Synthesis and acidity study of Na-ZSM-5 zeolite
Автор: Mikhailov Stepan, Monzharenko Margarita, Brovko Roman, Doluda Valentin
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Химические науки
Статья в выпуске: 9 т.8, 2022 года.
Бесплатный доступ
Zeolites are a special class of structured aluminosilicates with a developed inner surface and a large number of micropores, which allows them to be used as sorbents, membrane materials, and catalysts. Zeolite ZSM-5 is an aluminosilicate formed by parallel channels with a diameter of 5.3-5.6Å interconnected by sinusoidal channels with a diameter of 5.1-5.5Å. At the same time, the presence of aluminum atoms in the zeolite crystal lattice makes it possible to carry out dehydrogenation, esterification, isomerization, and aromatization reactions, and the presence of microchannels significantly increases the number of collisions of reacting compounds, which leads to a significant increase in the rate of chemical transformations in such systems. The synthesis of ZSM-5 zeolite using structure-directing agents has been known since the late 70s of the last centuries, while the high cost of structure-directing agents used and the long duration of hydrothermal synthesis and the need for subsequent post-synthetic procedures lead to a high cost of the resulting zeolite. One of the possible ways to reduce the cost of the synthesis of ZSM-5 zeolite is the direct use of ZSM-5 zeolite as a structure-forming agent, while the synthesis conditions play a decisive role in the structure of the resulting zeolite. The article presents the results of a study of the synthesis of ZSM-5 zeolite and a study of its acidic properties using locally produced reagents. Zeolite was prepared using pre-milled silica gel, sodium hydroxide, and sodium aluminate. The pre-weighed silica gel sample was filled with sodium hydroxide solution and stirred at 70 °C to form a colloidal solution, after which the pre-prepared sodium aluminate solution was added, and hydrothermal synthesis was carried out at 180 °C for 24 hours. During the synthesis, the Si/Al ratio was varied in the range from 7.5 to 2000, while the resulting acidity ranged from 0.002 to 0.265 mmol(NH3)/g(ZSM-5). It was found that the synthesis of ZSM-5 using pre-prepared reagent solutions is superior to the method using direct dissolution of solid reagents in an autoclave. An increase in the Si/Al ratio from 7.5 to 100 leads to a corresponding increase in surface acidity from 0.066 mmol(NH3)/g(ZSM-5) to 0.265 mmol(NH3)/g(ZSM-5). A further increase in the Si/Al ratio to 2000 leads to a decrease in surface acidity to 0.002-0.005 mmol(NH3)/g(ZSM-5). A reliable and simple method for the synthesis of ZSM-5 zeolite has been developed, providing a zeolite yield of up to 97%.
Zeolites, synthesis, acidity, chemosorption
Короткий адрес: https://sciup.org/14124807
IDR: 14124807 | УДК: 544.47 | DOI: 10.33619/2414-2948/82/03
Текст научной статьи Synthesis and acidity study of Na-ZSM-5 zeolite
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 544.47
Different zeolite and zeotype frameworks are of special interest due to their superior sorption and catalytic properties [1-3]. ZSM-5 zeolite has general formula |Na x (H 2 O) 16 | [AlxSi 96-x O 192 ]-(x<27), it’s structure consist from straight channels connected to other parallel channels via the sinusoidal channels[2]. ZSM-5 zeolite synthesis using structure directing agents is known since 1970 [1, 4], however due to high price of structure directing agents these methods are purely applicable for industrial applications. Therefor a lot of scientific teams were working on development of easy and reliable methods for template free synthesis of ZSM-5 zeolite [5-10]. In this article a template free method of ZSM-5 synthesis was developed using local reagents as reactive precursors.
Materials and methods
For NaZSM-5 zeolite synthesis chemical grade sodium hydroxide, sodium aluminate, silica gel and Na-ZSM-5 HKC with purity not less than 99%were purchased from local supplier and used as is. Distillate water was purified using DE-25 aqua distillation system. Prior to synthesis silica gel was crashed in laboratory milling machine to obtained 10-100 μm particles fraction. For synthesis of first sample (Table) sodium hydroxide, sodium aluminate, silica gel, one gram of Na-ZSM-5 sample, and distillate water were placed in Parr instrument top bench 4566 autoclave reactor equipped with impeller mixer. Mixer rotation rate was set to 600 rpm and temperature was set to 1800C. Hydrothermal synthesis was provided for 24 hours, then autoclave was cooled to ambient temperature. In all other zeolite synthesis (Table 1) sodium hydroxide and silica gel and 150 ml of water was placed in autoclave at 600 rpm and 700C for one hour to form gel. Then sodium aluminate solution in 100 ml of water and one gram of Na-ZSM-5 HKC was added to gel and temperature was set to 1800C for 24 hours. Reaction mixture was placed in IEC HN-SII centrifuge and zeolite was separated from reaction solution. Zeolite was washed with distillate water three times and dried in laboratory drier at 1400C. Synthesized samples have theoretical Si/Al rate from 7.5 to 2000 (Table).
Table
REACTION CONDITIONS FOR AND NA-ZSM-5 SYNTHESIS
|
Sample |
NaOH, g |
NaAlO 2 , g |
SiO 2 , g |
H 2 O, ml |
Si/Al |
m(zeolite), g |
Zeolite yield, % |
с(acid cites), mmol/g |
|
1 |
8.3264 |
4.1000 |
20.8330 |
250 |
7,5 |
15.33 |
61.5 |
0.055 |
|
2 |
8.3264 |
4.1000 |
20.8330 |
250 |
7,5 |
24.28 |
97.4 |
0.066 |
|
3 |
8.3264 |
1.4200 |
20.8330 |
250 |
20 |
13.24 |
59.5 |
0.116 |
|
4 |
8.3264 |
0.5680 |
20.8330 |
250 |
50 |
11.77 |
55.0 |
0.220 |
|
5 |
8.3264 |
0.2840 |
20.8330 |
250 |
100 |
8.71 |
41.3 |
0.265 |
|
6 |
8.3264 |
0.1420 |
20.8330 |
250 |
200 |
8.56 |
40.8 |
0.002 |
|
7 |
8.3264 |
0.0568 |
20.8330 |
250 |
500 |
15.07 |
72.1 |
0.002 |
|
8-1 |
8.3264 |
0.0284 |
20.8330 |
250 |
1000 |
8.11 |
38.9 |
0.005 |
|
8-2 |
8.3264 |
0.0284 |
20.8330 |
250 |
1000 |
13.95 |
66.9 |
0.002 |
|
9 |
8.3264 |
0.0142 |
20.8330 |
250 |
2000 |
8.20 |
39.3 |
0.002 |
Ammonia chemosorption experiments were made in order to evaluate acidic properties of synthesized samples using AutoChem HP chemosorption analyzer. For ammonia desorption experiments synthesized samples were placed in quartz cuvette and placed in analyzer module. Where sample was heated in argon atmosphere up to 800℃ cooled down to 1500C flashed with mixture of 10 v.% ammonia in helium for one hour followed by flashing with pure helium for one hour. Afterwards sample was heated to 8000C with temperature gradient of 100C /min and ammonia desorption curve was recorded (Figure 1). Quantity of acid cites were calculated according to quantity of chemosorbed ammonia using preliminary made calibration curve.
Yield of synthesized zeolite was made by dividing of dried solid weight on theoretical weight of Na-ZSM-5 sample.
Results and discussions
After Na-ZSM-5 hydrothermal synthesis crystallite solid residue of zeolite was formed (Figure 1). Synthesis of Na-ZSM-5 sample from solid precursors (Sample 1, Table) compared to synthesis from preliminary made precursors solutions (Sample 2, Table) showed lower for 35% yield, beside quantity of surface acid cites decreased for 15-16%. Therefor synthesis of Na-ZSM-5 from liquid solutions of precursors is superior compared to synthesis from solids with solutions formation directly in reaction autoclave.
Increasing of Si/Al ratio from 7.5 to 100 results in solid yield decrease from 97% to 41% and increase of surface acidity from 0.066 to 0.265 mmol/g (Table) that can be explained by optimal ratio of used precursors. Further increasing of Si/Al ratio up to 2000 lead to solid yield stabilization, however drastic decrease of surface acidity down to 0.002 mmol/g can be noticed, that can be explained by alumina ions lack in reaction solution.
Repeated synthesis of Na-ZSM-5 samples 8-1 and 8-2 showed high dispersion in solid yield and surface acidity that can be explained by different rates of zeolite species nucleation.

Figure 1. Samples of dried Na-ZSM-5
Time of hydrothermal synthesis was 24 hours under 180 ℃, one g of Na-ZSM-5 was added to reaction mixture before synthesis.
Ammonia desorption curves of Na-ZSM-5 sample 1, table 1 synthesized using solid precursor showed formation of broad peak from 2000C to 5000C that can be subscribed to weak and strong Bronsted acid sites (Figure 2). Sample 2, table 1 synthesized from precursor solutions showed presence of two partly imposed peaks, first in 200–350 C region and second one in 350–500 C. The increase of Si/Al ratio from 7.5 up to 100 results in appropriate increase of chemosorbed ammonia peak, broad peak shape remains practically the same. Further increase of Si/Al ratio up to 2000 results formation of small peak in 150–200 C that can be subscribed to physiosorbed ammonia in zeolite micropores.
Conclusions
Na-ZSM-5 synthesis using preliminary produced solutions of precursors was found to be more superior compared to direct solid precursors dissolution in reaction autoclave. Increasing of
Si/Al ratio from 7.5 up to 100 results in appropriate increase of surface acidity from 0.066 mmol (NH 3 )/g(ZSM-5) up to 0.265 mmol(NH 3 )/g(ZSM-5). Further increase of Si/Al ratio up to 2000 results in appropriate decrease of surface acidity down to 0.002-0.005 mmol (NH 3 )/g(ZSM-5). Reliable and easy method Na-ZSM-5 synthesis was designed providing zeolite yield up to 97%.
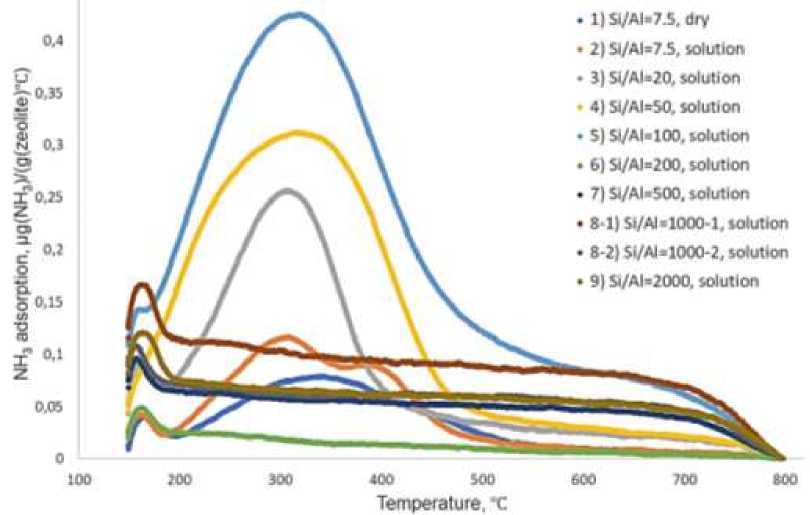
Figure 2. Ammonia chemosorption curves for synthesized Na-ZSM-5 samples
Acknowledgments
The reported study was jointly funded by Russian Foundation for Basic Research (RFBR), Sirius University of Science and Technology, JSC Russian Railways and Educational Fund “Talent and success”, project number 20-38-51001.
Список литературы Synthesis and acidity study of Na-ZSM-5 zeolite
- Kamaluddin H. S., Gong X., Ma, P., Narasimharao K., Chowdhury A. D., Mokhtar M. Influence of zeolite ZSM-5 synthesis protocols and physicochemical properties in the methanol-to-olefin process // Materials Today Chemistry. 2022. V. 26. P. 101061.
- Van der Gaag F. J., Jansen J. C., Van Bekkum H. Template variation in the synthesis of zeolite ZSM-5 // Applied catalysis. 1985. V. 17. №2. P. 261-271.
- Suzuki K., Kiyozumi Y., Matsuzaki K., Shin S. Influence of the synthesis conditions of H-ZSM-5 on its physical properties and catalytic activity in methanol conversion: the water content of the reaction mixture // Applied catalysis. 1987. V. 35. №2. P. 401-409.
- Moretti E., Leofanti G., Padovan M., Solari M., De Alberti G., Gatti F. A New Route to Zsm-5 Zeolite: Synthesis and Characterization // Studies in Surface Science and Catalysis. Elsevier, 1984. V. 18. P. 159-166.
- Beheshti M. S., Behzad M., Ahmadpour J., Arabi H. Modification of H-[B]-ZSM-5 zeolite for methanol to propylene (MTP) conversion: Investigation of extrusion and steaming treatments on physicochemical characteristics and catalytic performance // Microporous and Mesoporous Materials. 2020. V. 291. P. 109699.
- Shestakova D. O., Babina K. A., Sladkovskiy D. A., Parkhomchuk E. V. Seed-assisted synthesis of hierarchical zeolite ZSM-5 in the absence of organic templates // Materials Chemistry and Physics. 2022. V. 288. P. 126432.
- Chen K., Wu X., Zhao J., Zhao H., Li A., Zhang Q., Shen B. Organic-free modulation of the framework Al distribution in ZSM-5 zeolite by magnesium participated synthesis and its impact on the catalytic cracking reaction of alkanes // Journal of Catalysis. 2022. V. 413. P. 735-750.
- Mirshafiee F., Khoshbin R., Karimzadeh R. A green approach for template free synthesis of Beta zeolite incorporated in ZSM-5 zeolite to enhance catalytic activity in MTG reaction: Effect of seed nature and temperature // Journal of Cleaner Production. 2022. P. 132159.
- Zhang R., Wang Y., Chai C., Li F., Han L., Zhao L. Amine-free synthesis of fly ash based ZSM-5 via interzeolite transformation with related investigation of mechanism // Microporous and Mesoporous Materials. 2022. P. 111992.
- Yang S., Zhang Y., Guo W., Zhou L., Chen M., Ma J., Zhang Y. Synthesis of thin ZSM-5 zeolite membranes in a self-terminating mother liquor // Separation and Purification Technology. 2022. V. 299. P. 121721.


