Synthesis and antimicrobial activity hybrid systems with pyrazole and imidazole rings
Автор: Panasenko N.V.
Журнал: Теория и практика современной науки @modern-j
Рубрика: Основной раздел
Статья в выпуске: 8 (14), 2016 года.
Бесплатный доступ
By three-component condensation of 1-substituted 3-arylpyrazole-4-carbaldehydes, benzyl, and ammonium acetate in the medium of acetic acid 1-substituted 3-aryl-4-(4,5-diphenylimidazol-2-yl)pyrazoles were synthesized, among which the compounds with moderate antimicrobial activity were found.
Pyrazole-4-carbaldehydes, benzyl, ammonium acetate, 5-diphenylimidazol-2-yl)pyrazoles, condensation, antimicrobial activity
Короткий адрес: https://sciup.org/140269689
IDR: 140269689
Текст научной статьи Synthesis and antimicrobial activity hybrid systems with pyrazole and imidazole rings
The imidazole cycle is an important structural fragment of a considerable number of naturally occurring substances [1] and pharmaceutically active structures [2]. In the variety of imidazole compounds their polysubstituted representatives have a special position because of a wide range of their biological activities: antiinflammatory [3], antiallergenic [4], antimicrobial [5], antineoplastic [6], and analgesic [7]. Besides that, inhibitors of P38 MAP kinase [8] and glucagon receptors [9] were found among these compounds.
-
ІI. Formulation of the problem
It is known that acid catalyzed one-pot three-component condensation of aldehydes, α-diketones, and ammonium acetate is one of the effective methods of synthesizing 2-alkyl(aryl)-4,5-diarylimidazoles [10-12]. The usage of reactions of this type for their 2-heteryl analogues' synthesis, 2-pyrazolyl-4,5-diphenylimidazoles in particular, is represented in the literature with only one example [13]. At the same time, taking into account the pharmacological behavior of a row of heterocyclic ensembles that are a combination of imidazole and pyrazole nuclei [14,15], the design of new 4-imidazolylpyrazoles seems to be scientifically reasonable both from chemical and biological points of view. That's why the study of available [16,17] 3-aryl-4-formylpyrazoles (Іa-l) condensation with benzyl and ammonium acetate and testing of bactericidal activity of the synthesized compounds became the subject of our report.
-
III. Results
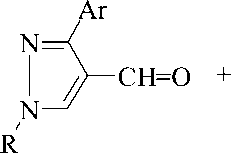
It was found that pyrazole-4-carbaldehydes (Ia-l) interact with benzyl and ammonium acetate in the ratio of 1:1:4 in the medium of boiling acetic acid, producing 4-(4,5-diphenylimidazol-2-yl)pyrazoles (IIa-l). The control of the reaction process using the TLC method has shown that in these conditions the reaction ends in 1 hour and leads to the target products with yields of 64-91 %.
Ph
Іa-l
OO
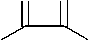
+ Ph
AcOH
2 NH OAc
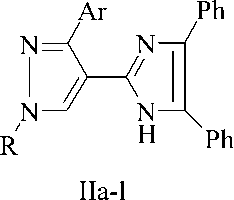
І, ІІ, R=Ph, Ar=Ph (a), 3-ClC 6 H 4 (b), 4-MeOC 6 H 4 (c), F 2 HCOC 6 H 4 (e), тієн-2-іл (f), pyridin-4-yl (е), benzofuran-2-yl (g) ; R=NCCH 2 CH 2 , Ar=3-ClC 6 H 4 (h), 4-BrC 6 H 4 (i); 3,4-Me 2 C 6 H 3 (j) ; R=HOOCCH 2 CH 2 , Ar=- 3-BrC 6 H 4 (k), 3-NO 2 -4-MeOC 6 H 3 (l).
The composition of the synthesized compounds was reliably proven by elemental analysis and mass-spectra (Table 1), and their structure by IR- and
1Н NMR spectroscopy (Table 2). The mass-spectra of all compounds are characterized by the maximum peaks of protonated molecular ions [M+1]+. In the IR-spectra the absorption bands of N-H bond of imidazole cycle in the interval of 3370-3385 cm-1 are present, and for the compounds (ІІj-l) – additional absorption bands of the C≡N groups (2255-2260 cm-1) or СООН groups (2490-2905 cm-1). In the 1Н NMR spectra, besides the typical signals of aryl and alkyl substitutes of the azole nuclei, the singlets of Н5 protons of the pyrazole cycle (8.26-9.07 ppm), and N-H singlets of the imidazole cycle (12.4112.65 ppm) are present.
We performed the testing of synthesized compounds for their bactericidal activity by the method of two-fold serial dilutions, using the standard strains cultures S.aureus and E.coli as test-microorganisms. The obtained values of minimal inhibiting bacteriostatic (MBsC) and bactericidal (MBcC) concentrations of compounds (ІІa-l ) are given in the Table 3, and show that they have moderate antimicrobial activity.
Experimental part
IR-spectra of the compounds in the KBr tablets were recorded with the UR-20 device. 1Н NMR spectra were measured using the Bruker Avance DRX (500.13 MHz) device, internal standard – TMS. Chromato-mass-spectra were recorded on the PE SCXAPI 150 EX device using UV (250 nm) and ELSOJ detectors.
1-Substituted 4-(4,5-diphenyl-1H-imidazol-2-yl)-3-aryl-1H-pyrazoles (ІІa-l). The mixture of 0.002 mole of aldehyde (Іa-l), 0.42 g (0.002 mole) of benzyl, and 0.62 g (0.008 mole) ammonium acetate in 5 ml of acetic acid were boiled for 1 hour. The reactional mixture was cooled down; the produced sediment was filtered, washed with water, dried, and crystallized from ethanol.
The study of antimicrobial activity
The antimicrobial activity of the synthesized compounds was determined by the micromethod that involved preparation of two-fold serial dilutions using the Takachi microtitrator with two test-cultures of microorganisms (grampositive and gram-negative) in disposable polystyrene plates.
The polystyrene plates were filled, using the Takachi microtitrator, with 4-hous old broth test-cultures of E.coli and S.aureus in the concentration of 105 microbial bodies per 1 ml. Two-fold serial dilutions (from 500 μg/ml to 7.8 μg/ml) were prepared from the studied compounds (in the concentration of 1000 μg/ml). The inoculations were incubated at 37 °C for 18-24 hours; after that they were visually assessed for presence or absence of microbial growth. The experiment was conducted three times.
The lowest dilution of the studied compound in the presence of which the inhibition of microorganism test-culture occurred was taken as the minimal bacteriostatic concentration (MBsC). It was expressed as numerical value of active substrate dilution.
The minimal bactericidal concentration (MBcC) was determined by inoculation of meat-and-peptone agar in the Petri dishes with the contents of the wells for which the growth was not observed.
The known antimicrobial drug, ethonium, was used as control.
-
IV. Conclusions
-
1. 1-Substituted 3-aryl-4-(4,5-diphenylimidazol-2-yl)pyrazoles were synthesized by three-component condensation of 1-substituted 3-arylpyrazole-4-carbaldehydes, benzyl, and ammonium acetate in the boiling acetic acid.
-
2. The testing of the produced compounds has shown that they demonstrate moderate antimicrobial activity.
Table 1
Characteristics of the compounds IIa-l
|
Compound |
Formula |
[М+1]+ |
Found , % |
T melt |
Yield, |
|
Calculated |
ºС . |
% |
|||||
|
C |
H |
N |
|||||
|
II a |
C 30 H 22 N 4 |
439 |
82.41 82.17 |
5.17 5.06 |
12.56 12.78 |
257-259 |
76 |
|
IIb |
C 30 H 21 ClN 4 |
473 |
76.38 76.18 |
4.36 4.48 |
11.64 11.85 |
294-296 |
81 |
|
IIc |
C 31 H 24 N 4 О |
469 |
79.18 79.47 |
5.27 5.16 |
12.21 11.96 |
254-256 |
64 |
|
IId |
C 31 H 22 F 2 N 4 О |
505 |
73.54 73.80 |
4.49 4.40 |
11.33 11.10 |
259-262 |
71 |
|
IIe |
C 28 H 20 N 4 S |
445 |
75.92 75.65 |
4.64 4.53 |
12.46 12.60 |
283-285 |
83 |
|
IIf |
C 29 H 21 N 5 |
404 |
78.96 79.25 |
4.93 4.82 |
15.75 15.93 |
276-278 |
87 |
|
IIg |
C 32 H 22 N 4 О |
479 |
80.03 80.32 |
4.55 4.63 |
11.95 11.71 |
236-237 |
91 |
|
IIh |
C 27 H 20 ClN 5 |
450 |
71.81 72.08 |
4.57 4.48 |
15.32 15.56 |
244-245 |
81 |
|
IIi |
C 27 H 20 BrN 5 |
495 |
65.87 65.60 |
4.19 4.08 |
13.94 14.17 |
275-278 |
76 |
|
IIj |
C 29 H 25 N 5 |
444 |
78.80 78.53 |
5.61 5.68 |
15.65 15.79 |
216-217 |
68 |
|
IIk |
C 27 H 21 BrN 4 О 2 |
514 |
62.88 63.17 |
4.23 4.12 |
10.69 10.91 |
227-228 |
73 |
|
IIl |
C 28 H 23 N 5 О 5 |
510 |
65.72 66.00 |
4.64 4.55 |
13.51 13.74 |
231-233 |
87 |
Table 2
IR and 1H NMR spectra of the compounds IIa-l
|
Comp ound |
IR spectrum, ν, cm-1 |
1H NMR spectra, δ. ppm( J , Hz) |
|
IIa |
3385 (NН) |
7.23-7.59 m (16Н arom .), 7.95 d (2H arom ., J 7.6 Hz), 8.10 d (2H arom ., J 7.6 Hz), 9.04 s (1Н, Н5 pyrazole ), 12.52 s (1Н, NН) |
|
IIb |
3370 (NН) |
7.56-7.96 m (15Н arom .), 7.96 d (2H arom ., J 8.0 Hz), 8.21 d (2H arom ., J 8.0 Hz), 9.03 s (1Н, Н5 pyrazole ), 12.54 s (1Н, NН) |
|
IIc |
3380 (NН) |
7.02 d (2H arom ., J 8.4 Hz), 7.23-7.60 m (13Н arom .), 7.93 d (2H arom ., J 8.4 Hz), 8.08 d (2H arom ., J 8.8 Hz), 8.96 s (1Н, Н5 pyrazole ), 12.49 s (1Н, NН) |
|
IId |
3370 (NН) |
7.30-7.62 m (16Н, 15Н arom .+СН), 7.94 d (2H arom ., J 8.0 Hz), 8.23 d (2H arom ., J 8.0 Hz), 9.01 s (1Н, Н5 pyrazole ), 12.53 s (1Н, NН) |
|
IIe |
3375 (NН) |
7.17-7.63 m (16Н arom .), 7.91 d (2H arom ., J 8.0 Hz), 8.56 d (2H thiophene ., J 4.0 Hz), 9.03 s (1Н, Н5 pyrazole ), 12.59 s (1Н, NН) |
|
IIf |
3370 (NН) |
7.32-7.64 m (13Н arom .), 7.97 d (2H arom ., J 8.0 Hz), 8.18 d (2H arom ., J 6.0 Hz), 8.68 d (2H arom ., J 6.0 Hz), 9.08 s (1Н, Н5 pyrazole ), 12.45 s (1Н, NН) |
|
IIg |
3380 (NН) |
7.37-7.77 m (17Н arom .), 7.98 m (2H arom ., J 8.0 Hz), 8.43 s (1Н, Н3 benzofuran ), 9.15 s (1Н, Н5 pyrazole ),12.65 s (1Н, NН) |
|
IIh |
3375 (NН) 2255 (С≡N) |
3.21 t (2Н, СН 2 , J 6.0 Hz), 4.55 t (2Н, СН 2 , J 6.0 Hz), 7.23 7.97 m (13Н arom .), 8.37 s (1Н arom. ), 8.52 s (1Н, Н5 pyrazole ), 12.51 s (1Н, NН) |
|
ІІi |
3380 (NН) 2255 (С≡N) |
3.19 t (2Н, СН 2 , J 6.8 Hz), 4.54 t (2Н, СН 2 , J 6.8 Hz), 7.227.53 m (10Н arom .), 7.62 d (2Н arom ., J 8.4 Hz), 7.99 d (2Н arom ., J 8.4 Hz), 8.32 s (1Н, Н5 pyrazole ), 12.47 s (1Н, NН) |
|
ІІj |
3385 (NН) 2260 (С≡N) |
2.24 s (3Н, СН 3 ), 2.26 s (3Н, СН 3 ), 3.18 t (2Н, J 6.4 Hz), 4.51 t (2Н, J 6.4 Hz), 7.14-7.65 m (12Н arom .), 7.86 s (1Н arom. ), 8.26 s (1Н, Н5 pyrazole ),12.38 s (1Н, NН) |
|
ІІk |
3375 (NН) 2490-2905 (СООН) |
2.93 t (2Н, СН 2 , J 6.4 Hz), 4.45 t (2Н, СН 2 , J 6.4 Hz), 7.22 7.55 m (11Н arom .), 8.27-8.35 m (2Н arom .), 9.02 s (1Н, Н5 pyrazole ), 12.46 m. s (2Н, NН+СООН) |
|
ІІl |
3375 (NН) 2540-2860 (СООН) |
2.93 t (2Н, СН 2 , J 6.2 Hz), 4.45 t (2Н, СН 2 , J 6.2 Hz), 7.22 7.99 m (13Н arom .), 8.30 s (1Н arom. ), 8.67 s (1Н, Н5 pyrazole ), 12.44 s (2Н, NН+СООН) |
Table 3
Bactericidal activity of the compounds IIa-l
|
Compound |
Test-cultures of microorganisms |
|||
|
S.aureus |
E.coli |
|||
|
MBsC |
MBcC |
MBsC |
MBcC |
|
|
IIa |
250 |
>500 |
>500 |
>500 |
|
IIb |
250 |
>500 |
>500 |
>500 |
|
IIc |
250 |
>500 |
>500 |
>500 |
|
IId |
250 |
>500 |
500 |
>500 |
|
IIe |
250 |
>500 |
>500 |
>500 |
|
IIf |
250 |
>500 |
>500 |
>500 |
|
IIg |
250 |
>500 |
500 |
>500 |
|
IIh |
125 |
>500 |
>500 |
>500 |
|
IIi |
>500 |
>500 |
>500 |
>500 |
|
IIj |
250 |
>500 |
>500 |
>500 |
|
IIk |
125 |
>500 |
>500 |
>500 |
|
IIl |
250 |
>500 |
250 |
>500 |
|
Ethonium |
7.8 |
31.2 |
125 |
250 |
Список литературы Synthesis and antimicrobial activity hybrid systems with pyrazole and imidazole rings
- Ho J.Z., Hohareb R.M., Ahn J.H. et.al. Enantiospecific synthesis of carbopentostatins // J. Org. Chem. -2003.- Vol.68.- P.109-114.
- Lombardino J.G., Wiseman E.H. Preparation and antiflammatory activity of some trisubstituted imidazoles // J. Med. Chem.- 1974.- Vol.17.- P.1182-1188.
- Mison M. Unique acid catalisis of heteropoly compounds (heteropolyoxymetalates) in solid state // Chem. Commun.- 2001.- P.1141-1152.
- Blank J.W., Durant G.L., Emmert J.C., Ganelin C.R. Sulfur-methylene isosterizm in the development of metiamide, a new histamine H2-receptor antagonist // Nature.- 1974.- Vol.248.- P.65-66.
- Antolini M., Bozzoli A., Ghiron C. et al. Analogues of 4,5-bis(3,5-dichlorophenyl)-2-trifluoromethyl-1H-imidazole as potential antibacterial agents // Bioorg. Med. Chem. Lett.- 1999.- Vol. 9.- P.1023-1028.
- Wang L., Woods K.W., Li Q. et al. Potent, orally active heterocycles-based cobbrestatin A-4 analogues: synthesis, structure-activity relationship, pharmacokinetics, and in vivo antitumor activitu evaluation // J. Med. Chem.- 2002.-Vol.45.- P.1697-1711.
- Ucucu U., Karaburum N.G., Iskdag I. Synthesis and analgesic activity of some 1-benzyl-2-substituted-4,5-diphenyl-1H-imidazole derivatives // Farmaco.- 2001.- Vol.56.- P. 285-290.
- Lee J.C., Laydon J.T., McDonnel D.C. et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis // Nature.- 1994.- Vol.372.- P.739-745.
- de Laszlo S.E., Hacker C., Li B. et al. Potent, orally absorbed glucagons receptor antagonist // Bioorg. Med. Chem. Lett.- 1999.- Vol.9.- P.641-646.
- Liu F.J., Chen J., Zhoo J. et al. A modified procedure for the Synthesis of 1-arylimadasoles // Synthesis.- 2003.- P.2661-2666.
- Satskar S., Siev D., Mjalli A.M.M. Imidazole libraries on solid support // Tetrahedron Lett.- 1996.- Vol.37.- P.835-838.
- Weinmann H., Hahhe M., Koenig K. et al. Efficient and environmentally friendly synthesis of 2-aminoimidazole // Tetrahedron Lett.- 2002.-Vol.43.-P.593-595.
- El Salam H.A.A., Shaker N.O., El-Telbani E.M., Nawwar G.A. Facile synthesis of heterocycles having bacteriocidal activity incorporating oleic acid residues // J. Chem. Reseach.- 2009.- Vol.6.- P.400-404.
- Pat. WO 2007038215 (A1). Tetracyclic inhibitors of Janus kinases / Arvanitis A.G., Rodgers J.D., Combs A.P., Sparks B., Robinson D.J., Fridman J.S., Vaddi K. (05.04.2007) // http: espacenet.com
- Pat. US 51090012 (A). 2-Imidazol(in)e substituted aryl-1,2,3-triazole pesticides / Roberts W.J., O'Mahong M.J., Bryan R. (28.04.92) // http: espacenet.com
- Bratenko M.K., Chernyuk I. N., Vovk M.V. 4-Functionalized 3-Heterylpyrazoles. I. 4-Formyl-3-heterylpyrazoles // Zh. Org. Khim., 1997, vol. 33, p. 1749-1751.
- Bratenko, M.K., Chornous, V.O. and Vovk, M.V. Polyfunctional pyrazoles. I. Synthesys of 1-(2-cyanoethyl)-3-(het)aryl-4-formylpyrazoles.


