Synergistic accumulative effect of salicylic acid and dibutyl phthalate on paclitaxel production in Corylus avellana cell culture
Автор: Rezaei A., Ghanati F., Behmanesh M., Safari M., Sharafi Y.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.9, 2013 года.
Бесплатный доступ
Suspension cell cultures of Corylus avellana were challenged with salicylic acid and its combined use with dibutyl phthalate solvent. Salicylic acid with concentrations of 12.5, 25 and 50 mg L –1 and 10% (v/v) dibutyl phthalate were used and added on day 8 and 10 of subculture, respectively. The results showed that growth, viability and protein content of cells were decreased by the treatments, compared to control. In all treatments, hydrogen peroxide content and lipid peroxidation rate of cells increased, compared to those of the control cells. Activity of phenylalanine ammonia-lyase increased by salicylic acid and, dibutyl phthalate exaggerated effect of salicylic acid. While flavonoids content decreased by the treatments, paclitaxel content increased significantly. The extracellular paclitaxel was more affected, compared to cell-associated paclitaxel and all treatments increased paclitaxel release and specific yield compared to that of the control. The most production of paclitaxel and specific yield of it were observed under effect of combined use of salicylic acid (50 mg L –1) and dibutyl phthalate, suggesting a synergistic accumulative effect.
Cell culture, corylus avellana, in situ extraction, paclitaxel, salicylic acid
Короткий адрес: https://sciup.org/14323709
IDR: 14323709
Текст научной статьи Synergistic accumulative effect of salicylic acid and dibutyl phthalate on paclitaxel production in Corylus avellana cell culture
Paclitaxel as a most efficient antitumor agent by a unique way of stabilizing microtubules is used in the treatment of various cancers. The restricted availability of the yew trees and the small amounts that can be extracted from each tree joint to the environmental protests originated from its various exploitation forced researchers to look for other abundant source of the substance. Recently, a number of studies have shown hazelnut as a new source of paclitaxel (and related taxane) among angiosperms (Bestoso et al. 2006; Hoffman and Shahidi, 2009; Rezaei et al. 2011). However, the production efficiency of paclitaxel is very low because of the inherent characteristics of plant cells. This prompts deep efforts to develop other methods for improved paclitaxel production. Numerous strategies have been proposed for improving plant cell productivity and secondary metabolite production in suspension-cultured cells, including precursor and nutrient feeding, in situ extraction (two–phase culture), and treatment with elicitors.
Elicitation efficiently activates the expression of defense-related genes and also the pathways of defense-related secondary metabolites such as alkaloids, terpenoids, flavonoids, phenolic compounds and phytoalexins (Zhao et al, 2005). SA is considered one of the key endogenous signals involved in regulating a number of processes in plants and vigorously stimulates secondary metabolism. It has been shown that SA induced paclitaxel production in Taxus suspension cultures (Wang et al. , 2007) and affected metabolic profile of Catharanthus roseus cell cultures (Mustafa et al. 2009).
The organic solvents as second phases are introduced to the culture systems for the in situ removal of the cell products. It has been reported that during culture the accumulation of paclitaxel in cells resulted in feedback inhibition and product degradation (Lidija and Gordana 2000). Therefore, in situ removal of paclitaxel simplifies downstream processing and is essential to improving its production (Kwon et al. 1998). However, no report is available neither on in situ enhancement of paclitaxel production and its concurrent removal from hazelnut cells. The present study was undertaken in order to investigate on the effect of SA with or without application of two-phase culture on some physiological parameters of hazelnut cells and their paclitaxel yields.
MATERIALS AND METHODS
Cell culture establishment
Calli were induced from seeds of hazelnut ( C. avellana L. cv. Gerd Eshkevar ) (an Iranian cultivar), on solidified MS media, containing 3% (w/v) sucrose, and supplemented with 1 mg L–1 2,4-dichlorophenoxyacetic acid and 0.5 mg L–1 benzyladenine, pH 5.5. Cell suspension cultures were established from the calli in the same media without agar. The cultures were incubated at 25oC in the darkness, on orbital shakers (120 rpm) and were subcultured every 2 weeks. All chemicals used for culture, treatments and biochemical analysis were purchased from Merck, Germany.
Elicitation with SA and Two-phase culture
Ethanolic solution of SA (70%, v/v) was sterilized by filtration (0.2 µm) and added to media at final concentrations of 12.5, 25 and 50 mg L–1 on day 8 of subculture, when the cells were in the middle of their rapid growth phase. The control cells were treated with the same volume of ethanol. The organic solvent DBP (n-butyl phthalate, specific gravity 1.04) was used for in situ extraction of paclitaxel. It was sterilized by autoclave and added to the culture flasks at 10% by volume on day 10 post inoculation. In preliminary experiments, higher concentrations of DBP or its earlier application excreted noticeably inhibited cell growth. For combined tests, a group of cells were treated with 50 mg L–1 SA on day 8 and then exposed to DBP on day 10. The cells were harvested on day 14 and were frozen in liquid N 2 and kept at -80 0C until used for biochemical analysis.
Growth measurement and biochemical analysis
Cell growth was evaluated by measuring the increase of dry weight. Viability assay was performed with Evans Blue (Smith et al. 1984). Level of damage of membranes was determined by measuring malondialdehyde (MDA) as the end product of peroxidation of membrane lipids (De Vos et al. 1991). Protein concentration was determined by Bradford (1976) method using BSA as standard. Hydrogen peroxide content was assayed according to the method of Velikova et al. (2000). The flavonoid content of cells was determined according to the method as described (Djeridane et al. 2006). PAL activity was determined based on the rate of cinnamic acid production as described by (Ochoa-Alejo and Gomez-Peralta 1993).
Paclitaxel extraction and assay
Paclitaxel was extracted from medium and powdered dried cells by methods as previously described (Wu and Lin 2003). The paclitaxel content in the extract solution (of methanol, or DBP from the two-phase culture) was analyzed by HPLC system (Knauer, Germany), which was equipped a C-18 column (Perfectsil Target ODS-3 (5µm), 250×4.6 mm) MZ-Analysentechnik, Mainz, Germany). Paclitaxel was eluted at a flow rate of 1 ml acetonitrile and water (45:55, v/v)/min and was detected at 227 nm using a UV detector (PDA, Germany). Identification of paclitaxel was accomplished by comparison of retention times with authentic standard (Sigma).
RESULTS AND DISCUSSION
Effects of SA on growth and production of paclitaxel
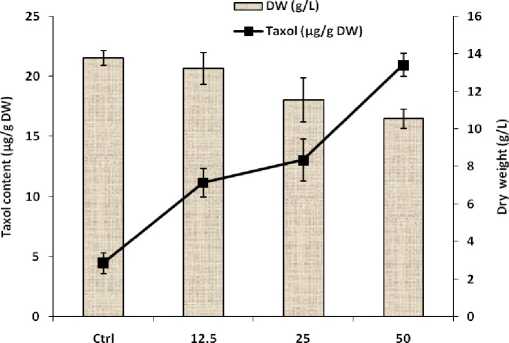
Figure 1 shows while the SA concentrations repressed the growth of cells, they resulted in a higher production of paclitaxel. Total paclitaxel production by 12.5, 25 and 50 mg L–1 SA- treated cells were respectively 2.5, 3 and 4.7 folds higher than that of the control cells. According to the results obtained here, the results are in agreement with those found by Dong et al. (2010) on the effects of SA on Salvia miltiorrhiza cultured cell, and imply that response to SA is dose dependent. In agreement with our results, Zhao et al. (2010) showed that the stimulated tanshinone production in Salvia miltiorrhiza cell culture by most elicitors was associated with notable growth suppression. In addition, SA induced paclitaxel production in Taxus cell culture after elicitation (Wang et al. 2007). It also has been reported that elicitation with SA down regulated primary metabolism of Hypericum perforatum, which is directly connected with biomass production, in benefit to secondary metabolism (Conceicao et al. 2006).
The SA treatment with 50 mg L–1 had significant effect on induction of paclitaxel production compared to other concentrations; therefore it was selected for subsequent combined experiments with DBP.
Effects of SA and DBP on viability, lipid peroxidation rate and H 2 O 2 production
There was a significant decrease in viability under all treatments compared to that of the control culture (Figure 2a). The minimum cell viability was in cultures treated with the SA and its combined use with DBP. Although the cells viability was decreased by DBP, but it was less affected compared to SA and its combined use with DBP. Exposure to all treatments significantly increased the MDA level compared to control (Figure 2a). The maximum MDA content was observed in cultures treated with SA, indicating that SA caused more damage to the cells. The Figure 2a shows that more the level of MDA, more the decrease in viability. It has been shown that SA inhibits mitochondrial electron transport leading to ATP exhaustion (Xie et al. 1999). Combination of low ATP levels with ROS such as H2O2 which induced by SA (Figure 2b), may resulted in viability loss. In respect to DBP, since the primary action site of organic solvents is the cell membrane, a straight contact between it and cells may result in significant losses in viability. The absorbed organic solvent by cell membranes can induce an alteration of their permeability, resulting in enzyme inhibition, protein deactivation, or a breakdown of transport mechanisms causing their disruption and compromising the cell viability (Mojaat et al. 2008).
MDA is the terminal product and important marker of cells membrane integrity, lipid peroxidation, oxidative stress, the extent of damage to the membrane, and the amount of solute leakage from cells resulting from several abiotic stresses. By generating changes in unsaturated fatty acids that affect membrane structure and properties, this enhanced free radical formation and lipid peroxidation under the treatments may have also brought about an increase in membrane permeability or loss of membrane integrity, as evidenced by the increase in extracellular paclitaxel (Table 1). The decrease in biomass and viability might be due to membrane lipid peroxidation induced by the treatments, as the degree of biomass and viability decrease was strongly parallel to membrane lipid peroxidation (Figure 2a and Table 1). Exposure to DBP significantly increased the content of MDA in hazelnut cells. Huang et al. (2006) reported that DBP significantly increased MDA content of duckweeds such as Spirodela polyrhiza and Lemna minor . Membrane lipid peroxides are generated from membrane fractions of dying cells which may be part of the signaling system that activates defense responses (Farmer and Ryan 1992).
Production of H2O2 was affected by all treatments (Figure 2b). While it was increased significantly by SA and DBP alone compared to control, the SA effect was potentiated by DBP in combined use treatment. It has been reported that the concentration of H2O2 increased after SA treatment, and the increase in H2O2 was followed by an increase in secondary metabolite artemisinin concentration (Pu et al., 2009). The present study demonstrated that DBP enhanced the generation of H2O2 by hazelnut cells. Similarly, it has been reported that DBP derivatives such as di(2-ethylhexyl)phthalate improved ROS production by human cells (Palleschi et al. 2009). In general, reactive oxygen species (ROS) are kept at stable basal levels in control cells, but their levels temporally or persistently increase under different stress conditions or in reaction to developmental signals. The ROS induced by SA, DBP, and both of them in hazelnut cells show that defense responses have stimulated in treated cells, which resulted in elevation of MDA content, cell death, and finally production of paclitaxel as defense–related compound.
In our experiments, SA treatment potentiated the H 2 O 2 production in cells upon DBP addition (Figure 2b). As this is the case reported by Borsani et al. (2001), that SA potentiated the generation of ROS and oxidative damage during seed germination and photosynthesis of Arabidopsis during abiotic stresses. Considering the Figures 2a, 2b and Table 1 it is suggested that a positive correlation exists between H 2 O 2 generation, lipid peroxidation, and paclitaxel production in hazelnut cell cultures. Thoma et al. (2003) showed that ROS induced lipid peroxidation of polyunsaturated fatty acids and resulted in accumulation of sanguinarine in the Eschscholtzia californica cell cultures.
Effects of SA and DBP on protein content and phenylalanine ammonia-lyase (PAL) activity
Figure 3 shows that in the presence of elicitor, DBP or both concurrently the protein content of cells was lower than that of the control culture, indicating an inhibitory effect of them to protein content. SA and its combined use with DBP resulted in the further decrease in protein content, but DBP had a moderate effect on the parameter. The intracellular PAL activity of cells was also stimulated significantly by all treatments, especially when SA applied with DBP to the cultures. Our results showed that the protein content of cells decreased by SA and DBP. As we know, protein synthesis as a part of primary metabolism is tightly correlated to growth. Considering that the treatments decreased growth, thus its loss was predictable. Similarly, the protein content in duckweeds exposed to DBP was significantly lower than that of the control (Huang et al. 2006).
PAL is a branch point enzyme between the primary and the secondary metabolism, and a key enzyme for regulating the influx of phenylalanine to the biosynthesis of phenolic compounds (Dixon and Paiva 1995). The increase in PAL activity indicates the improved secondary metabolism activity of the cells. The results showed that the organic solvent like SA induced the secondary metabolic activity of cells. This effect may be associated with the potentially poisonous effect or distorting action of the DBP on the cell membrane, which stimulates the stress response of the cells with consequent secondary metabolism. Since paclitaxel side chain provided by phenylpropanoid pathway, thus increased PAL activity may be associated with increase in paclitaxel production (Table 1).
Effects of SA and DBP on biomass, flavonoids and paclitaxel production
The dry matter or growth and flavonoid content in hazelnut suspension cultures were affected by the treatments (Figure 4). Biomass production of hazelnut cells was decreased after elicitation with SA and using DBP alone and combined with SA. Interestingly, accumulation of flavonoids in cells was negatively affected by the all treatments compared to control culture. Flavonoids accumulation in cells even more negatively was affected when cell cultures exposured to DBP in the presence of SA compared to the two factors alone and control. It is remarkable to mention that the decrease in flavonoids content coincided with the enhancement of paclitaxel production by cells (Table 1).
While all treatments decreased biomass production, they led to significant increase in the extracellular, cell-associated and total paclitaxel accumulation compared to control cultures (Table 1). SA was more effective in stimulating cell-associated paclitaxel production and increased the total paclitaxel production significantly about 2.6-fold that of the control culture. The treatment of cells with DBP solvent during the culture cycle had significant effect especially on extracellular paclitaxel production. As a result, the total paclitaxel yield under the treatment was closely related to the amount of extracellular paclitaxel which was about 28 times higher than that of the control. In general, DBP was more effective than SA in stimulating total paclitaxel production. The exposure of DBP to cultures treated with SA led to more increase in both type of paclitaxel production. In other word DBP potentiated the SA effect on paclitaxel production (Table 1). Especially, combined treatment of DBP and SA caused significant improvement in extracellular paclitaxel production. The total paclitaxel production under the combined treatment was about 16.3 times higher than that of the culture treated with SA, 1.5 times higher than that of the culture elicited with DBP, and 42 times higher than that of the control culture. It was observed that the paclitaxel release from cells was increased under treatments. The DBP and its combined use with SA were more effective than SA in this respect. The specific yield was also improved by all treatments and most increase observed by combined treatment, which was about 50 times higher than that of the control.
The results showed that in the presence of DBP as case of SA the dry weight was lower than that of the control culture, indicating a poisonous effect of DBP to cell growth. One of the interesting results in this study was decrease in flavonoids content under effect of treatments. Conceicao et al. (2006) showed that accumulation of flavonoid compounds was lesser than xanthone accumulation in the Hypericum perforatum cells treated with SA alone or primed with SA followed by fungal elicitation. The enhancement of paclitaxel accumulation observed in hazelnut cell cultures after the treatment with SA, DBP or both of them (Table 1) could be the reason why flavonoids became lesser after elicitation. As the biosynthesis of paclitaxel consists of two parts, syntheses of the nucleus, baccatin-III, and the side chain of phenylpropanoid, phenylisoserine (Fleming et al. 1993), so those precursors could have been channeled for the phenolics biosynthesis such as phenylisoserine, the side chain of paclitaxel, in detriment to the flavonoid pathway resulting in a low flavonoids production.
Paclitaxel production, release and specific yield were induced by DBP, like SA. But due to their different nature, mechanism action appears to be different. SA is a well–known inducer of plant systematic acquired resistance (SAR) in plantpathogen interaction, but it is not a universal inducer for production of plant defensive metabolites (Zhao et al. 2005). Induced biosynthesis of paclitaxel by SA has been reported in Taxus cell cultures (Wang et al. 2007). Similarly, elicitation of cell cultures of hazelnut with elicitors such as methyl jasmonate and chitosan effectively resulted in increase in paclitaxel and other taxanes production (Bestoso et al. 2006). Amount of paclitaxel production by leaves and brown shell of hazelnut plants has been reported about o.74 and 1.9 µg g–1 dry weight, respectively (Hoffman and Shahidi 2009). In addition, Bestoso et al. (2006) found 1 mg L–1 of paclitaxel in hazelnut plant cell cultures. Our study showed that paclitaxel production by control cultures 5.5 µg g–1 dry weight and in the treated cultures, depending on treatment much higher production values of the compound were observed, for example in SA and DBP combined treatment, paclitaxel content was achieved about 275 µg g–1 dry weight. These data imply that elicitation could be a good strategy to achieve greater production of paclitaxel and related compounds from hazelnut cell cultures.
A second phase, generally a non-polar phase, is introduced to the culture system for the in situ absorbance of the extracellular products. It is suggested that the remarkable improvement of paclitaxel yield with in situ solvent extraction was not only attributed to the benefits of product excretion and instant removal from the cells and medium, such as the elimination of feedback inhibition and product degradation. Rather, the solvent may also have stimulated the defense reactions and secondary metabolism of the cells.
Similarly, an improved paclitaxel production has been reported in T. cuspidata (Kwon et al. 1998) cell suspension cultures by in situ extraction. In addition, combined elicitation with yeast elicitor and in situ adsorption increased the specific and total tanshinone yields in S. miltiorrhiza hairy root cultures (Yan et al . 2005). DBP augmented effect of
SA especially in respect to paclitaxel production, release and specific yield significantly, suggesting a synergistic accumulative effect. There are reports showing that elicitor mixtures application often could result in higher secondary metabolite production in plant cell cultures (Zhang et al. 2000).
Salicylic acid (mg/L)
Figure 1: Effect of SA on growth and paclitaxel production of suspension-cultured hazelnut cells. (SA was treated on day 8 post inoculation). SA = Salicylic acid, DW= dry weight. Data are mean ± SD, n = 3.
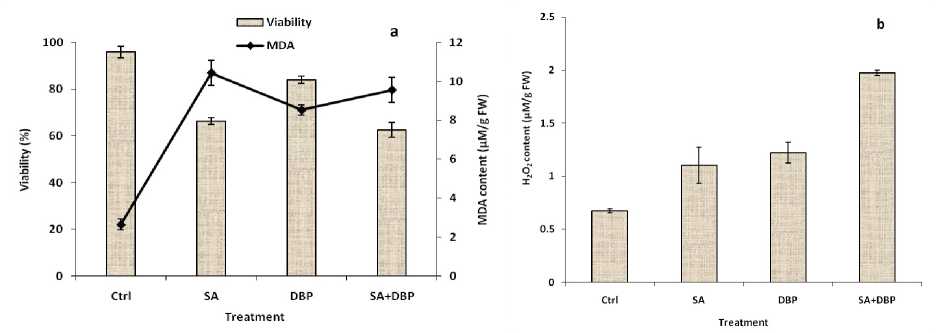
Figure 2: Effect of SA and DBP on a) cell viability and MDA content, and b) hydrogen peroxide content of suspension-cultured hazelnut cells. (SA and DBP were treated on day 8 and 10 post inoculation respectively). SA = Salicylic acid (50 mg L–1), DBP = Dibutyl phthalate (10%, v/v). Data are mean ± SD, n = 3.
Table 1. Effects of SA and DBP on paclitaxel production, release and specific yield in suspension-cultured hazelnut cells. SA and DBP were treated on days 8 and 10 post inoculation respectively. SA = Salicylic acid (50 mg L–1), DBP = Dibutyl phthalate (10%, v/v), DW= dry weight. Data are mean ± SD, n = 3.
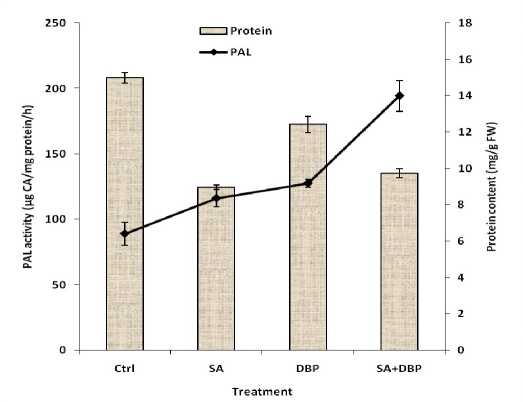
Figure 3: Effect of SA and DBP on protein content and PAL activity of suspension-cultured hazelnut cells. (SA and DBP were treated on day 8 and 10 post inoculation respectively). SA = Salicylic acid (50 mg L–1), DBP = Dibutyl phthalate (10%, v/v). Data are mean ± SD, n = 3.
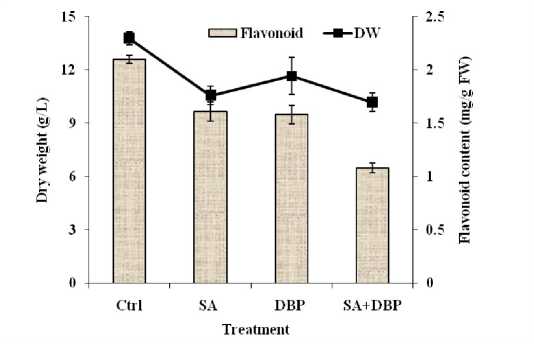
Figure 4: Effect of SA and DBP on growth and flavonoid content of suspension-cultured hazelnut cells. (SA and DBP were treated on day 8 and 10 post inoculation respectively). SA = Salicylic acid (50 mg L–1), DBP = Dibutyl phthalate (10%, v/v). Data are mean ± SD, n = 3.
CONCLUSIONS
In hazelnut cell cultures, it was observed the greater part of paclitaxel produced by the cells was
the addition of an extraction solvent to the culture medium together with elicitation can efficiently stimulate product release from cells and allow for in situ product removal from the culture medium. The in situ product removal strategy may have multiple benefits for the culture process such as, facilitating the product recovery, prohibiting product degradation, and feed-back inhibition.
REFRENCES
Borsani, O., Valpuesta, V. and Botella, M.A. (2001) Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol., 126 , 1024–1030.
Bradford, M. (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem ., 72 , 248–254.
Conceicao, L.F.R., Ferreres, F., Tavares, R.M. and Dias, A.C.P. (2006) Induction of phenolic compounds in Hypericum perforatum L. cells by Colletotrichum gloeosporioides elicitation. Phytochemistry , 67 , 149–155.
De Vos, C.H.R., Schat, H., De Waal, M.A.D., Vooijs, R. and Ernst, W.H.O. (1991) Increased resistance to copper–induced damage of the root plasma membrane in copper tolerant Silene cucubalus . Physiol. Plantarum , 82 , 523–528.
Dixon, R.A. and Paiva, N.L. (1995) Stress–induced phenylpropanoids metabolism. Plant Cell , 7 , 1085–1097.
Djeridane, A., Yousfi, M., Nadjemi, B., Boutassouna, D., Stocker, P. and Vidal, N. (2006) Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem ., 97 , 654–660.
Dong, J., Wan, G. and Liang, Z. (2010) Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol., 148 , 99–104.
Farmer, E.E. and Ryan, C.A. (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound inducible proteinase inhibitors. Plant Cell , 4 , 129 –134.
Fleming, P.E., Mocek, U. and Floss, H.G. (1993) Biosynthesis of taxiods. Mode of formation of the paclitaxel side chain. J. Am. Chem. Soc., 115 , 805–807.
Hoffman, A. and Shahidi, F. (2009) Paclitaxel and other taxanes in hazelnut. J. Funct. Food., 1 , 33–37.
Huang, Q., Wng, Q., Tan, W., Song, G., Lu, G. and Li, F. (2006) Biochemical responses of two typical duckweeds exposed to dibutyl phthalate. J. Environ. Sci. Heal . A., 41 , 1615 – 1626.
Kwon, I.C., Yoo, Y.J., Lee, J.H. and Hyun, J.O. (1998) Enhancement of paclitaxel production by in situ recovery of product. Process Biochem ., 33 , 701–707.
Lidija, S. and Gordana, V.N. (2000) Extractive bioconversion in a four–phase external–loop airlift bioreactor. AIChE J., 46 , 1368–1375.
Mojaat, M., Foucault, A., Pruvost, J. and Legrand, J. (2008) Optimal selection of organic solvents for biocompatible extraction of β–carotene from Dunaliella salina. J. Biotechnol., 133, 433–441.
Mustafa, N.R., Kim, H.K., Choi, Y.H. and Verpoorte, R. (2009) Metabolic changes of salicylic acid– elicited Catharanthus roseus cell suspension cultures monitored by NMR–based metabolomics. Biotechnol. Lett ., 31 , 1967– 1974.
Ochoa-Alejo, N. and Gómez-Peralta, J.E. (1993) Activity of enzymes involved in capsaicin biosynthesis in callus tissue and fruits of chili pepper ( Capsicum annuum L.). J. Plant Physiol., 141 , 147–152.
Palleschi, S., Rossi, B., Diana, L. and Silvestroni, L. (2009) Di (2-ethylhexyl) phthalate stimulates Ca2+ entry, chemotaxis and ROS production in human granulocytes. Toxicol. Lett., 187 , 52–57.
Pu, G.B., Ma, D.M., Chen, J.L., Ma, L.Q., Wang, H., Li, G.F., Ye, H.C. and Liu, B.Y. (2009) Salicylic acid activates artemisinin biosynthesis in Artemisia annua L. Plant Cell Rep., 28 , 1127– 1135.
Rezaei, A., Ghanati, F. and Behmanesh, M. (2011) Increased taxol production and release by methyl jasmonate, ultrasound, and dibutyl phthalate in hazelnut ( Corylus avellana L.) cell culture. IJPB. , 3 , 55–72
Smith, M.A.L., Palta, J.P. and Mc Cown, B.H. (1984) The measurement of isotonicity and maintenance of osmotic balance in plant protoplast manipulations. Plant Sci. Lett ., 33 , 249–258.
Thoma, I., Loeffler, C., Sinha, A.K., Gupta, M., Krischke, M. and Steffan, B. (2003) Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J., 34, 363–375.
Velikova, V., Yordanov, I. and Edreva, A. (2000) Oxidative stress and some antioxidant systems in acid rain–treated been plants protective role of exogenous polyamines. Plant Sci., 151 , 59–66.
Wang, Y.D., Wu, J.C. and Yuan, Y.J. (2007) Salicylic acid–induced paclitaxel production and isopentenyl pyrophosphate biosynthesis in suspension cultures of Taxus chinenesis var mairei . Cell Biol. Int., 31 , 1179–1183.
Wu, J. and Lin, L. (2003) Enhancement of paclitaxel production and release in Taxus chinensis cell cultures by ultrasound, methyl jasmonate and in situ solvent extraction. Appl. Microbiol. Biotechnol., 62 , 151–155.
Xie, Z. and Chen, Z. (1999) Salicylic acid induces rapid inhibition of mtochondrial electron transport and oxidative phosphorylation in tobacco cells. Plant Physiol ., 120 , 217–226.
Yan, Q., Hu, Z., Xiang, T.R. and Wu, J. (2005) Efficient production and recovery of diterpenoid tanshinones in Salvia miltiorrhiza hairy root cultures with in situ adsorption, elicitation and semi–continuous operation. J. Biotechnol., 119 , 416–424.
Zhang, C.H., Mei, X.G., Liu, L. and Yu, L.J. (2000) Enhanced paclitaxel production induced by the combination of elicitors in cell suspension cultures of Taxus chinensis . Biotechnol. Lett., 22 , 1561–1564.
Zhao, J., Davis, L.C. and Verpoorte, R. (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv., 23 , 283–333.
Список литературы Synergistic accumulative effect of salicylic acid and dibutyl phthalate on paclitaxel production in Corylus avellana cell culture
- Borsani, O., Valpuesta, V. and Botella, M.A. (2001) Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol., 126, 1024-1030.
- Bradford, M. (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248-254.
- Cavalli, F., Ledda, B. and Miele, M. (2006) In vitro cell cultures obtained from different explants of Corylus avellana produce paclitaxel and taxanes. BMC Biotechnol., DOI: 10.1186/1472-6750-6-45
- Conceicao, L.F.R., Ferreres, F., Tavares, R.M. and Dias, A.C.P. (2006) Induction of phenolic compounds in Hypericum perforatum L. cells by Colletotrichum gloeosporioides elicitation. Phytochemistry, 67, 149-155.
- De Vos, C.H.R., Schat, H., De Waal, M.A.D., Vooijs, R. and Ernst, W.H.O. (1991) Increased resistance to copper-induced damage of the root plasma membrane in copper tolerant Silene cucubalus. Physiol. Plantarum, 82, 523-528.
- Dixon, R.A. and Paiva, N.L. (1995) Stress-induced phenylpropanoids metabolism. Plant Cell, 7, 1085-1097.
- Djeridane, A., Yousfi, M., Nadjemi, B., Boutassouna, D., Stocker, P. and Vidal, N. (2006) Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem., 97, 654-660.
- Dong, J., Wan, G. and Liang, Z. (2010) Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol., 148, 99-104.
- Farmer, E.E. and Ryan, C.A. (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound inducible proteinase inhibitors. Plant Cell, 4, 129 -134.
- Fleming, P.E., Mocek, U. and Floss, H.G. (1993) Biosynthesis of taxiods. Mode of formation of the paclitaxel side chain. J. Am. Chem. Soc., 115, 805-807.
- Hoffman, A. and Shahidi, F. (2009) Paclitaxel and other taxanes in hazelnut. J. Funct. Food., 1, 33-37.
- Huang, Q., Wng, Q., Tan, W., Song, G., Lu, G. and Li, F. (2006) Biochemical responses of two typical duckweeds exposed to dibutyl phthalate. J. Environ. Sci. Heal. A., 41, 1615 -1626.
- Kwon, I.C., Yoo, Y.J., Lee, J.H. and Hyun, J.O. (1998) Enhancement of paclitaxel production by in situ recovery of product. Process Biochem., 33, 701-707.
- Lidija, S. and Gordana, V.N. (2000) Extractive bioconversion in a four-phase external-loop airlift bioreactor. AIChE J., 46, 1368-1375.
- Mojaat, M., Foucault, A., Pruvost, J. and Legrand, J. (2008) Optimal selection of organic solvents for biocompatible extraction of β-carotene from Dunaliella salina. J. Biotechnol., 133, 433-441.
- Mustafa, N.R., Kim, H.K., Choi, Y.H. and Verpoorte, R. (2009) Metabolic changes of salicylic acid-elicited Catharanthus roseus cell suspension cultures monitored by NMR-based metabolomics. Biotechnol. Lett., 31, 1967-1974.
- Ochoa-Alejo, N. and Gómez-Peralta, J.E. (1993) Activity of enzymes involved in capsaicin biosynthesis in callus tissue and fruits of chili pepper (Capsicum annuum L.). J. Plant Physiol., 141, 147-152.
- Palleschi, S., Rossi, B., Diana, L. and Silvestroni, L. (2009) Di (2-ethylhexyl) phthalate stimulates Ca2+ entry, chemotaxis and ROS production in human granulocytes. Toxicol. Lett., 187, 52-57.
- Pu, G.B., Ma, D.M., Chen, J.L., Ma, L.Q., Wang, H., Li, G.F., Ye, H.C. and Liu, B.Y. (2009) Salicylic acid activates artemisinin biosynthesis in Artemisia annua L. Plant Cell Rep., 28, 1127-1135.
- Rezaei, A., Ghanati, F. and Behmanesh, M. (2011) Increased taxol production and release by methyl jasmonate, ultrasound, and dibutyl phthalate in hazelnut (Corylus avellana L.) cell culture. IJPB., 3, 55-72
- Smith, M.A.L., Palta, J.P. and Mc Cown, B.H. (1984) The measurement of isotonicity and maintenance of osmotic balance in plant protoplast manipulations. Plant Sci. Lett., 33, 249-258.
- Thoma, I., Loeffler, C., Sinha, A.K., Gupta, M., Krischke, M. and Steffan, B. (2003) Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J., 34, 363-375.
- Velikova, V., Yordanov, I. and Edreva, A. (2000) Oxidative stress and some antioxidant systems in acid rain-treated been plants protective role of exogenous polyamines. Plant Sci., 151, 59-66.
- Wang, Y.D., Wu, J.C. and Yuan, Y.J. (2007) Salicylic acid-induced paclitaxel production and isopentenyl pyrophosphate biosynthesis in suspension cultures of Taxus chinenesis var mairei. Cell Biol. Int., 31, 1179-1183.
- Wu, J. and Lin, L. (2003) Enhancement of paclitaxel production and release in Taxus chinensis cell cultures by ultrasound, methyl jasmonate and in situ solvent extraction. Appl. Microbiol. Biotechnol., 62, 151-155.
- Xie, Z. and Chen, Z. (1999) Salicylic acid induces rapid inhibition of mtochondrial electron transport and oxidative phosphorylation in tobacco cells. Plant Physiol., 120, 217-226.
- Yan, Q., Hu, Z., Xiang, T.R. and Wu, J. (2005) Efficient production and recovery of diterpenoid tanshinones in Salvia miltiorrhiza hairy root cultures with in situ adsorption, elicitation and semi-continuous operation. J. Biotechnol., 119, 416-424.
- Zhang, C.H., Mei, X.G., Liu, L. and Yu, L.J. (2000) Enhanced paclitaxel production induced by the combination of elicitors in cell suspension cultures of Taxus chinensis. Biotechnol. Lett., 22, 1561-1564.
- Zhao, J., Davis, L.C. and Verpoorte, R. (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv., 23, 283-333.
- Zhao, J.L., Zhou, L.G. and Wu, J.Y. (2010) Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol., 87, 137-144.


