Synthesis and structure of bismuth complexes [p-Tol 4E] +3[Bi 3I 12] 3-•HOCH 2CH 2OC 2H 5 E=P, Sb
Автор: Sharutin V.V., Sharutina O.K., Senchurin V.S., Khisamov R.M., Mosunova T.V.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Металлоорганическая химия
Статья в выпуске: 4 т.7, 2015 года.
Бесплатный доступ
The interaction of equimolar amounts of tetra- p-tolylphosphonium and tetra- p-tolylstybonium iodides with bismuth triiodide in 2-ethoxyethanol leads to formation of the complexes [p-Tol 4E] +3[Bi 3I 12] 3-∙HOCH 2CH 2OC 2H 5 E=P (I), Sb (II). X-ray diffraction analysis of compounds (I) and (II) has shown that in the cations of complexes I and II the coordination of phosphorus and antimony atoms is tetrahedral (angles equal: CPC 107.3(3)°-113.4(4)° (I), CSbC 105.4(3)°-113.7(3)° (II); bond lengths for P-C and Sb-C are 1.761(9)-1.815(7) Å and 2.085(8)-2.099(8) Å, respectively). In trinuclear anions [Bi 3I 12] 3- the terminal fragments BiI 3 (Bi-Iterm 2.8714(5)-2.9181(5) Å (I), 2.8867(5)-2.9248(6) Å (II)) are bonded to the central bismuth atom through six μ2-bridging iodine atoms (Bi-Ibr 3.0454(6)-3.3891(6) Å (I), 3.0595(4)-3.3694(6) Å (II)).
Bismuth triiodide, комплексы висмута [p-tol 4e] +3[bi 3i 12] 3-∙hoch2ch2oc2h5, bismuth complexes [p-tol 4e] +3[bi 3i 12] 3-∙hoch2ch2oc2h5, e=p, sb, synthesis, structure
Короткий адрес: https://sciup.org/147160329
IDR: 147160329 | УДК: 547.53.024+549.242+548.312.5 | DOI: 10.14529/chem150406
Текст научной статьи Synthesis and structure of bismuth complexes [p-Tol 4E] +3[Bi 3I 12] 3-•HOCH 2CH 2OC 2H 5 E=P, Sb
Ionic bismuth complexes with linear anions [Bi 3 I 12 ]3–, in which the bismuth atoms are bonded through six μ 2 -bridging iodine atoms, are described in the literature as separate examples, where nitrogen-containing acyclic or heterocyclic cations stand as counter-ions [1–3]. Thus far only one similar complex with phosphonium cation is known [4]; like complexes with organoantimony cations are not known.
In the present paper we have synthesized two new phosphorus- and antimony-containing bismuth complexes [p-Tol 4 E]+ 3 [Bi 3 I 12 ]3–∙HOCH 2 CH 2 OC 2 H 5 (E=P, Sb) with the linear anion [Bi 3 I 12 ]3– and carried out X-ray diffraction analysis of these.
Experimental tris(Tetra-p-tolylphosphonium) hexakis(μ2-iodo)-hexaiodo-tribismuth 2-ethoxyethanol solvate (I). The solution of 0.100 g (0.19 mmol) tetra-p-tolylphosphonium and 0.113 g (0.19 mmol) bismuth triiodide in 15 mL 2-ethoxyethanol was obtained. The solvent was slowly evaporated. The yield was 0.142 g (65%) of orange-red crystals of complex I with decomposition temperature 195 °C. Found, %: C 30.83, Н 2.74. For С88Н94О2P3Bi3I12 calculated,%: C 30.54, H 2.85.
tris(Tetra- p -tolylstibonium) hexakis(μ 2 -iodo)-hexaiodo-tribismuth 2-ethoxyethanol solvate (II) . It was obtained by the similar procedure. Orange-red crystals of complex II were isolated (48%) with decomposition temperature 219 ° C. Found, %: C 28.56, Н 2.54. For C88H94O2Sb3Bi3I i2 calculated, %: C 28.43, H 2.62.
The X-ray diffraction analyses of crystals I and II were performed on the Bruker D8 QUEST automatic four-circle diffractometer (Mo K « -emission, X = 0.71073 A, graphite monochromator). The data were collected and analyzed, the unit cell parameters were refined, and the absorption correction was applied using the SMART and SAINT- Plus programs [5]. All calculations for structure determination and refinement were performed using the SHELXL/PC [6] and OLEX2 programs [7]. The structures were determined by the direct method and refined by the least-squares method in the anisotropic approximation for non-hydrogen atoms. The main crystallographic data and refinement results for the structure are listed in Table 1, the selected bond lengths and bond angles are given in Table 2.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1053856, 1049481; ; .
Table 1
Crystallographic data and the experimental and structure refinement parameters for compounds I, II
|
Parameter |
Value |
|
|
I |
II |
|
|
Empirical formula |
C 88 H 94 O 2 P 3 I 12 Bi 3 |
C 88 H 94 O 2 Sb 3 I 12 Bi 3 |
|
Formula weight |
3426.28 |
3698.62 |
|
Т , К |
296(2) |
296(2) |
|
Crystal system |
Triclinic |
Triclinic |
|
Space group |
P¯ 1 |
P¯ 1 |
|
a , Å |
12.4517(6) |
12.4055(4) |
|
b , Å |
18.0680(9) |
18.3805(5) |
|
c, Å |
24.1711(10) |
24.6523(7) |
|
α , deg |
87.532(2) |
87.401(2) |
|
β, deg |
87.039(2) |
87.049(2) |
|
γ , deg |
75.281(2) |
74.124(2) |
|
V , Å3 |
5250.0(4) |
5396.8(3) |
|
Z |
4 |
2 |
|
ρ (calcd.), g/сm3 |
2.167 |
2.276 |
|
µ , mm - 1 |
8.630 |
9.085 |
|
F (000) |
3136.0 |
3352.0 |
|
Crystal size, mm |
0.26×0.25×0.16 |
0.33×0.3×0.14 |
|
2 θ Range of data collection, deg |
4.04 – 46.56 |
4.1 – 53.54 |
|
Range of refraction indices |
–13 ≤ h ≤ 13 –20 ≤ k ≤ 20 –26 ≤ l ≤ 26 |
–15 ≤ h ≤ 15 –23 ≤ k ≤ 23 –31 ≤ l ≤ 31 |
|
Measured reflections |
94609 |
102068 |
|
Independent reflections |
15056 |
22967 |
|
R int |
0.0486 |
0.0587 |
|
Refinement variables |
990 |
990 |
|
GOOF |
1.036 |
1.007 |
|
R factors for F2 > 2 σ (F2) |
R 1 = 0.0361 wR 2 = 0.0817 |
R 1 = 0.0382 wR 2 = 0.0705 |
|
R factors for all reflections |
R 1 = 0.0579 wR 2 = 0.0941 |
R 1 = 0.0711 wR 2 = 0.0811 |
|
Residual electron density (min/max), e /Å3 |
1.42/–0.77 |
1.29/–0.92 |
Table 2
Selected bond lengths and bond angles in the structure of compounds 1, 2
|
Bond d, Å |
Angle ω , deg |
||
|
I |
|||
|
P(1) – С(1) |
1.788(8) |
C(1)P(1)C(11) |
109.0(4) |
|
P(1) – С(11) |
1.800(7) |
C(1)P(1)C(21) |
110.0(4) |
|
P(1) – С(21) |
1.761(9) |
C(1)P(1)C(31) |
109.6(4) |
|
P(1) – С(31) |
1.799(8) |
C(11)P(1)C(21) |
110.3(4) |
|
P(2) – С(41) |
1.804(7) |
C(11)P(1)C(31) |
109.9(3) |
|
P(2) – С(51) |
1.791(6) |
C(21)P(1)C(31) |
108.0(4) |
Organometallic chemistry
Table (continued)
|
Bond d, к |
Angle ω , deg |
||
|
I |
|||
|
P(2) – С(61) |
1.770(7) |
C(41)P(2)C(51) |
107.5(3) |
|
P(2) – С(71) |
1.795(7) |
C(41)P(2)C(61) |
109.6(3) |
|
P(3) – С(81) |
1.800(7) |
C(41)P(2)C(71) |
111.7(3) |
|
P(3) – С(91) |
1.791(7) |
C(51)P(2)C(61) |
109.3(3) |
|
P(3) – С(101) |
1.815(7) |
C(51)P(2)C(71) |
111.4(3) |
|
P(3) – С(111) |
1.800(8) |
C(61)P(2)C(71) |
107.3(3) |
|
Bi(1) – I (1) |
3.0748(5) |
C(81)P(3)C(91) |
109.7(3) |
|
Bi(1) – I (2) |
3.0706(5) |
C(81)P(3)C(101) |
109.1(3) |
|
Bi(1) – I (3) |
3.0444(4) |
C(81)P(3)C(111) |
107.7(4) |
|
Bi(2) – I (1) |
3.3046(5) |
C(91)P(3)C(101) |
107.8(3) |
|
Bi(2) – I (2) |
3.2715(5) |
C(91)P(3)C(111) |
109.1(3) |
|
Bi(2) – I (3) |
3.3556(5) |
C(101)P(3)C(111) |
113.4(4) |
|
Bi(2) – I (4) |
2.9181(5) |
I(1)Bi(1)I(2) |
91.611(13) |
|
Bi(2) – I (5) |
2.8714(5) |
I(7)Bi(3)I(9) |
88.583(16) |
|
Bi(2) – I (6) |
2.9049(5) |
I(7)Bi(3)I(8) |
85.096(15) |
|
Bi(3) – I (7) |
3.0669(5) |
I(8)Bi(3)I(9) |
88.406(15) |
|
Bi(3) – I (8) |
3.0654(5) |
I(2)Bi(2)I(3) |
79.092(13) |
|
Bi(3) – I (9) |
3.0454(6) |
I(2)Bi(2)I(4) |
88.012(16) |
|
Bi(4) – I (7) |
3.3153(6) |
I(2)Bi(2)I(5) |
93.250(16) |
|
Bi(4) – I (8) |
3.3891(6) |
I(2)Bi(2)I(6) |
169.597(15) |
|
Bi(4) – I (9) |
3.2907(7) |
I(3)Bi(2)I(4) |
94.399(15) |
|
Bi(4) – I (10) |
2.8958(6) |
I(3)Bi(2)I(5) |
165.035(15) |
|
Bi(4) – I (11) |
2.9040(7) |
I(3)Bi(2)I(6) |
92.370(15) |
|
Bi(4) – I (12) |
2.9088(7) |
I(4)Bi(2)I(5) |
98.194(16) |
|
I(4)Bi(2)I(6) |
98.652(17) |
||
|
I(5)Bi(2)I(6) |
93.689(17) |
||
|
I(7)Bi(4)I(8) |
76.406(14) |
||
|
I(7)Bi(4)I(9) |
80.500(15) |
||
|
I(7)Bi(4)I(10) |
87.219(17) |
||
|
I(7)Bi(4)I(11) |
93.121(18) |
||
|
I(7)Bi(4)I(12) |
170.78(2) |
||
|
I(8)Bi(4)I(9) |
79.245(15) |
||
|
I(8)Bi(4)I(10) |
163.130(16) |
||
|
I(8)Bi(4)I(11) |
87.587(17) |
||
|
I(8)Bi(4)I(12) |
101.94(2) |
||
|
I(9)Bi(4)I(10) |
94.251(17) |
||
|
I(9)Bi(4)I(11) |
166.381(18) |
||
|
I(9)Bi(4)I(12) |
90.28(2) |
||
|
I(10)Bi(4)I(11) |
97.464(19) |
||
|
I(10)Bi(4)I(12) |
93.58(2) |
||
|
I(11)Bi(4)I(12) |
95.88(2) |
||
|
II |
|||
|
Sb(1) – С(1) |
2.089(6) |
C(1)Sb(1)C(11) |
107.6(3) |
|
Sb(1) – С(11) |
2.099(8) |
C(1)Sb(1)C(21) |
110.8(3) |
|
Sb(1) – С(21) |
2.091(7) |
C(1)Sb(1)C(31) |
108.8(3) |
Table (end)
|
Bond d, к |
Angle ω , deg |
||
|
II |
|||
|
Sb(1) – С(31) |
2.085(8) |
C(11)Sb(1)C(21) |
112.0(3) |
|
Sb(2) – С(41) |
2.086(7) |
C(11)Sb(1)C(31) |
110.9(3) |
|
Sb(2) – С(51) |
2.097(7) |
C(21)Sb(1)C(31) |
106.9(3) |
|
Sb(2) – С(61) |
2.097(7) |
C(41)Sb(2)C(51) |
109.7(3) |
|
Sb(2) – С(71) |
2.086(7) |
C(41)Sb(2)C(61) |
106.1(3) |
|
Sb(3) – С(81) |
2.092(8) |
C(41)Sb(2)C(71) |
113.6(3) |
|
Sb(3) – С(91) |
2.073(9) |
C(51)Sb(2)C(61) |
110.2(3) |
|
Sb(3) – С(101) |
2.090(9) |
C(51)Sb(2)C(71) |
105.6(3) |
|
Sb(3) – С(111) |
2.085(8) |
C(61)Sb(2)C(71) |
111.7(3) |
|
Bi(1) – I (1) |
3.0785(5) |
C(81)Sb(3)C(91) |
113.7(3) |
|
Bi(1) – I (2) |
3.0866(5) |
C(81)Sb(3)C(101) |
113.1(3) |
|
Bi(1) – I (3) |
3.0595(4) |
C(81)Sb(3)C(111) |
105.4(3) |
|
Bi(2) – I (2) |
3.3341(5) |
C(91)Sb(3)C(101) |
107.9(3) |
|
Bi(2) – I (3) |
3.3376(5) |
C(91)Sb(3)C(111) |
109.6(3) |
|
Bi(2) – I (4) |
2.9191(5) |
C(101)Sb(3)C(111) |
106.8(4) |
|
Bi(2) – I (5) |
2.9234(6) |
I(1)Bi(1)I(2) |
92.813(14) |
|
Bi(2) – I (6) |
2.8867(5) |
I(1)Bi(1)I(3) |
93.202(13) |
|
Bi(3) – I (7) |
3.0754(5) |
I(2)Bi(2)I(3) |
78.388(13) |
|
Bi(3) – I (8) |
3.0672(5) |
I(2)Bi(2)I(4) |
92.235(15) |
|
Bi(3) – I (9) |
3.0750(6) |
I(2)Bi(2)I(5) |
167.390(16) |
|
Bi(4) – I (8) |
3.3694(6) |
I(2)Bi(2)I(6) |
89.205(16) |
|
Bi(4) – I (9) |
3.2947(6) |
I(3)Bi(2)I(4) |
91.055(14) |
|
Bi(4) – I (10) |
2.9086(8) |
I(3)Bi(2)I(5) |
93.163(15) |
|
Bi(4) – I (11) |
2.9248(6) |
I(3)Bi(2)I(6) |
166.958(16) |
|
Bi(4) – I (12) |
2.9032(6) |
I(4)Bi(2)I(5) |
97.312(17) |
|
I(4)Bi(2)I(6) |
93.459(17) |
||
|
I(5)Bi(2)I(6) |
98.383(17) |
||
|
I(7)Bi(3)I(8) |
94.620(15) |
||
|
I(7)Bi(3)I(9) |
91.955(16) |
||
|
I(8)Bi(3)I(9) |
88.963(16) |
||
|
I(8)Bi(4)I(9) |
80.444(15) |
||
|
I(8)Bi(4)I(10) |
101.90(2) |
||
|
I(8)Bi(4)I(11) |
86.408(17) |
||
|
I(8)Bi(4)I(12) |
163.561(19) |
||
|
I(9)Bi(4)I(10) |
89.58(2) |
||
|
I(9)Bi(4)I(11) |
166.666(19) |
||
|
I(9)Bi(4)I(12) |
94.318(16) |
||
|
I(10)Bi(4)I(11) |
95.33(2) |
||
|
I(10)Bi(4)I(12) |
93.58(2) |
||
|
I(11)Bi(4)I(12) |
97.730(19) |
||
Results and Discussion
It has been shown that the interaction of equimolar amounts of tetra-p-tolylphosphonium and tetra-p-tolylstybonium iodides with bismuth triiodide in 2-ethoxyetanol leads to formation of the ionic bismuth complexes with the anion [Bi 3 I 12 ]3–, which contain the solvate molecule of the solvent:
Organometallic chemistry
HOCH 2 CH 2 OEt
3 p -Tol 4 EI + 3 BiI 3 → [ p -Tol 4 E]+ 3 [Bi 3 I 12 ]3–∙HOCH 2 CH 2 OEt
E = P ( I ); Sb ( II ).
According to X-ray diffraction data, the phosphorus and antimony atoms of the cations have weakly distorted tetrahedral coordination (Fig. 1 and 2). Angles CPC and CSbC equal 107.3(3)°–113.4(4)° and 105.4(3)°–113.7(3)°, respectively. Bond lengths P–C (1.761(9) - 1.815(7) Å) and Sb–C (2.085(8) - 2.099(8) Å) are near to the sums of covalent radii of phosphorus, carbon (1.88 Å) and antimony, carbon (2.19 Å) [8]. In trinuclear centrosymmetrical anions [Bi 3 I 12 ]3– the terminal fragments BiI 3 (Bi - I term 2.8714(5) - 2.9181(5) Å ( I ), 2.8867(5) - 2.9248(6) Å ( II )) are bonded to the central bismuth atom through six μ 2 -bridging iodine atoms (Bi–I br 3.0454(6)–3.3891(6) Å ( I ), 3.0595(4)-3.3694(6) Å ( II )). The terminal fragments BiI 3 are in the masked conformation.
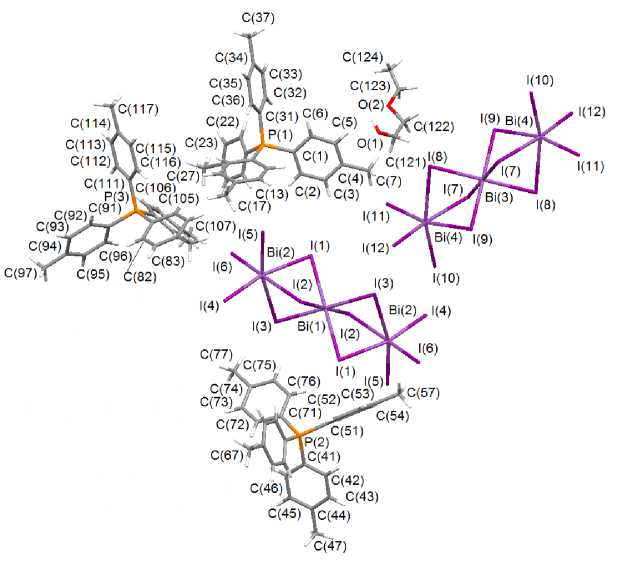
Fig. 1. The structure of complex I
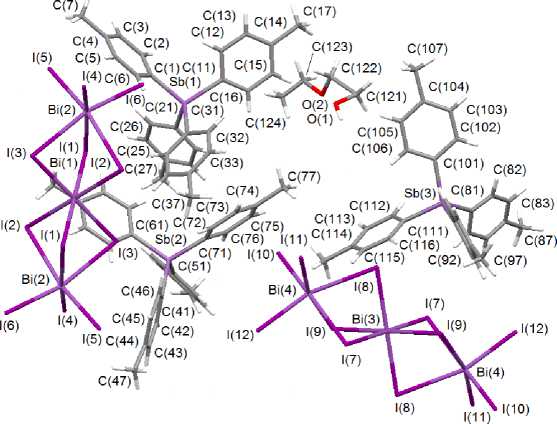
Fig. 2. The structure of complex II
The structural organization in crystals I and II results from weak interactions of the type H∙∙∙I and H∙∙∙O. In I the bridging μ 2 -atoms of iodine in anions are bonded (2.86–3.16 Å) to the hydrogen atoms of phosphonium cations, in II the similar bonds are formed with participation of both bridging (3.10 Å) and terminal (3.15 Å) atoms of iodine (which is somewhat less than the sum of Van der Waals radii of hydrogen and iodine, namely, 3.3 Å [8]). No significant close contacts of the ions with the solvent molecules have been observed, but the molecules of 2-ethoxyethanol are bonded with each other by hydrogen bonds H∙∙∙O (2.33 Å ( I ) and 2.62 Å ( II )) (Fig. 3).
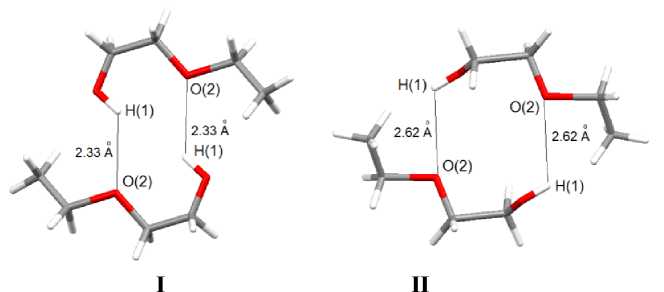
Fig. 3. Association of solvent molecules in crystals I and II
Conclusions
Complexes [p-Tol 4 E]+ 3 [Bi 3 I 12 ]3- ∙ HOCH 2 CH 2 OC 2 H 5 E=P ( I ), Sb ( II ) have been synthesized by interaction of equimolar amounts of tetra- p -tolylphosphonium and tetra- p -tolylstibonium iodides with bismuth triiodide in 2-ethoxyethanol. The structure of the products has been established by X-ray diffraction analysis.
Список литературы Synthesis and structure of bismuth complexes [p-Tol 4E] +3[Bi 3I 12] 3-•HOCH 2CH 2OC 2H 5 E=P, Sb
- Structure of a New Iodobismuthate: Tetra(n-butyl)ammonium 1,2;1,2;1,2;2,3;2,3;2,3-hexa-μ-iodo-1,1,1,3,3,3-hexaiodotribismuthate (III) (3:1)/U. Geiser, E. Wade, H.H. Wang, J.M. Williams//Acta Crystallographica Section C, 1990. -V. 46, № 8. -P. 1547-1549.
- Carmalt, C.J. Structural Studies on some Iodoantimonate and Iodobismuthate Anions/C.J. Carmalt, L.J. Farrugia, N.C. Norman//Zeitschrift für anorganische und allgemeine Chemie. -1995. -V. 621, №. 47. -Р. 47-56.
- Intercalated Iodobismuthate in the Layers of Azoimidazoles. Structure, Photochromism and DFT Computation/D. Mallick, K.K. Sarker, R. Saha, T.K. Mondal et al.//Polyhedron. -2013. -V. 54, P. 147-157.
- Synthesis and Structure of Bismuth Complexes 6+ 3-3-• H2O2, 3+ 3-, 3+ 3-, 3+ 3-• 2Me2C=O, and 3+ 3-/V.V. Sharutin, I.V. Egorova, N.N. Klepikov, E.A. Boyarkina et al//Russian Journal of Inorganic Chemistry. -2009. -V. 54, № 1. -P. 52-68.
- Bruker (1998). SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (1998). SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures From Diffraction Data. Bruker AXS Inc., Madison, Wisconsin, USA.
- OLEX2: a Complete Structure Solution, Refinement and Analysis Program/O.V. Dolomanov, L.J. Bourhis, R.J. Gildea et al.//J. Appl. Cryst. -2009. -V. 42. -P. 339-341.
- Бацанов, С.С. Атомные радиусы элементов/С.С. Бацанов//Журн. неорган. химии. -1991. -Т. 36. -№ 12. -С. 3015-3037.

![Synthesis and structure of bismuth complexes [p-Tol 4E] +3[Bi 3I 12] 3-•HOCH 2CH 2OC 2H 5 E=P, Sb Synthesis and structure of bismuth complexes [p-Tol 4E] +3[Bi 3I 12] 3-•HOCH 2CH 2OC 2H 5 E=P, Sb](/file/cover/147160329/synthesis-and-structure-of-bismuth-complexes-p-tol-4e-3-bi-3i-12-3-hoch-2ch.png)
