic swimming, frequent bottom to surface movements, state of motionless, swirling, and sluggish movements sudden darts, aggression, loss of reflex, loss of equilibrium and surface activity increased, these behaviours increased as concentration and time of exposure increased. Similar behavioural alterations were reported by Ladipo, (20 ), Nafi’u et. al., (2022).
According to reports, fish's homeostasis and electron transfer mechanisms are affected by the nitrogen-based herbicide paraquat (Akinsorontan et al. , 20 9). The loss of reflex considered in the experimental fish could be a result of an alteration in the fish's homeostasis during exposure to paraquat. Fish attempting to adjust their metabolism to tolerate the harmful effects of paraquat may be the cause of the observed deviations from normal behaviour (Aghoghovwia and Izah, 20 8). According to Mishra (20 ), the fish's ability to tolerate the water quality is affected by a decrease in carbohydrate metabolism caused by their erratic swimming and faster opercular movement corresponding to the oxygen depletion in the treated water. Lack of nerve and muscle coordination causes erratic movements and abnormal swimming; this problem may be caused by acetylcholine accumulation in the synaptic and neuromuscular junctions (Rao et al. , 2005).
When exposed to varying concentrations of PD and during the exposure period, Anabas testudineus displayed significant respiratory abnormalities. Fish in the control unit do not exhibit any signs of respiratory distress. At 00 mgL- and 50 mgL- , Fish first showed signs of air gulping, opercula movement, mucus secretion, snout extension, and air bubble production throughout a 24-hour exposure. The duration of exposure and the PD concentration increase as this respiratory distress behaviour accelerates the rate. The fish's increased snout extension, opercula movement, air gulping, and generation of bubbles were explained by their high oxygen demand under stressful circumstances and their need to protect themselves from breathing in toxic water. Patil and David (2008), Norhan et al. (20 9), and Nafi'u et al. (202 ) reported similar behaviours. Low oxygen consumption and aerobic respiration have been linked to respiratory distress, according to Dube and Hosetti (20 0). Tilak et al. (2007) found that as xenobiotic concentration increased, there was a significant decrease in both haemoglobin level and oxygen consumption rate. The quantity of total haemoglobin per volume of whole blood is known as haemoglobin concentration, and it is crucial for the transfer of oxygen from gas-exchange organs to peripheral tissues (De Souza and Bonilla-Rodriguez 2007). Velliyath Ligina et al., (2022) report that during exposer to acrylamide for 96 hours, in Anabas testudineus, RBC decreased as the concentration increased. The fish were anemic as a result of the decreased red blood cell count, which suggested that the fish's hemopoietic system had been destroyed and that erythropoiesis was inhibited. According to Soldatov (2005), erythrocytes from fish are susceptible to environmental toxicants, and their morphological assessment serves as a bioindicator in toxicity studies.
Different exposure times to different concentrations of paraquat dichloride result in morphological changes in Anabas testudineus . Fish in the control treatments during this study showed no morphological changes. First, fish with varying morphological alterations were seen at a 24-hour exposure to the maximum dose of 250 mgL- . The fish were sticky; sclerosis mostly affects the head and tail areas of the fish, these regions are red as well, and the eyes became red, blood drips from the gills at 200 mgL- and 250 mgL- of PD for 72–96 hours of exposure showed these morphological changes mild to moderate but the fish's whole body was covered with a film of mucous layer that as PD concentration and exposure duration increased, thicker. Velliyath Ligina reported a similar observation in 2022. It minimizes the Anabas testudineus 's direct contact with a toxic environment by serving as an extra barrier. Fish that secrete an excessive amount of mucus all over their bodies do so as a non-specific defense against toxins and as a means of minimizing toxicant contact (Singh et al. , 2009). According to Eizadi-Mood et al. (20 ), the secretion may also act as a barrier to lessen the herbicide's irritating effect on the fish's body.
Based on the current research, the experimental fish's exposure to paraquat dichloride increased mortality and decreased survival rates at upgrading concentrations. Ladipo M.K. (20 ) reported a similar observation. According to the investigations by Kumar et al. (20 6), Hansen et al. (2002), Gholami-Seyedkolaei et al. (20 3), Silva et al. (20 3), Norhan et al. (20 9), and other researchers, the mortality of Anabas testudineus was directly correlated with the concentration of PD and the duration of time of exposure. These effects are caused by bioaccumulation as well as the biomagnification power of PD that impacts liver detoxification activity lower than toxicant uptake, enzyme inhibition including acetylcholinesterase inhibition, cellular activity like energy production, and physiological effects like behavioural changes and death. In this investigation, the LC50 value for paraquat dichloride toxicity on Anabas testudineus is 6.94 mgL-. An acute toxicity test can assess the toxicant toxicosis by measuring the LC50 value, which is equivalent to 50 % of test subjects dying within the entire test period. Different LC50 values were found for the same toxicant in many research investigations on acute toxicity involving different fish species. For example, Clarias gariepinus 60 mgL- (Ladipo, 20 ), Orechromis niloticus 7 mgL-(Fidelis et al., 20 2), Channa punctatus 65.87 mgL-(Badroo et al., 20 9), and Clarias gariepinus 28.03 mgL- (Ogunwole et al., 20 8). Labeo rohita 25.7 mgL-(ArivuI et al.,20 6).
The length of exposure, the sensitivity of herbicides, and the different surfactants in the paraquat are all linked to the variations in the LC 50 values that were found. It might also result from the fish's demonstrated chemical susceptibility (Ezenwosu et al. , 2020). Numerous LC 50 values can be obtained from the same herbicide and fish species, suggesting that variation in the LC 50 could be caused by the fish's age, size, or physiological state (Ullah et al. , 20 9). In toxicology, the sensitivity is referred to as a dose-response relationship, which is essential (Oulmi et al. , 995).
Based on this experiment, the toxicity of paraquat dichloride varies with temperature; at 50 mgL- in the winter, the percentage of deaths rises to 7 from 20 in the summer. Anabas testudineus, 7 died in the winter and 80 in the summer after 96 hours of exposure to 200 mgL- PD. According to Cairns et al. ( 975), Lydyet al. ( 990b), and Brecken-Folse et al. ( 994), alterations in abiotic variables like temperature can have a significant impact on a chemical's toxicity. Giese ( 968) also reported that for every 0°C increase in temperature, the metabolism of ectothermic organisms will increase by almost twofold.
CONCLUSION
Based on the current investigation, it can be determined that Anabas testudineus exposed to paraquat dichloride noticed morphological and behavioral changes in addition to a decrease in survival. It was discovered that the toxicity increased with paraquat concentration. Due to the high LC 50 value, A. testudineus appears to have a high chance of surviving in contaminated environments. Fish, in particular, can be used as an efficient model to determine the possible risks associated with acute herbicide exposure in aquatic environments. Furthermore, the use of herbicides on farmlands not only kills desired organisms but also represents a risk to human health and may eradicate other non-target organisms. Thus, this study can help estimate the possible health risks that herbicide exposure can cause to non-target organisms.
ACKNOWLEDGEMENT
We would like to extend our sincere gratitude to the Department of Fishery Sciences (Vidyasagar University, Midnapore) for providing a wide lab facility and adequate space for the experimental setup. Furthermore, we want to extend our gratitude to the University Grant Commission (Government of India) for providing the financial assistance needed to complete the experiment.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Study on the effect of paraquat dichloride’s (PD) acute toxicity on Anabas testudineus (Bloch, 1792)
- Aghoghovwia, O.A., and Izah, S.C. (2018). Acute toxicity of Paraquat dichloride based herbicide against Heterobranchus bidorsalis fingerlings. EC Agriculture, 4(2): 128-132.
- Akinsorotan, A.M. (2015). Histological Studies of African Catfish (Clarias gariepinus) Exposed to Varying Concentrations of Dizensate (Glyphosate: N-phosphonomethyl glycine). Ph.D Thesis.Delta State University, Abraka-Nigeria. P132.
- Almeida, J. R., Gravato, C., & Guilhermino, L. (2012). Challenges in assessing the toxic effects of polycyclic aromatic hydrocarbons to marine organisms: a case study on the acute toxicity of pyrene to the European seabass (Dicentrarchus labrax L.). Chemosphere, 86(9), 926-937.
- Arivu, I., Muthulingam, M., & Jiyavudeen, M. (2016). Toxicity of paraquat on freshwater fingerlings of Labeo rohita (Hamilton). International Journal of Scientific and Engineering Research, 7(10), 1965-1971.
- Badroo, I. A., Nandurkar, H. P., & Khanday, A. H. (2020). Toxicological impacts of herbicide paraquat dichloride on histological profile (gills, liver, and kidney) of freshwater fish Channa punctatus (Bloch). Environmental Science and Pollution Research, 27, 39054-39067.
- Bagheri, F. (2007). Study of pesticide residues (Diazinon, Azinphosmethyl) in the rivers of Golestan province (GorganRoud and Gharehsou) (Doctoral dissertation, M. Sc. Thesis, Tehran University of Medical Science. Tehran,
- Banaee, M., Sureda, A., Mirvaghefi, A. R., & Ahmadi, K. (2013). Biochemical and histological changes in the liver tissue of rainbow trout (Oncorhynchus mykiss) exposed to sub-lethal concentrations of diazinon. Fish physiology and biochemistry, 39, 489-501.
- Brecken-Folse, J. A., Mayer, F. L., Pedigo, L. E., & Marking, L. L. (1994). Acute toxicity of 4-nitrophenol, 2, 4-dinitrophenol, terbufos and trichlorfon to grass shrimp (Palaemonetes spp.) and sheepshead minnows (Cyprinodon variegatus) as affected by salinity and temperature. Environmental Toxicology and Chemistry: An International Journal, 13(1), 67-77.
- Brewer, S. K., Little, E. E., DeLonay, A. J., Beauvais, S. L., Jones, S. B., & Ellersieck, M. R. (2001). Behavioral dysfunctions correlate to altered physiology in rainbow trout (Oncorynchus mykiss) exposed to cholinesterase-inhibiting chemicals. Archives of Environmental Contamination and Toxicology, 40, 70-76.
- Cailleaud, K., Michalec, F. G., Forget-Leray, J., Budzinski, H., Hwang, J. S., Schmitt, F. G., & Souissi, S. (2011). Changes in the swimming behavior of Eurytemora affinis (Copepoda, Calanoida) in response to a sub-lethal exposure to nonylphenols. Aquatic toxicology, 102(3-4), 228231.
- Cairns, J., Heath, A. G., & Parker, B. C. (1975). The effects of temperature upon the toxicity of chemicals to aquatic organisms. Hydrobiologia, 47, 135-171.
- De Souza, P. C., & Bonilla-Rodriguez, G. O. (2007). Fish hemoglobins. Brazilian Journal of Medical and Biological Research, 40, 769-778.
- Dube, P. N., & Hosetti, B. B. (2010). Behavior surveillance and oxygen consumption in the freshwater fish Labeo rohita (Hamilton) exposed to sodium cyanide. Biotechnology in Animal Husbandry, 26(1-2), 91-103.
- Dutta, H. M., Nassar, S. S. T., Munshi, J. S. D., & Richmonds, C. (1994). Behavioral changes in an air-breathing fish, Anabas testudineus, exposed to malathion. Bulletin of environmental contamination and toxicology, 52, 80-86.
- Eisler, R. (1990). Paraquat hazards to fish, wildlife, and invertebrates: a synoptic review (No. 22). US Department of the Interior, Fish and Wildlife Service.
- Eizadi-Mood, N., Sabzghabaee, A. M and Badri, S. S. (2011). Paraquat Poisoning: What the Acute Care Physician Needs to Know? J. Isfahan Med. School, 29(1): 997-1006.
- Ezenwosu, S. U., Nnamonu, E. I., Odo, G. E., Ani, O. C., Egilibe, O. C., Ogbodo, G. U., & Ebe, J. F. (2020). Lambda-Cyhalothrin induced hepato-nephro toxicity potentials and post treatment recovery in Badroo, I. A., Nandurkar, H. P., & Khanday, A. H. Iran).
- Ballesteros, M. L., Wunderlin, D. A., & Bistoni, M. A. Banaee, M., Sureda, A., Mirvagefei, A. R., & Ahmadi, K. Clarias garipinus. African Journal of Biochemistry Research, 14(1), 18-26.
- Farah, M. A., Ateeq, B., Ali, M. N., Sabir, R., & Ahmad, W. (2004). Studies on lethal concentrations and toxicity stress of some xenobiotics on aquatic organisms. Chemosphere, 55(2), 257-265.
- Haematological, biological and behavioural changes in Oreochromis niloticus (Linne 1757) juveniles exposed to Paraquat herbicide. Journal of Environmental Chemistry and Ecotoxicology, 4(3), excessive growth of Azolla in the Anzali Lagoon and its control. Iranian Journal of Natural Resources, 55(1), 65-80 Finney, D. J. (1971). Probit analysis: 3d ed. Cambridge
- University Press. Gholami-Seyedkolaei, S. J., Mirvaghefi, A., Farahmand, H., & Kosari, A. A. (2013). Effect of a glyphosate-based herbicide in Cyprinus carpio: assessment of acetylcholinesterase activity, hematological responses and serum biochemical parameters. Ecotoxicology and environmental safety, 98, 135-141. Giese AC (1968) Cell physiology. WB Saunders,
- Philadelphia, PA Hansen, J. A., Lipton, J., Welsh, P. G., Morris, J., Cacela, D., & Suedkamp, M. J. (2002). Relationship between exposure duration, tissue residues, growth, and mortality in rainbow trout (Oncorhynchus mykiss) juveniles sub-chronically exposed to copper. Aquatic Toxicology, 58(3-4), 175-188.
- HESIS. and LOHP. (1986). Understanding Toxic Substances: An Introduction to Chemical Hazards in the Workplace. Hazard Evaluation System & Information Service: Land pamphlets, 58,(7), 38 p. Retrieved from www.cdph.ca.gov/programs/hesis
- Evaluation of herbicide pollution in the kerian ricefields of Perak, Malaysia. World Applied Sciences Journal, 15(1), 05-13.
- Kearney, P. C., Ruth, J. M., Zeng, Q., & Mazzocchi, P. (1985). UV ozonation of paraquat. Journal of Agricultural and Food Chemistry, 33(5), 953-957.
- Kumar, N., Ambasankar, K., Krishnani, K. K., Gupta, S. K., Bhushan, S., & Minhas, P. S. (2016). Acute toxicity, biochemical and histopathological responses of endosulfan in Chanos chanos. Ecotoxicology and environmental safety, 131, 7988.
- Ladipo, M. K., Doherty, V. F., & Oyebadejo, S. A. (2011). Acute toxicity, behavioural changes and histopathological effect of paraquat dichloride on tissues of catfish (Clarias gariepinus). International Journal of Biology, 3(2), 67-74.
- Ligina, V., Martin, R., Aiswarya, M. V., Mashirin, K. R., & Chitra, K. C. (2022). Acute and sublethal effects of acrylamide on the freshwater fish Anabas testudineus (Bloch, 1792). Environmental Science and Pollution Research, 29(60), 90835-90851.
- Lydy, M. J., Bruner, K. A., Fry, D. M., & Fisher, S. W. (1990). Effects of sediment and the route of exposure on the toxicity and accumulation of neutral lipophilic and moderately water soluble metabolizable compounds in the midge, Chironomus riparius. Aquatic toxicology and risk assessment, 13, 140-164.
- Martin-Rubi, J. C., Marruecos-Sant, L., Palomar-Martinez, M., & Martinez-Escobar, S. (2007). Immunosuppressive treatment due to paraquat poisoning. Medicina intensiva, 31(6), 331-334.
- Mishra, A., Tripathi, C. P. M., Dwivedi, A. K., & Dubey, V. K. (2011). Acute toxicity and behavioral response of freshwater fish, Mystus vittatus exposed to pulp mill effluent. J. Environ. Chem. Ecotoxicol, 3(6), 167-172.
- Nafi'u, S. A., Suleiman, K., Ahmad, M. K., & Zakariyya, M. (2022) Effect of Paraquat Herbicide on Oxidative Stress Biomaker Enzyme Activities in C. Gariepinus. Dutse Journal of Pure and Applied Sciences, 7(3b), 48-59.
- OECD. (2012). Fish Toxicity Testing Framework: Series on Testing and Assessment, No. 171. Organisation for Economic Cooperation and Development. Paris, France.
- Ogunwole, G. A., Uju, S., & Saliu, J. K. (2018). Paraquat toxicity on selected biomarkers in Clarias gariepinus. IOSR Journal of Environmental Science, Toxicology and Food Technology, 12(5),
- Cytopathology of liver and kidney in rainbow trout Oncorhynchus mykiss after long-term exposure to sublethal concentrations of linuron. Diseases of aquatic organisms, 21(1), 35-52.
- Kushwaha, B., & Lakra, W. S. (2011). Investigation on acute toxicity and behavioral changes in Channa punctatus (Bloch) due to organophosphate pesticide profenofos. Drug and chemical toxicology, 34(4), 424-428.
- Patil, V. K., & David, M. (2008). Behaviour and respiratory dysfunction as an index of malathion toxicity in the freshwater fish, Labeo rohita (Hamilton). Turkish Journal of fisheries and aquatic sciences, 8(2).
- PIC (2017). Paraquat Information Center, available at paraquat.com, accessed Sept, 27th 2017.
- Senapati, T., Samanta, P., Mandal, S., & Ghosh, A. R. (2013). Study on histopathological, histochemical and enzymological alterations in stomach and intestine of Anabas testudineus (Cuvier) exposed to Almix 20WP herbicide. International Journal of Food, Agriculture and Veterinary Sciences, 3(2), 100-111.
- Silva, C., Oliveira, C., Gravato, C., & Almeida, J. R. (2013). Behaviour and biomarkers as tools to assess the acute toxicity of benzo (a) pyrene in the common prawn Palaemon serratus. Marine environmental research, 90, 39-46.
- Singh, R. N., Pandey, R. K., Singh, N. N., & Das, V. K. (2009). Acute toxicity and behavioral responses of common carp Cyprinus carpio (Linn.) to an organophosphate (Dimethoate). World Journal of Zoology, 4(2), 70-75.
- Soldatov, A. A. (2005). Peculiarities of organization and functioning of the fish red blood system. Journal of Evolutionary Biochemistry and Physiology, 41, 272-281.
- Sureda, A., Box, A., Ensenat, M., Alou, E., Tauler, P., Deudero, S., & Pons, A. (2006). Enzymatic antioxidant response of a labrid fish (Coris julis) liver to environmental caulerpenyne. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 144(2), 191-196.
- Tilak, K. S., Veeraiah, K., & Raju, J. M. P. (2007). Effects of ammonia, nitrite and nitrate on hemoglobin content and oxygen consumption of freshwater fish, Cyprinus carpio (Linnaeus). Journal of Environmental Biology, 28(1), 45-47.
- Rao, J. V., Begum, G., Pallela, R., Usman, P. K., & Rao, R. N. (2005). Changes in behavior and brain acetylcholinesterase activity in mosquito fish, Gambusia affinis in response to the sub-lethal
- Ullah, S., Li, Z., Zuberi, A., Arifeen, M. Z. U., & Baig, M. M. F. A. (2019). Biomarkers of pyrethroid toxicity in 66-75.
- Oulmi, Y., Negele, R. D., & Braunbeck, T. (1995). Pandey, A. K., Nagpure, N. S., Trivedi, S. P., Kumar, R., exposure to chlorpyrifos. International Journal of fish. Environmental chemistry letters, 17, 945-973.
- Environmental Research and public health, 2(3), 478-483.


