Synthesis and acidity study of mixed MFI-mord type zeolite
Автор: Brovko Roman, Lakina Natalia, Doluda Valentin
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Химические науки
Статья в выпуске: 12 т.8, 2022 года.
Бесплатный доступ
Zeolites of various nature are widely used in the chemical industry, the fuel and energy sector of the economy as sorbents, catalysts and materials for the creation of inorganic membranes for various purposes. At the same time, it is possible to change the acid properties of the surface of zeolites both by varying the ratio of silicon to aluminum or silicon to phosphorus, and by joint synthesis of zeolites of various types with different acidic properties. The presented article provides a method for the sequential production of a zeolite of a mixed structure type MFI and mordenite. Synthesis of the original MFI type zeolite was carried out using seed grains by the hydrothermal method for 72 hours, followed by washing and drying of the zeolite. To obtain a layer of mordenite on the surface of the MFI type zeolite, the initial zeolite was pretreated with alkali and then treated with n-butylamine. In this way, nine samples of zeolite with different acidic surface properties were obtained. Determination of the acidic properties of the surface was carried out by the method of ammonia chemisorption followed by its desorption from the surface of the zeolite. For this purpose, the test sample was loaded into a quartz cuvette, purged with argon at a temperature of 800°С, after which the temperature dropped to 150°С, and the surface of the zeolite was treated with ammonia. Subsequently, the test sample was heated up to 800°С with registration of desorption curves. The amount of adsorbed ammonia was carried out according to previously prepared calibration curves. The synthesized samples of zeolites had different acidity from 0.48 to 0.72 mmol(NH3)/g(sample). In this case, the total acidity of the samples correlated with the ratio of silicon to aluminum in the zeolite samples. Also, depending on the ratio of the MFI and mordenite structures in the zeolite sample, it is possible to vary not only the number, but also the strength of the formed acid sites. So, an increase in the content of mordenite contributes to an increase in the strength of acid centers. The developed method for the synthesis of mixed structure zeolites of the MFI type mordenite made it possible to control the surface acidity of the synthesized samples.
Zeolites, synthesis, acidity
Короткий адрес: https://sciup.org/14126176
IDR: 14126176 | УДК: 544.47 | DOI: 10.33619/2414-2948/85/07
Текст научной статьи Synthesis and acidity study of mixed MFI-mord type zeolite
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 544.47
Zeolites are widely applied as sorbents, membrane materials, catalysts in chemical industry, fuel and energy production sectors of economy [1-3]. Typically, acidic and morphological properties of zeolites can be varied by changing Si/Al ratio for typical zeolites and/or Si/P ratio for zeotype [46]. However, control of zeolites and zeotype morphological and diffusion properties is rather complex problem [7-12], due to their rigid structure. One possible way to solve this problem is to provide synthesis of mixed structure zeolites. Mixed zeolite systems are of special interest due to possibility of accurate control of acidic and sorption properties for synthesized material. MFI type zeolites are characterized by three-dimensional structure of straight channels along [010] axis connected to one another via the sinusoidal channels along [100] axis with diameter 5.1-5.6 Å. Mordenite is characterized by two-dimensional channels structure with six ring pores opening acceptable for molecules diffusion with following dimensional parameters a: 1.57 Å, b: 2.95 Å, c:
6.45 Å. Therefor by vary ratio of MFI to mordenite it is possible to obtain different surface morphology and surface acidic properties that is of special importance for organic sorption and catalysis application.
Materials and Methods
For obtaining mixed structure material consecutive MFI-mordenite synthesis was provided. For obtaining initial MFI zeolite chemical grade sodium hydroxide, sodium aluminate, silica gel and MFI zeolite seeds with purity not less than 99% were purchased from local supplier. Distillate water was purified using DE-25 aqua distillation system. Prior to synthesis silica gel was crashed in laboratory milling machine to obtained 10-100 μm particles fraction. In zeolite synthesis (Table 1) sodium hydroxide, silica gel and 150 ml of water was placed in autoclave at 600 rpm and 70°С for one hour to form gel.
Table 1 REACTION CONDITIONS FOR INITIAL MFI ZEOLITE SYNTHESIS
|
Sample |
NaOH, g |
NaAlO 2 , g |
SiO 2 , g |
H 2 O, ml |
Si/Al |
|
1 |
9.54 |
0.58 |
21.8 |
250 |
54 |
|
2 |
9.54 |
0.32 |
21.8 |
250 |
98 |
|
3 |
9.54 |
0.16 |
21.8 |
250 |
180 |
Then sodium aluminate solution in 100 ml of water and one gram of MFI zeolite was added to gel and temperature was set to 240°С for 72 hours. Reaction mixture was placed in IEC HN-SII centrifuge and initial zeolite was separated from reaction solution. Zeolite was washed with distillate water three times and dried in laboratory drier at 140°C.
For mordenite synthesis ten grams of dried MFI zeolite samples were placed in autoclave and treated with two hundred milliliters of 0.1M solution of sodium hydroxide for one hour at 50°С for desoldering initial zeolite surface structure. Then suspension was placed on shell for sedimentation for two hours and solution was decantated and twenty milliliters of n-butylamine was added and stirred for three hours. Then mixture of reagents showed in table 2 was added to suspension and suspension was sealed, heated to 240°C for 72 hours.
Table 2 REACTION CONDITIONS FOR INITIAL MORDENITE SYNTHESIS
|
Sample |
NaOH, g |
NaAlO 2 , g |
SiO 2 , g |
H 2 O, ml |
Si/Al |
|
1 |
1.58 |
0.98 |
9.15 |
150 |
11 |
|
2 |
1.58 |
0.46 |
9.15 |
150 |
24 |
|
3 |
1.58 |
0.24 |
9.15 |
150 |
48 |
Then reaction mixture was placed in IEC HN-SII centrifuge and mixed zeolite was separated from reaction solution. Zeolite was washed with distillate water three times and dried in laboratory drier at 140°C. Synthesized MFI mordenite samples were designated according to synthesis procedure MFI1-MORD1, MFI1-MORD2, MFI1-MORD3, MFI2-MORD1, MFI2-MORD2, MFI2-MORD3, MFI3-MORD1, MFI3-MORD2, MFI3-MORD3.
Ammonia chemosorption experiments were made in order to evaluate acidic properties of synthesized samples using AutoChem HP chemosorption analyzer. For ammonia desorption experiments synthesized samples were placed in quartz cuvette and placed in analyzer module. Where sample was heated in argon atmosphere up to 800°C cooled down to 150°C flashed with mixture of 10 v.% ammonia in helium for one hour followed by flashing with pure helium for one hour. Afterwards sample was heated to 800°C with temperature gradient of 10°C/min and ammonia desorption curve was recorded. Quantity of acid cites were calculated according to quantity of chemosorbed ammonia using preliminary made calibration curve.
Yield of synthesized zeolite was made by dividing of dried solid weight on theoretical weight of zeolite samples.
Results and Discussions
Ammonia desorption curves (Figure 1) for initial MFI zeolite samples shows increasing of zeolite acidity from 0.15 mmol(NH 3 )/g for MFI3, to 0.24 mmol(NH 3 )/g for MFI2 sample and 0.39 mmol(NH 3 )/g for MFI1. Increasing of initial MFI zeolite acidity correlates with decrease of Si/Al ratio from 180 for MFI3 sample, to 98 for MFI2 sample and to 54 for MFI1 sample.
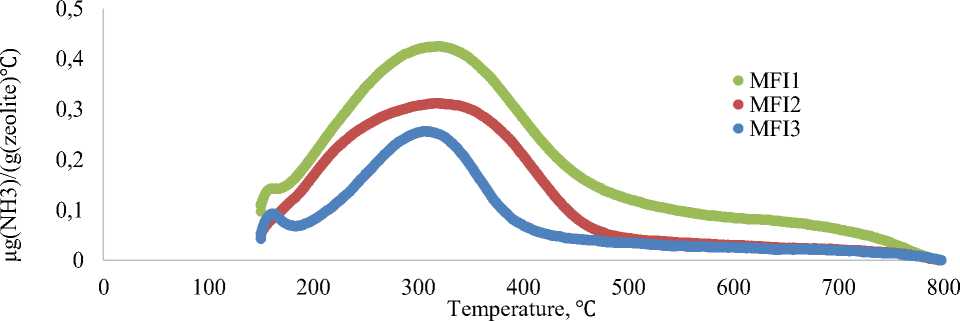
Figure 1. Ammonia chemosorption curves for initial MFI zeolites samples
Ammonia desorption curves for MFI1-MORD1, MFI1-MORD2, MFI1-MORD3 samples (Figure 2) shows increasing of zeolite acidity from 0.49 mmol(NH 3 )/g for MFI1-MORD3 to 0.54 mmol(NH 3 )/g for MFI1-MORD2 and to 0.72 mmol(NH 3 )/g for MFI1-MORD1. Increasing of initial MFI1-MORD1-3 zeolite acidity correlates with decreasing of Si/Al ratio in mordenite from 48 to 11.
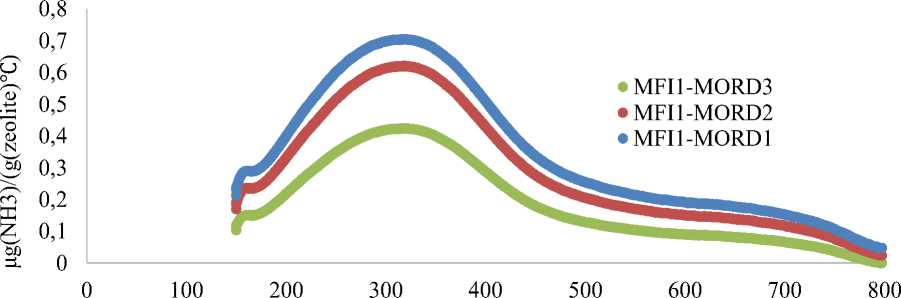
Temperature, °C
Figure 2. Ammonia chemosorption curves for MFI1-MORD1-3 zeolites samples
Ammonia desorption curves for MFI2-MORD1, MFI2-MORD2, MFI2-MORD3 samples (Figure 3) shows broad peaks increasing of zeolite acidity from 0.45 mmol(NH 3 )/g for MFI2-MORD3 to 0.5 mmol(NH 3 )/g for MFI2-MORD2 and to 0.65 mmol(NH 3 )/g for MFI2-MORD1. Increasing of initial MFI2-MORD1-3 zeolite acidity correlates with decreasing of Si/Al ratio.
Ammonia desorption curves for MFI3-MORD1, MFI3-MORD2, MFI3-MORD3 samples (Figure 4) shows broad peaks increasing of zeolite acidity from 0.42 mmol(NH 3 )/g for MFI3-MORD3 to 0.48 mmol(NH 3 )/g for MFI3-MORD2 and to 0.52 mmol(NH 3 )/g for MFI3-MORD1. Increasing of initial MFI3-MORD1-3 zeolite acidity correlates with decreasing of Si/Al ratio.
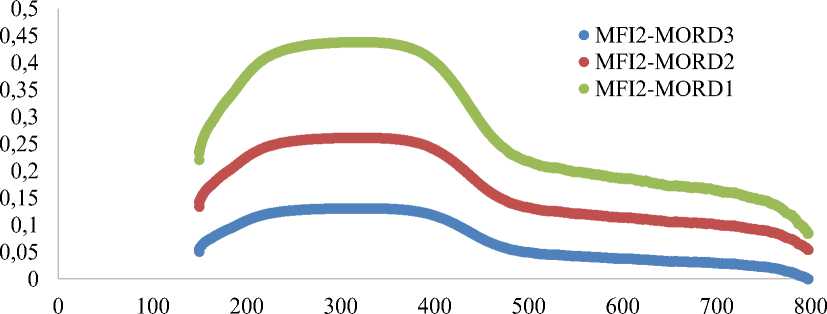
Temperature, °C
Figure 3. Ammonia chemosorption curves for MFI2-MORD1-3 zeolites samples
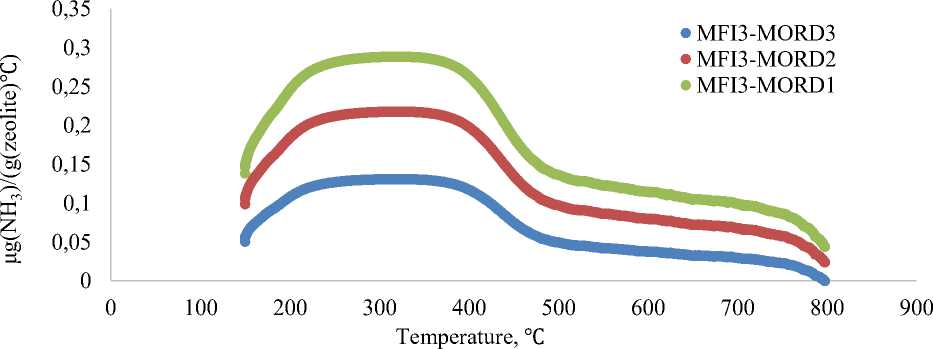
Figure 4. Ammonia chemosorption curves for MFI3-MORD1-3 zeolites samples
Therefor joint acidity of mixed MFI mordenite zeolites shows strong correlation with acidity of initial MFI and mordenite zeolites.
Conclusions
Mixed structure MFI mordenite zeolites were synthesized using mixed seeds and organic template methodology. Synthesized samples MFI mordenite samples showed high acidity. Increasing of Si/Al ratio from 11 up to 180 results in appropriate decrease of surface acidity from 0.72 mmol(NH 3 )/g down to to 0.48 mmol(NH 3 )/g. Reliable and easy method of MFI mordenite synthesis was designed providing high zeolite yield.
Acknowledgments: The reported study was funded by Russian Foundation for Basic Research (RFBR), project number 20-08-00191.
Список литературы Synthesis and acidity study of mixed MFI-mord type zeolite
- Pilar, R., Moravkova, J., Sadovska, G., Sklenak, S., Brabec, L., Pastvova, J., & Sazama, P. (2022). Controlling the competitive growth of zeolite phases without using an organic structuredirecting agent. Synthesis of Al-rich* BEA. Microporous and Mesoporous Materials, 333, 111726. https://doi.org/10.1016/j.micromeso.2022.111726
- Yu, Q., Cheng, H., Tang, X., Yi, H., Ren, X., & Li, Z. (2022). Progress in the synthesis of small-pore zeolites for purifying NOx from motor vehicle exhaust. Journal of Cleaner Production, 135119. https://doi.org/10.1016/j.jclepro.2022.135119
- Li, Z., Liu, Y., Dou, T., Li, X., Di, C., & Chen, S. L. (2022). Sustainable synthesis of AEI/CHA intergrowth zeolites for methanol-to-olefins conversion. Microporous and Mesoporous Materials, 344, 112201. https://doi.org/10.1016/j.micromeso.2022.112201
- Probst, J., Couperthwaite, S. J., Millar, G. J., & Kaparaju, P. (2022). Critical evaluation of zeolite N synthesis parameters which influence process design. Journal of Environmental Chemical Engineering, 10(5), 108347. https://doi.org/10.1016/j.jece.2022.108347
- Indira, V., & Abhitha, K. (2022). A review on recent developments in Zeolite A synthesis for improved carbon dioxide capture: Implications for the water-energy nexus. Energy Nexus, 100095. https://doi.org/10.1016/j.nexus.2022.100095
- Aguirre-Cruz, G., Legorreta-Garcia, F., Aguirre-Cruz, G., Stanciu, L., & Aguirre-Alvarez, G. (2022). Synthesis of hierarchical silica zeolites for heterogenous catalysis and adsorption. Microporous and Mesoporous Materials, 112274. https://doi.org/10.1016/j.micromeso.2022.112274
- Chen, X., Wang, Y., Wang, C., Xu, J., Li, T., Yue, Y., ... & Bao, X. (2022). Synthesis of NaA zeolite via the mesoscale reorganization of submolten salt depolymerized kaolin: A mechanistic study. Chemical Engineering Journal, 140243. https://doi.org/10.1016/j.cej.2022.140243
- Ma, D., Li, X., Liang, J., Wang, Z., & Yang, W. (2022). Distilling seed-assisted zeolite synthesis conditions by machine learning. Microporous and Mesoporous Materials, 339, 112029. https://doi.org/10.1016/j.micromeso.2022.112029
- Gao, X., Wang, Z., Chen, T., Hu, L., Yang, S., & Kawi, S. (2022). State-of-art designs and synthesis of zeolite membranes for CO2 capture. Carbon Capture Science & Technology, 100073. https://doi.org/10.1016/j.ccst.2022.100073
- Conroy, B., Nayak, R., Hidalgo, A. L. R., & Millar, G. J. (2022). Evaluation and application of machine learning principles to Zeolite LTA synthesis. Microporous and Mesoporous Materials, 335, 111802. https://doi.org/10.1016/j.micromeso.2022.111802
- Outram, J. G., Collins, F. J., Millar, G. J., Couperthwaite, S. J., & Beer, G. (2022). Process Optimisation of Low Silica Zeolite Synthesis from Spodumene Leachate Residue. Chemical Engineering Research and Design. https://doi.org/10.1016/j.cherd.2022.11.015
- Samanta, N. S., Das, P. P., Mondal, P., Changmai, M., & Purkait, M. K. (2022). Critical review on the synthesis and advancement of industrial and biomass waste-based zeolites and their applications in gas adsorption and biomedical studies. Journal of the Indian Chemical Society, 100761. https://doi.org/10.1016/j.jics.2022.100761


