Synthesis and structure of gold complexes [ p-Tol 4Sb][ p-TolAuCl 3] and [ p-Tol 4Sb][AuCl 4]
Автор: Sharutin V.V., Sharutina O.K., Senchurin V.S.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Металлоорганическая химия
Статья в выпуске: 4 т.7, 2015 года.
Бесплатный доступ
Gold complexes [ p-Tol 4Sb] +[ p-TolAuCl 3] - (1) and [ p-Tol 4Sb] +[AuCl 4] - (2) have been synthesized by interaction of tetra- para-tolylstibonium chloride and aurichlorohydric acid tetrahydrate in acetone. In 1 the tetra- para-tolylstibonium cations are tetrahedral (Sb-C 2.091(7)-2.097(6) Å, CSbC 106.1(3)°-112.7(3)°), the [p-TolAuCl3]- anions have square-planar structure (Au-Cl 2.286(2)-2.389(2) Å, Au-C 2.028(7) Å, ClAuCl 89.92(9)°, 92.42(9)°, 176.68(8)°, CAuCl 88.27(18)°, 89.57(18)°, 175.59(19)°). In crystal 2 the geometrical characteristics of cations (Sb-C 2.076(7)-2.097(9), CSbC 106.5(3)°-111.5(4)°) and [AuCl 4] - square-planar anions (Au-Cl 2.248(3)-2.278(3) Å, ClAuCl 89.17(9)°-90.74(13)°, 177.24(16)°, 177.61(13)°) are similar to those in 1.
Synthesis, structure, gold compounds, x-ray diffraction analysis
Короткий адрес: https://sciup.org/147160338
IDR: 147160338 | УДК: 541.49+546.593+546.865+547.53.024+548.312.2 | DOI: 10.14529/chem150413
Текст научной статьи Synthesis and structure of gold complexes [ p-Tol 4Sb][ p-TolAuCl 3] and [ p-Tol 4Sb][AuCl 4]
Synthesis and structure of complex compounds of gold, containing [AuHal4] - anions in their composition, were mentioned in the literature [1-5]. In the present paper the synthesis and structure of gold complexes [ p -Tol 4 Sb][AuCl 4 ] and [ p -Tol 4 Sb][ p -TolAuCl 3 ] are described.
Experimental
Synthesis of [ р -Тоl 4 Sb]+[ р -ТоlAuCl 3 ]- (1). Tetra- para -tolylantimony chloride 0.250 g (0.48 mmol) and aurichlorohydric acid tetrahydrate 0.197 g (0.48 mmol) were dissolved in 10 mL acetone, then 5 ml toluene was added. The solution was left to stand for 12 h at 20 ° C. After evaporation of the solvent 0.287 g (68%) of tetra- para -tolylantimony para -tolyltrichloroaurate was obtained (mp 156 ° C). IR spectrum (v, cm - 1): 484, 587, 800, 1007, 1072, 1194, 1305, 1401, 1492, 1588, 1627, 1905, 2926. Found, %: С 47,38, Н 4,03. Calculated for C 35 H 35 AuCl 3 , %: С 47,72, Н 3,98.
Synthesis of [ р -Тоl 4 Sb]+[AuCl 4 ]- (2). a) The mixture of tetra-para-tolylantimony chloride 0.137 g (0.26 mmol) and and aurichlorohydric acid tetrahydrate 0.108 g (0.26 mmol) in 10 mL acetone stood for 24 h at room temperature. The solvent was evaporated. The yield is 0.205 g (95%) of tetra- para -tolylantimony tetra- para -chloroaurate with decomposition temperature 148-150 ° C. IR spectrum (v, cm - 1): 483, 529, 585, 633, 697, 809, 1101, 1040, 1066, 1123, 1190, 1212, 1313, 1396, 1444, 1493, 1589, 1708, 2863, 2918, 2953, 3045. Found, %: С 40.52, Н 3.49. Calculated for C 28 H 28 SbAuCl 4 , %: С 40.75, Н 3.40.
-
b) The mixture of penta- para -tolylantimony 0.181 g (0.31 mmol) and aurichloric acid tetrahydrate 0.129 g (0.31 mmol) in 10 mL acetone stood for 24 h at room temperature. The solvent was evaporated. The yield is 0.232 g (90%) of pale yellow tetra- para -tolylantimony tetrachloroaurate, the melting point and IR spectrum coincide with tetra- para -tolylantimony tetrachloroaurate obtained according to method a. Found: %: С 40.54, Н 3.52. Calculated for C 28 H 28 SbAuCl 4 , %: С 40.75, Н 3.40.
IR spectra were recorded on the Bruker Tensor 27 in KBr pellets.
The X-ray diffraction analyses of crystals 1 and 2 were performed on the Bruker D8 QUEST automatic four-circle diffractometer (Mo K „ -emission, X = 0.71073 A, graphite monochromator). The data were collected and analyzed, the unit cell parameters were refined, and the absorption correction was applied using the SMART and SAINT-Plus programs [6]. All calculations for structure determination and refinement were performed using the SHELXL/PC programs [7]. The structures were determined by the direct method and refined by the least-squares method in the anisotropic approximation for nonhydrogen atoms.
The main crystallographic data and refinement results for the structures are listed in Table 1. The selected bond lengths and bond angles are given in Table 2.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1000139 for 1, CCDC 1000140 for 2; ; .
Table 1
Crystallographic data, the experimental and structure refinement parameters for compounds 1, 2
|
Parameter |
Value |
|
|
1 |
2 |
|
|
М |
880.70 |
825.02 |
|
Crystal system |
Monoclinic |
Monoclinic |
|
Space group |
P2 1 /n |
P2 1 /n |
|
a , Å |
12.748(5) |
12.7962(5) |
|
b , Å |
14.941(5) |
14.8701(5) |
|
c, Å |
18.19 8(10) |
16.1167(5) |
|
α , град |
90.00 |
90.00 |
|
β , град |
102.87(4) |
101.8270(10) |
|
γ , град |
90.00 |
90.00 |
|
V , Å3 |
3379(3) |
3001.59(18) |
|
Z |
4 |
4 |
|
ρ (calcd.), г/см3 |
1.731 |
1.826 |
|
µ , mm–1 |
5.395 |
6.153 |
|
F (000) |
1704.0 |
1576.0 |
|
Crystal size, mm |
0.54 × 0.36 × 0.34 |
0.99 × 0.61 × 0.5 |
|
2 θ Range of data collection, deg |
4.48 - 62.1° |
6.16 - 60.1° |
|
Range of refraction indices |
–18 ≤ h ≤ 18, –16 ≤ k ≤ 21, –25 ≤ l ≤ 26 |
–17 ≤ h ≤ 13, –20 ≤ k ≤ 7, –22 ≤ l ≤ 20 |
|
Measured reflections |
38599 |
7733 |
|
Independent reflections |
10659 (R int = 0.0819) |
5051 (R int = 0.0298) |
|
Refinement variables |
366 |
311 |
|
GOOF |
1.011 |
0.993 |
|
R -factors for F2 > 2 σ (F2) |
R 1 = 0.0653, wR 2 = 0.1145 |
R 1 = 0.0416, wR 2 = 0.0913 |
|
R -factors for all reflections |
R 1 = 0.1352, wR 2 = 0.1342 |
R 1 = 0.1046, wR 2 = 0.1137 |
|
Residual electron density (min/max), e/A3 |
2.06/–0.82 |
0.55/–0.85 |
Table 2
Selected bond lengths ( d ) and bond angles ( ω ) in the structures of compounds 1, 2
|
Bond d, Å |
Angle ω , deg |
|||
|
1 |
||||
|
Au(1) - Cl(1) |
2.286(2) |
Cl(1)Au(1)Cl(2) |
176.68(8) |
|
|
Au(1) - Cl(2) |
2.292(2) |
C(41)Au(1)Cl(3) |
175.59(19) |
|
|
Au(1) - Cl(3) |
2.389(2) |
C(1)Sb(1)C1(1) |
110.6(3) |
|
|
Au(1) - C(41) |
2.028(7) |
C(1)Sb(1)C(21) |
106.1(3) |
|
|
Sb(1) - C(1) |
2.094(6) |
C(21)Sb(1)C(11) |
112.7(3) |
|
|
Sb(1) - C(11) |
2.097(6) |
C(31)Sb(1)C(1) |
111.3(3) |
|
|
Sb(1) - C(21) |
2.095(7) |
C(31)Sb(1)C(11) |
108.1(3) |
|
|
Sb(1) - C(31) |
2.091(7) |
C(31)Sb(1)C(21) |
108.0(3) |
|
|
2 |
||||
|
Au(1) - Cl(1) |
2.266(3) |
Cl(1)Au(1)Cl(2) |
90.74(13) |
|
|
Au(1) - Cl(2) |
2.269(3) |
Cl(1)Au(1)Cl(3) |
89.58(13) |
|
|
Au(1) - Cl(3) |
2.278(3) |
Cl(2)Au(1)Cl(3) |
177.61(13) |
|
|
Au(1) - Cl(4) |
2.248(3) |
Cl(4)Au(1)Cl(1) |
177.24(16) |
|
Organometallic chemistry
Table 2 (end)
|
Bond d, Å |
Angle to , deg |
||
|
Sb(1) - C(1) |
2.076(9) |
Cl(4)Au(1)Cl(2) |
89.17(13) |
|
Sb(1) - C(11) |
2.095(8) |
Cl(4)Au(1)Cl(3) |
90.62(13) |
|
Sb(1) - C(31) |
2.097(9) |
C(1)Sb(1)C1(1) |
109.5(3) |
|
Sb(1) - C(21) |
2.086(8) |
C(1)Sb(1)C(31) |
111.5(4) |
|
C(1) - C(2) |
1.391(13) |
C(1)Sb(1)C(21) |
109.4(3) |
|
C(1) - C(7) |
1.370(12) |
C(11)Sb(1)C(31) |
106.5(3) |
|
C(2) - C(3) |
1.375(16) |
C(21)Sb(1)C(11) |
111.2(3) |
|
C(4) - C(5) |
1.501(16) |
C(21)Sb(1)C(31) |
108.6(4) |
Results and Discussion
It is known that the product of pentaphenylantimony reaction with aurichlorohydric acid (hydrogen tetrachloroaurate (III)) at 20 ° С in acetone is tetraphenylantimony tetrachloroaurate, separated from the reaction mixture with 84 % yield [2].
We have found that in similar conditions the interaction of aurichlorohydric acid, obtained by dissolving jewelry gold in aqua regis, with tetra- para -tolylantimony chloride runs with formation of tetra- para -tolylantimony para -tolyltrichloroaurate ( 1 ):
p -To^SbCl + H[AuCl 4 ] + TolH ^ [ p -Tol 4 Sb]+[ p -TolAuCy -
If chemically pure aurichlorohydric acid is used, such an unusual transmetalation reaction between antimony and gold compounds is not observed. In this case the formation of tetra- para -tolylantimony tetrachloroaurate ( 2 ) occurs:
p -To^SbCl + HAuCLJ • 4 H 2 O ^ [ p -ToUSbJ + LAuCLJ - + HCl + 4 H 2 O
Complex 2 has also been obtained from penta- para -tolylantimony and aurichlorohydric acid: p -To^Sb + H[AuCl 4 ] • 4 H 2 O ^ [ p -Tol 4 Sb]+[AuCl 4 ] - + TolH + 4 H 2 O
It is obvious that the presence of impurities in the initial jewelry gold and, consequentially, in the used acid affects the direction of the chemical reaction between aurichlorohydric acid and tetra- para -tolylantimony chloride.
According to the X-ray analysis data, the crystal of complex 1 consists of tetra- para -tolylstibonium tetrahedral cations and [ p -TolAuCl 3 ] - square-planar anions (Fig. 1).
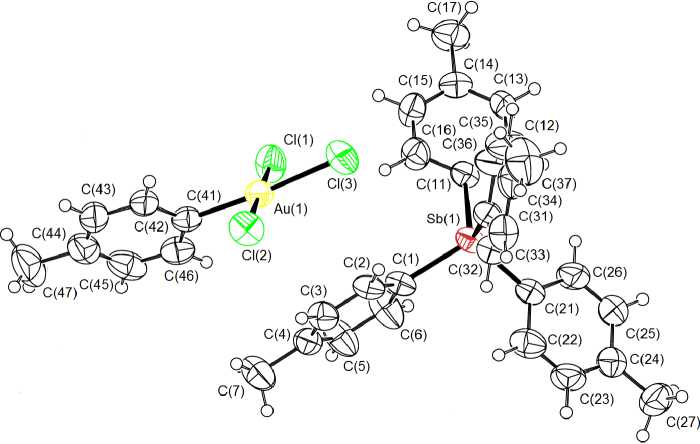
Fig. 1. The structure of the molecule of compound 1
In crystal 1 tetra- para -tolylstibonium tetrahedral cations (Sb-C 2.091(7) - 2.097(6) A, CSbC 106.1(3) °- 112.7(3) ° ) are present, as well as [ p -TolAuCl3] - anions of square-planar structure (Au - Cl 2.286(2) - 2.389(2) A, Au - C 2.028(7) A, ClAuCl 89.92(9) ° , 92.42(9) ° , 176.68(8) ° , CAuCl 88.27(18) ° , 89.57(18) ° , 175.59(19) ° ). In crystal 2 the geometric characteristics of cations (Sb - C 2.076(7) - 2.097(9), CSbC 106.5(3) °- 111.5(4) ° ) and [AuCl 4 ] - square-planar anions (Au - Cl 2.248(3) - 2.278(3) A, ClAuCl 89.17(9) °- 90.74(13) ° , 177.24(16) ° , 177.61(13) ° ) (Fig. 2, Table 2) are similar to those found in 1 .
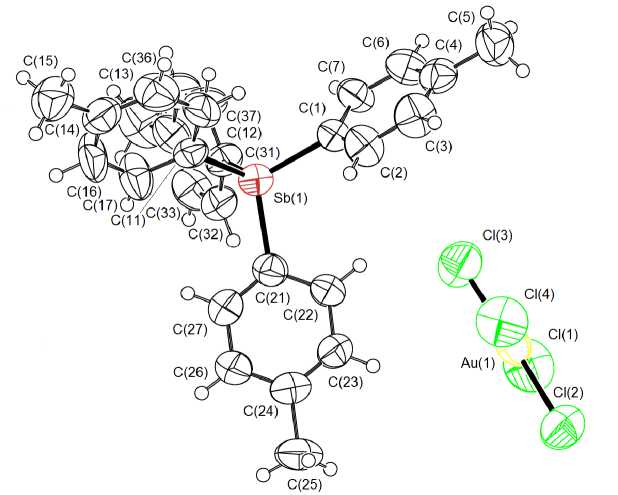
Fig. 2. The structure of complex 2
The structural organization in crystals I and II is caused by weak interionic hydrogen bonds. At that in I the single interionic contact is observed: ortho -H Ph (16)∙∙∙Cl(1) (2.92 Å). In complex II each anion is bonded with four cations through Cl --- H bonds, formed with the use of all four chlorine atoms and ortho -, meta - and methyl hydrogen atoms of tolyl substituents (2.81 - 2.88 A) (Fig. 3).
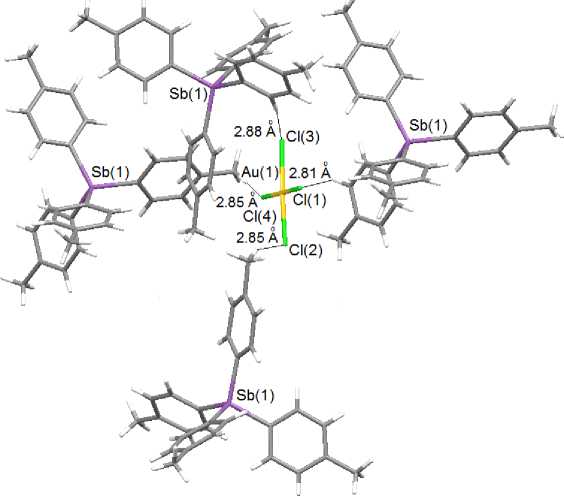
Fig. 3. Interionic hydrogen bonds in the crystal of complex 2
Organometallic chemistry
Conclusions
Thus, the ionic complexes of gold with tetra- para -stibonium tetrahedral cation and [ p -TolAuCl3] - and [AuCl 4 ] square-planar anions have been synthesized and structurally described for the first time. The first example of transmetalation reaction with the use of antimony and gold compounds has been described.
Список литературы Synthesis and structure of gold complexes [ p-Tol 4Sb][ p-TolAuCl 3] and [ p-Tol 4Sb][AuCl 4]
- Синтез и строение смешаннолигандных комплексных соединений сурьмы 4+ 3-1-(Hal = I, Br) и золота 2+1-1-(Hal = Cl, Br)/В.В. Шарутин, В.С. Сенчурин, О.А. Фастовец и др.//Бутлеровские сообщения. -2007. -T. 11, № 2. -С. 43-48.
- Синтез и кристаллические структуры гексахлороплатината, тетрахлороаурата и гексахлоростанната тетрафенилсурьмы (V) +22-, +-, +2 2-/В.В. Шарутин, В.С. Сенчурин, О.А. Фастовец и др.//Коорд. химия. -2008. -Т. 34, № 5. -С. 373-379.
- Синтез и кристаллическая структура тетрахлороаурата бутилтрифенилфосфония +-/В.В. Шарутин, В.С. Сенчурин, О.К. Шарутина, Л.Н. Винокурова//Бутлеровские сообщения. -2011. -Т. 27, № 16. -С. 68-71.
- Шарутин, В.В. Синтез и кристаллическая структура тетрахлороаурата ацетонилтрифенилфосфония +-/В.В. Шарутин, О.К. Шарутина, В.С. Сенчурин//Бутлеровские сообщения. -2014. -Т. 38, № 5. -С. 151-154.
- Cambridge Crystallographic Data Center. -2015. -http://deposit@ccdc.cam.ac.uk/http://www/ccdc.cam.ac.uk.
- SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. -Madison (Wisconsin, USA): Bruker AXS Inc., 1998.
- SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures From Diffraction Data. -Madison (Wisconsin, USA): Bruker AXS Inc., 1998.
- OLEX2: a complete structure solution, refinement and analysis program/O.V. Dolomanov, L.J. Bourhis, R.J. Gildea et al.//J. Appl. Cryst. -2009. -V. 42. -P. 339-341.

![Synthesis and structure of gold complexes [ p-Tol 4Sb][ p-TolAuCl 3] and [ p-Tol 4Sb][AuCl 4] Synthesis and structure of gold complexes [ p-Tol 4Sb][ p-TolAuCl 3] and [ p-Tol 4Sb][AuCl 4]](/file/cover/147160338/synthesis-and-structure-of-gold-complexes-p-tol-4sb-p-tolaucl-3-and-p-tol-4sb.png)
