Subcellular Redistribution of Calcium Ions in Zea mays L. Leaves under Hypoxia
Автор: Moskvina P.P., Anokhina G.B., Eprintsev A.T.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.21, 2025 года.
Бесплатный доступ
Previous studies on the functioning of the γ-aminobutyric acid (GABA) shunt under hypoxic conditions have demonstrated the activation of catalytic activity in enzymes that bypass two reactions of the citric acid cycle. However, the mechanism responsible for transmitting the signal of the onset of hypoxic stress into the cell remains unclear. It is known that calcium ions can act as messengers of intracellular signals. In this study, we analyzed changes in calcium ion content in maize leaves under hypoxia. It was found that under oxygen deficiency, the level of the target cation in the total cellular fraction increases 3,8-fold compared to the control. At 3 hours of incubation, the calcium content in the cytosolic fraction increases slightly, reaching its maximum value by 24 hours. In the mitochondrial fraction of the treated samples, a decrease in the concentration of free calcium was observed, reaching 2,2 times lower than the control. The rise in cytosolic Ca2+ levels, along with the rapid reestablishment of ionic homeostasis, is thought to act as a hypoxic stress signal. The obtained results will expand our knowledge of the mechanisms underlying the primary stress response in plant cells to oxygen deficiency in the environment, which may ultimately contribute to the development of more resilient agricultural crops.
GABA shunt, hypoxic stress, calcium, Zea mays L.
Короткий адрес: https://sciup.org/143185136
IDR: 143185136
Текст научной статьи Subcellular Redistribution of Calcium Ions in Zea mays L. Leaves under Hypoxia
Calcium plays a crucial role in various plant cellular processes, such as cell division, growth, and development under normal conditions and during adaptation to abiotic and biotic stresses. Increased cytosolic calcium concentrations can be sensed by calmodulin, calcium-dependent protein kinases, and other calcium-binding proteins. These proteins trigger downstream responses, which in turn can elicit specific physiological reactions. Calmodulin signals can induce mRNA and gene expression, either directly or by binding to specific transcription factors (Lindberg et al. , 2012).
Under hypoxic stress, the plant root system is unable to absorb sufficient oxygen and nutrients from the environment, which leads to an imbalance in osmotic pressure and affects plant growth and development in general (Vartapetyan, 2007). Hypoxia caused by soil flooding in maize plants leads to a number of metabolic changes in cells: programmed cell death in root cells, which occurs as an adaptation to oxygen deficiency, leading to the formation of aerenchyma – extensive intercellular spaces necessary for gas exchange and mechanical support of the plant in a loose substrate (Gunawardena et al. , 2001). In response to stress, various rearrangements of metabolic pathways occur in the body's cells, allowing the plant to survive. Complete removal of O 2 leads to an immediate cessation of protein synthesis in maize seedlings ( Zea mays L.), followed by the synthesis of about twenty anaerobic-induced proteins. These included enzymes involved in glycolysis and related processes. However, genes involved in other processes were also induced (Subbaiah, Sachs, 2003).
O 2 deficiency leads to disturbances in the ionic balance of plant cells, reflecting energy depletion and membrane depolarization (Roberts et al. , 1984; Buwalda et al. , 1988). In addition, hypoxia is known to be accompanied by acidification of the cytoplasm.
It is known that in response to abiotic stresses, including lack of oxygen, a bypass pathway of the tricarboxylic acid cycle, the GABA shunt, is activated, the work of which is carried out by three enzymes: glutamate decarboxylase (GDC, EC 4.1.1.15), GABA transaminase (GABA-T, EC 2.6.1.19), succinate semialdehyde dehydrogenase (SSADH, EC 1.2.1.24) (Yuan et al., 2023; Moskvina et al., 2024; Carillo, 2018).
Glutamate decarboxylase, which is localized in the cytoplasm, unlike other enzymes of the GABA shunt, has a C-terminal domain containing a calmodulin-binding domain, which regulates enzyme activity depending on the presence of calcium in the medium (Gut et al. , 2009). Hypoxia depolarizes the mitochondrial membrane due to a weakening of the electron transport chain (ETC), causing the release of Ca2+ from the mitochondria into the cytosol. Depolarization of the plasma membrane suppresses potassium channels, which leads to an influx of Ca2+ into the cytosol (Wang et al. , 2017). Based on this, it can be hypothesized that plant cells can use calcium ions as a hypoxic signaling messenger, activating additional stress pathways through the induction of calcium-dependent enzymes (Subbaiah et al. , 1994).
Thus, the aim of our study was to investigate the dynamics of calcium levels and its possible redistribution between the cytoplasm and mitochondria in maize leaves exposed to low oxygen concentrations.
MATERIALS AND METHODS
The object of the study were 14-day-old leaves of corn ( Zea mays L.) of the Voronezhskaya-76 variety, grown hydroponically under a 10-hour daylight period, with a luminous flux intensity of 25 W/m2 at a temperature of 25°C.
Setting up an experiment to create hypoxic conditions. Fourteen-day-old corn seedlings ( Zea mays L.) were root-removed and then placed in a vacuum desiccator. Gas environments were supplied to the vacuum desiccator: the control group of seedlings received oxygen (group No. 1), and the experimental samples received nitrogen (group No. 2) from a commercial 10150U cylinder (GOST 94973, Russia, UZGPO) at an ambient temperature of 25°C. The first samples were collected before the experiment (0); after the start of incubation, samples for research were collected at 1, 3, 6, and 24 hours from the start of the experiment.
Obtaining a total cell fraction. A sample of green maize leaves was homogenized in a medium containing 0.150 M potassium phosphate buffer (pH 7.4) at a ratio of 1:10. The homogenate was centrifuged in an Eppendorf Centrifuge 5804 R (Eppendorf, Germany) to remove cell walls for 3 minutes at 5000 rpm. The supernatant was transferred to a new Eppendorf centrifuge and used for spectrophotometric measurement of calcium concentration (Eprintsev, Anokhina, 2023).
Mitochondria isolation. A sample of green corn leaves was homogenized in a medium containing 0.4 M sucrose, 4 mM MgCl 2 , 2.5 mM EDTA, 0.15 M potassium phosphate buffer (pH 7.4), and 1 mM KCl (1:10). The homogenate was then centrifuged at 4°C in an Eppendorf Centrifuge 5804 R (Eppendorf, Germany) for 3 minutes at 5,000 rpm. The supernatant was transferred to a new tube and centrifuged at 12,000 rpm for 30 minutes. The supernatant was used as the cytoplasmic fraction, and the precipitated mitochondria were resuspended in 0.15 M Tris-HCl buffer (pH 7.4) (Shahov et al. , 2023). The isolated cytoplasmic and mitochondrial fractions were used to determine calcium concentration.
Determination of calcium ion concentration. Calcium concentration was determined colorimetrically. The total reaction mixture contained 50 mM Tris-HCl buffer (pH 6.0), 0.25 mM Arsenazo III, and 100 mM sodium acetate. 20 μl of deionized water were added to blank samples, 20 μl of 2.5 mM CaCO 3 to calibration samples, and 20 μl of the test sample to the experimental samples. Changes in the optical density of the solution were recorded on an Evolution 260 Bio spectrophotometer (Thermo Fisher Scientific, USA) at 613 nm. Magnesium binding by the Arsenazo III reagent was excluded by carrying out the reaction under acidic conditions at pH 6.0. Calcium concentration was calculated using the formula:
C= (2.5*(E sample/E calib.))/m sample where E sample is the change in the optical density of the sample at 613 nm, E calib. is the change in the optical density of the calibrator at 613 nm, m sample is the mass of the sample of green leaves.
Statistical data processing. The experiments were conducted in triplicate, with each sample analyzed in three analytical replicates. The obtained data were statistically processed using STATISTICA 12.0. The Student's t-test was used to assess the reliability of the obtained results due to the normal data distribution. The differences presented in the study are statistically significant (p < 0.05).
RESULTS
Analysis of the total cell fraction revealed that incubation of corn seedlings in a low-oxygen environment resulted in a 3.8-fold increase in free calcium levels from the first hour of exposure compared to the control, where levels remained consistent until the sixth hour of the experiment. However, after 24 hours of hypoxic exposure, a significant decrease in the concentration of the studied cation was observed in the experimental group of plants (Fig. 1).
It was shown that incubation of maize seedlings in the dark for a long time (more than 24 hours) leads to a decrease in the calcium level in the cytoplasm by 3.7 times, remaining at a low level throughout the entire experiment (Fig. 2), which is consistent with the information known in the world literature on light-induced calcium transport from the cytoplasm to the cell nucleus (Bossen et al. , 1988). Under hypoxia, a decrease in the calcium content in the cytoplasmic fraction also occurs in the first hour of the experiment, however, the decline is not as significant – a decrease in calcium concentration by only 1.5 times. At the same time, at the 3rd hour of incubation, an insignificant increase in the studied parameter is observed, which by 24 hours of the experiment reaches its maximum value, rising to the level of the initial values, thereby stabilizing. Thus, it can be assumed that calcium is involved in the development of the adaptive response of cellular metabolism in response to a decrease in oxygen levels in the environment.
In the mitochondria of the control group, there is virtually no change in the level of free calcium; however, a decrease in oxygen in the incubation medium leads to an outflow of Ca2+ from the mitochondria.
Starting from the first hour of the experiment, the calcium content in the experimental samples decreased by 2.2 times compared to the samples of the control group (Fig. 3). Our data are consistent with the data obtained for isolated wheat mitochondria – isolated wheat mitochondria also respond to anoxia by immediately releasing Ca2+ into the medium (Virolainen et al., 2002). Perhaps, the outflow of calcium from the mitochondria into the cytoplasm is one of the signals for the development of hypoxic stress, thereby facilitating the activation of calcium-dependent enzymatic systems, including cytosolic glutamate decarboxylase, the activity of which, as previously shown, increases in maize leaves with a decrease in oxygen in the environment (Moskvina et al., 2024).
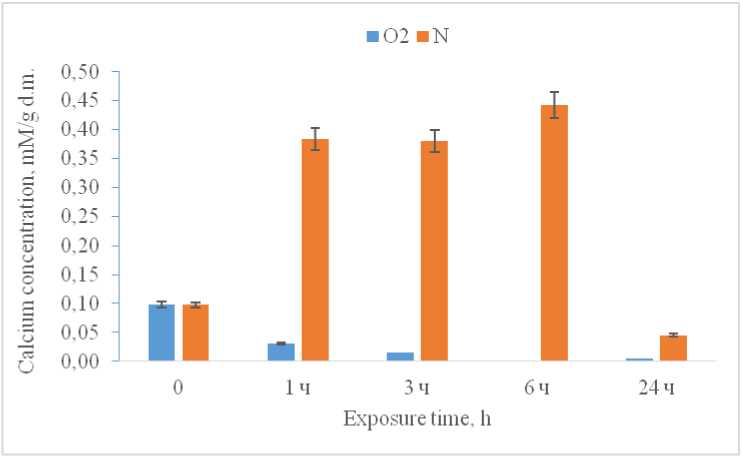
Figure 1. Concentration of Ca2+ in the homogenate under hypoxia
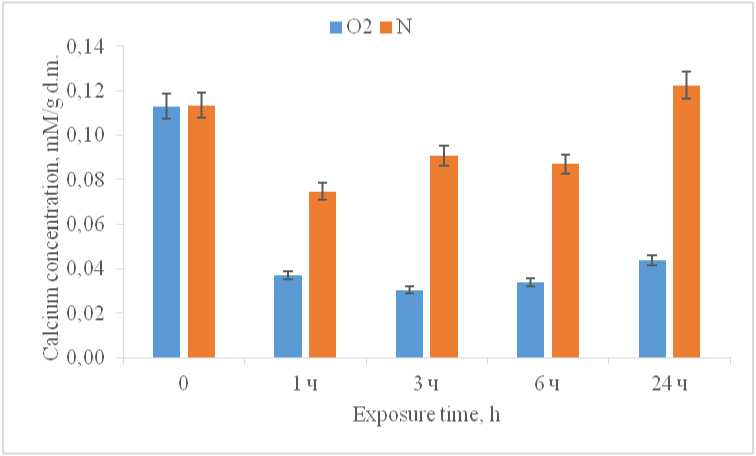
Figure 2. Concentration of Ca2+ in the cytoplasm during hypoxia
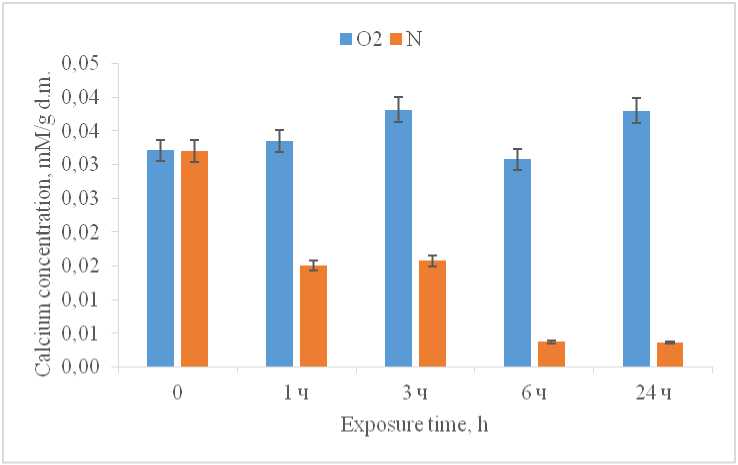
Figure 3. Concentration of Ca2+ in mitochondria during hypoxia
DISCUSSION
Ca2+ is known to act as a second messenger in cells, carrying information between the receptor and reactive components of various cellular signaling systems. The study revealed changes in calcium levels in corn leaf cells in response to decreased oxygen concentrations in the environment.
Hypoxic conditions promote an increase in the level of free calcium in the total cellular fraction. Changes in the concentration of free calcium ions may also be due to the release of calcium from bound calcium stores (Xiong et al. , 2004).
Moreover, it was found that a decrease in the oxygen concentration in the medium leads to a redistribution of Ca2+ between the cytoplasmic and mitochondrial fractions. Interestingly, prolonged (more than 24 hours) exposure of maize seedlings to darkness is accompanied by a significant decrease in the level of free calcium, probably due to its transport from the cytoplasm to the nucleus. Ca2+ transport and its uptake by the cell is a light-induced process (Bossen et al., 1988). Among the transport systems that maintain a constant Ca2+ level in the cell, Ca2+-ATPase is under the control of phytochrome. The opening of calcium channels leads to the movement of calcium. In addition, changes in Ca2+ concentration regulate the level of cGMP (Sokolovskij, 1996; Volotovski et al., 1998). Phytochrome controls the content of cGMP in the cytoplasm (Sokolovskij, 1996) through guanylate cyclase (Dubovskaya et al., 2002; Dubovskaya, Volotovsky, 2004).
A significant decrease in Ca2+ concentration was observed in the mitochondria of green corn leaves exposed to hypoxic stress, likely due to the transport of the studied cation into the cytoplasm. In the cytoplasmic fraction, the dynamics were reversed: the initial decrease in calcium concentration stabilized, and by 24 hours of hypoxic exposure, calcium homeostasis was restored. Given that cytosolic glutamate decarboxylase (an enzyme involved in the Krebs cycle bypass pathway — the γ-aminobutyric acid shunt) is a calciumcalmodulin-dependent protein, these data may indicate the involvement of the cation in cellular stress signal transmission.
CONCLUSIONS
This confirms the involvement of calcium in the cellular response to stress caused by a lack of oxygen in the environment.
CONFLICTS OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.


