Synthesis and structure of tri- and tetraphenylantimony aroxides: Ph 3Sb[OC 6H 3(Br 2-2,6)(Me-4)] 2 and Ph 4SbOC 6H 3Br 2-2,6, Me-4
Автор: Sharutin V.V., Sharutina O.K., Senchurin V.S.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Металлоорганическая химия
Статья в выпуске: 4 т.7, 2015 года.
Бесплатный доступ
Interaction of pentaphenylantimony with 2,6-dibromo,4-methylphenol or with bis(2,6-dibromo,4-methylphenoxy)triphenylantimony (1) leads to formation of 2,6-dibromo,4-methylphenoxytetraphenylantimony (2). In molecules of compounds 1 and 2 the antimony atoms have distorted trigonal-bipyramidal coordination with the oxygen atoms in axial positions (OSbO 179.20(15)° (1), OSbC 179.7(9)° (2)). The bond lengths Sb-C and Sb-O equal 2.090(5), 2.101(5), 2.110(5) and 2.088(4), 2.088(4) Å (1), 2.11(2), 2.11(2), 2.11(2), 2.17(3) and 2.234(18), 2.234(18) Å (2), respectively.
Pentaphenylantimony, triphenylantimony, 4-methylphenol, bis(2, molecular structure, x-ray diffraction analysis, 6-dibromo, tertbutyl hydroperoxide, 4-methylphenoxy)triphenylantimony, 4-methylphenoxy)tetraphenylantimony
Короткий адрес: https://sciup.org/147160335
IDR: 147160335 | УДК: 546.865+547.56+547.53.024+548.312.5 | DOI: 10.14529/chem150411
Текст научной статьи Synthesis and structure of tri- and tetraphenylantimony aroxides: Ph 3Sb[OC 6H 3(Br 2-2,6)(Me-4)] 2 and Ph 4SbOC 6H 3Br 2-2,6, Me-4
It is known that redistribution of organic radicals between pentaphenylantimony and symmetrical derivatives of antimony with the general formula Ph 3 SbX 2 leads to formation of the single organoanti-mony compound with the general formula Ph 4 SbX, yielding up to 99 % [1–6].
Ph5Sb + Ph 3 SbX 2 ^ 2 Ph 4 SbX
X = F, Cl, Br, NO 3 , OC(O)R, OSO 2 Ar, SCN, ONCRR’, OAr
This reaction is of universal character, since it is valid for compounds containing substituents (X) of various nature, but for tetraphenylantimony aroxides it has been demonstrated by one example only [5].
In the course of research on characteristic features of ligand redistribution we have studied the reactions of pentaphenylantimony with bis (2,6-dibromo,4-methylphenoxy)triphenylantimony ( 1 ) and 2,6-dibromo,4-methylphenol. The structures of the products have been established by X-ray diffraction analysis.
Experimental
Synthesis of bis (2,6-dibromo,4-methylphenoxy)triphenylantimony (1). The mixture of 0.353 g (1.00 mmol) triphenylantimony, 0.532 g (2.00 mmol) 2,6-dibromo,4-methylphenol and 0.129 g 70% solution of tert -butyl hydroperoxide in 10 mL ether stood for 18 h at 24 ° C. After slow evaporation of the solvent, large crystals 1 with melting point 214 ºС were formed to yield 0.786 g (89%). Found, %: С 43.37; Н 2.99. Calculated for С 32 H 25 O 2 Br 4 Sb, %: С 43.49; Н 2.83.
Interaction of pentaphenylantimony with bis (2,6-dibromo,4-methylphenoxy) triphenylantimony. The mixture of 0.250 g (0.50 mmol) pentaphenylantimony, 0.441 g (0.50 mmol) bis (2,6-dibromo,4-methylphenoxy)triphenylantimony and 2 mL toluene in glass ampoule was heated in boiling water bath for 1 hour. The progress of the reaction was controlled by thin-layer chromatography. The solution was decanted from the crystals, and the solvent was evaporated. Formation of large crystals 2 with decomposition temperature 224 ºС was observed. IR spectrum (ν, cm‾1): 3048, 2917, 2361, 2342, 1577, 1447, 1434, 1302, 1291, 1240, 1176, 1069, 1020, 996, 849, 799, 730, 692, 570, 563, 491, 467, 458, 447. Found, %: С 54.43; Н 3.71. Calculated for C 31 H 25 OBr 2 Sb, %: С 53.54, Н 3.60.
Interaction of pentaphenylantimony with 2,6-dibromo,4-methylphenol. The mixture of 0.25 g (0.49 mmol) pentaphenylantimony, 0.131 g (0.49 mmol) 2,6-dibromo-4-methylphenol and 2 mL toluene in glass ampoule was heated in boiling water bath for 1 hour. The solution was decanted from the crystals, the solvent was evaporated. Colorless crystals with decomposition temperature 224 °С were formed to yield 0.274 g (80%). IR spectrum is identical to the IR spectrum of the compound obtained by the abovementioned procedure.
IR spectra were recorded on the Fourier-transform spectrometer Bruker Tensor 27 in KBr pellets.
The X-ray diffraction analyses of crystals 1 and 2 were performed on the Bruker D8 Quest diffractometer (Mo K α -emission, λ = 0.71073 Å, graphite monochromator). The data were collected and analyzed, the unit cell parameters were refined, and the absorption correction was applied using the SMART and SAINT- Plus programs [7]. All calculations for structure determination and refinement were performed using the SHELXL/PC [8] and OLEX2 programs [9]. The structures were determined by the direct method and refined by the least-squares method in the anisotropic approximation for nonhydrogen atoms. The main crystallographic data and refinement results for the structures are listed in Table 1, the selected bond lengths and bond angles are given in Table 2.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1043497, 1009712; ; .
Table 1
Crystallographic data and the experimental and structure refinement parameters for compounds 1, 2
|
Parameter |
Value |
|
|
1 |
2 |
|
|
Empirical formula |
C 32 H 25 O 2 Br 4 Sb |
C 31 H 25 OBr 2 Sb |
|
Formula weight |
882.91 |
695.08 |
|
Т , К |
273.15 |
273.15 |
|
Crystal system |
triclinic |
orthorhombic |
|
Space group |
P-1 |
Pnma |
|
a , Å |
10.1942(6) |
16.3869(9) |
|
b , Å |
11.0622(6) |
11.6579(6) |
|
c, Å |
15.3136(8) |
14.5305(7) |
|
α , deg |
87.547(3) |
90.00 |
|
β, deg |
78.460(3) |
90.00 |
|
γ , deg |
69.703(3) |
90.00 |
|
V , Å3 |
1586.28(15) |
2775.9(2) |
|
Z |
2 |
4 |
|
ρ (calcd.), g/сm3 |
1.848 |
1.663 |
|
-1 µ , mm |
5.934 |
3.894 |
|
F (000) |
848.0 |
1360.0 |
|
Crystal size, mm |
0.35 × 0.18 × 0.09 |
0.68 × 0.45 × 0.43 |
|
2 θ Range of data collection, deg |
5.56 - 53.02° |
6.08 - 52.9° |
|
Range of refraction indices |
–12 ≤ h ≤ 12, –13 ≤ k ≤ 13, –19 ≤ l ≤ 19 |
–20 ≤ h ≤ 20, –14 ≤ k ≤ 14, –18 ≤ l ≤ 18 |
|
Measured reflections |
31896 |
67786 |
|
Independent reflections |
6504 |
2998 |
|
R int |
0.0801 |
0.0474 |
|
Refinement variables |
354 |
184 |
|
GOOF |
1.018 |
1.050 |
|
R factors for F2 > 2 σ (F2) |
R 1 = 0.0477, wR 2 = 0.1073 |
R 1 = 0.0324, wR 2 = 0.0785 |
|
R factors for all reflections |
R 1 = 0.0857, wR 2 = 0.1236 |
R 1 = 0.0419, wR 2 = 0.0881 |
|
Residual electron density (min/max), e /Å3 |
1.35/–0.91 |
0.60/–0.83 |
Organometallic chemistry
Table 2
Selected bond lengths and bond angles in the structure of compounds 1, 2
|
Bond \ |
d , Å \ |
Angle \ |
ω , deg |
|
1 |
|||
|
Sb(1) - O(1) |
2.088(4) |
O(1)Sb(1)O(2) |
179.20(15) |
|
Sb(1) - O(2) |
2.089(4) |
O(1)Sb(1)C(1) |
89.6(2) |
|
Sb(1) - C(1) |
2.110(5) |
O(1)Sb(1)C(11) |
89.39(18) |
|
Sb(1) - C(11) |
2.101(5) |
O(1)Sb(1)C(21) |
92.17(19) |
|
Sb(1) - C(21) |
2.090(5) |
O(2)Sb(1)C(1) |
89.6(2) |
|
Br(1) - C(32) |
1.892(6) |
O(2)Sb(1)C(11) |
91.17(19) |
|
Br(2) - C(36) |
1.892(6) |
O(2)Sb(1)C(21) |
88.0(2) |
|
Br(3) - C(46) |
1.886(7) |
C(11)Sb(1)C(1) |
121.3(2) |
|
Br(4) - C(42) |
1.894(6) |
C(21)Sb(1)C(1) |
116.9(2) |
|
O(1) - C(31) |
1.338(7) |
C(21)Sb(1)C(11) |
121.8(2) |
|
O(2) - C(41) |
1.339(7) |
C(31)O(1)Sb(1) |
130.1(3) |
|
Sb(1) ⋅⋅⋅ Br(4) |
3.914(4) |
C(41)O(2)Sb(1) |
130.3(3) |
|
Sb(1) ⋅⋅⋅ Br(2) |
3.984(4) |
C(2)C(1)Sb(1) |
119.2(4) |
|
2 |
|||
|
Sb(1) - O(1) |
2.234(18) |
C(1)Sb(1)O1 |
179.7(9) |
|
Sb(1) - C(1) |
2.17(3) |
C(31)Sb(1)O1 |
85.0(6) |
|
Sb(1) - C(311) |
2.11(2) |
C(31)Sb(1)C1 |
95.2(7) |
|
Sb(1) - C(31) |
2.11(2) |
C(31)Sb(1)C21 |
122.7(6) |
|
Sb(1) - C(21) |
2.11(3) |
C(311)Sb(1)C21 |
122.7(6) |
|
Br(1) - C(12) |
1.90(2) |
C(21)Sb(1)O1 |
84.2(9) |
|
O(1) - C(11) |
1.31(3) |
C(21)Sb(1)C1 |
95.4(11) |
|
Symmetry operations: 1 +x, 1/2 |
y, +z |
||
Results and Discussion
The way of synthesis of antimony compounds with the general formula Ph 4 SbX by ligand redistribution reaction is particularly attractive due to easy isolation and high yield of the target product, the amount of which reaches two moles for one mole of initial pentaphenylantimony amount. The similar reaction with the use of pentaphenylantimony and triphenylantimony diaroxides was previously studied on the single example of tetraphenylantimony 4-nitrophenoxide [5].
It has been suggested that this disproportionation reaction proceeds through formation of the intermediate labile complex of ionic structure with tetraphenylstibonium cation and an anion containing two electronegative groups X, apart from four phenyl ligands. The following transition of electronegative group X to tetraphenylstibonium cation is accompanied by formation of the target product Ar 4 SbX. It is obvious that aryl compounds of antimony easily enter into disproportionation reactions with groups X, whose electronegativity is high enough.
We have studied the possibility of radical redistribution reaction between pentaphenylantimony and bis (2,6-dibromo,4-methylphenoxy)triphenylantimony ( 1 ).
The reaction was carried out in toluene solution (1 h, 100 оC). The progress of the reaction was controlled by thin-layer chromatography (Silufol UV-254, o -xylene as eluent). The reaction was considered completed after the disappearence of the spot peculiar to pentaphenylantimony. It has been established that the interaction between the abovementioned reactants proceeds with formation of (2,6-dibromo,4-methylphenoxy)tetraphenylantimony ( 2 ).
Ph 5 Sb + Ph 3 Sb(OAr) 2 → 2 Ph 4 SbOAr
Ar = C 6 H 2 Br 2 -2,6,Me-4
Tetraphenylantimony aroxide 2 , obtained by the radical redistribution reaction, was identical in its characteristics (melting point, IR spectrum) to the compound synthesized from pentaphenylantimony and 2,6-dibromo,4-methylphenol.
The parent antimony compound 1 was obtained by triphenylantimony oxidation with the use of tret -butyl hydroperoxide in the presence of 2,6-dibromo,4-methylphenol, according to the procedure described in [6]:
Ph 3 Sb + 2 НОС б Н з Вг 2 -2,6,Ме-4 + t -BuOOH ^ Ph 3 Sb(OC 6 H 3 Br 2 -2,6,Me-4) 2 + t -BuOH + H 2 O
From the X-ray analysis data it follows that in molecule 1 the antimony atoms have distorted tri-gonal-bipyramidal coordination with aroxyl ligands in axial positions (Fig. 1).
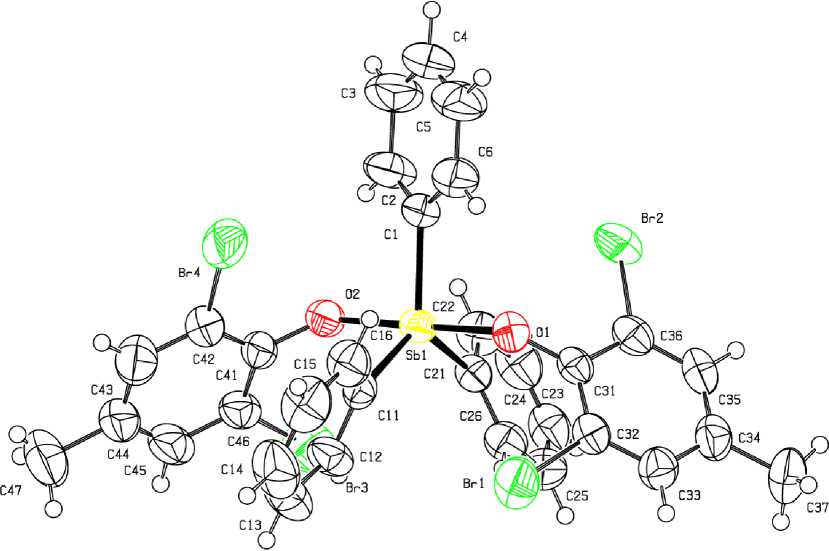
Fig. 1. The structure of compound 1
The axial angle OSbO measures 179.20(15) ° , the sum of angles CSbC in equatorial plane equals 360 ° . The angles between axial and equatorial bonds OSbC vary in the range 88.0(2) - 92.17(19)°. The lengths of equatorial bonds Sb–C (2.090(5), 2.101(5), 2.110(5) Å) exceed the values of axial distances Sb–O (2.088(4), 2.089(4) Å) and the sum of antimony and oxygen covalent radii (2.07 Å [10]). In 1 the close intermolecular contacts between the central atom and one of bromine atoms of aroxyl ligand are observed (Sb --- Br(2) 3.984(6), Sb --- Br(4) 3.914(6) A, while the sum of Van der Waals radii of antimony and bromine is 4.15 Å [10]). The structural organization of the crystal is formed with the participation of weak hydrogen bonds of the type H --- O (2.61 A) and H --- Br (3.02 - 3.03 A).
Geometrical characteristics of compound 2 (Fig. 2) differ from those found in 1 , obviously because of the presence of different ligands in axial positions.
For example, the axial angle OSbC equals 179.7(9) ° , while the sum of angles CSbC in equatorial plane (357.5 ° ) differs from the ideal value of 360 ° . The lengths of equatorial bonds Sb - C (2.11(2), 2.11(2), 2.11(3) A) are less than the values of axial distances Sb - О (2.234(18) A) and Sb - C (2.17(3) A). Intra- and intermolecular interactions H --- Br in crystal 2 are nonexistent, though the structure-forming contacts H --- 0 (2.53 A) are stronger than in 1 .
Organometallic chemistry
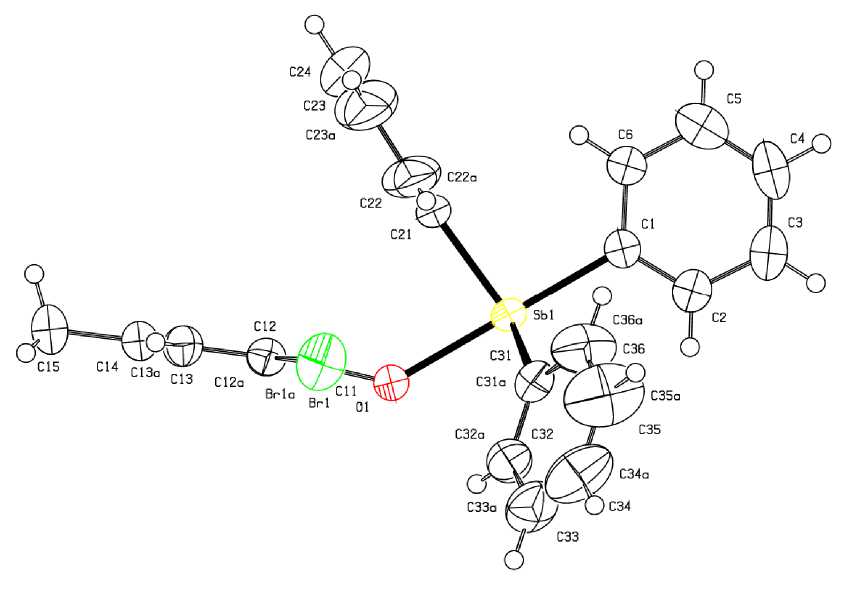
Fig. 2. The structure of compound 2
Conclusions
To summarize, the ligand redistribution reaction with participation of pentaphenylantimony and bis (2,6-dibromo,4-methylphenoxy)triphenylantimony, leading to formation of (2,6-dibromo,4-methylphenoxy)tetraphenylantimony, confirms the general character of the abovementioned reaction. In the molecules of bis (2,6-dibromo,4-methylphenoxy)triphenylantimony and (2,6-dibromo,4-methylphenoxy)tetraphenylantimony the antimony atoms have distorted trigonal-bipyramidal coordination with aroxyl ligands in axial positions. The compound 1 is characterized by short axial bonds Sb–O (2.088(4), 2.089(4) Å), intramolecular contacts Sb ⋅⋅⋅ Br (3.914(6), 3.984(6) Å).
Список литературы Synthesis and structure of tri- and tetraphenylantimony aroxides: Ph 3Sb[OC 6H 3(Br 2-2,6)(Me-4)] 2 and Ph 4SbOC 6H 3Br 2-2,6, Me-4
- Способ получения солей тетрафенилстибония общей формулы Ph4SbX /В.В. Шарутин, В.С. Сенчурин, О.К. Шарутина и др.//Журн. общ. химии. -1996. -Т. 66, № 10. -С. 1755-1756.
- Сульфонаты тетра-и триарилсурьмы/В.В. Шарутин, О.К. Шарутина, Л.П. Панова, В.К. Бельский//Журн. общ. химии. -1997. -Т. 67, № 9. -С. 1531-1535.
- Реакции пентаарилсурьмы с диацилатами триарилсурьмы/В.В. Шарутин, О.К. Шарутина, А.П. Пакусина В.К. Бельский//Журн. общ. химии. -1997. -Т. 67. -№ 9. -С. 1536-1541.
- Шарутин, В.В. Синтез и строение оксиматов тетра-и триарилсурьмы/В.В. Шарутин, О.К. Шарутина, О.В. Молокова и др.//Журн. общ. химии. -2001. -Т. 71, № 8. -C. 1317-1321.
- Новый метод синтеза арокситетраарильных соединений сурьмы/В.В. Шарутин, О.К. Шарутина, П.Е. Осипов, O.В. Субачева//Журн. общ. химии. -2001. -Т. 71, № 6. -C. 1045-1046.
- Шарутин, В.В. Именные реакции в химии элементоорганических соединений: справочник/В.В. Шарутин, В.С. Сенчурин. -Челябинск: Издательский центр ЮУрГУ, 2011. -427 с.
- Bruker (1998). SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (1998). SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures From Diffraction Data. Bruker AXS Inc., Madison, Wisconsin, USA.
- OLEX2: a Complete Structure Solution, Refinement and Analysis Program/O.V. Dolomanov, L.J. Bourhis, R.J. Gildea et al.//J. Appl. Cryst. -2009. -V. 42. -P. 339-341.
- Бацанов, С.С. Атомные радиусы элементов/С.С. Бацанов//Журн. неорган. химии. -1991. -Т. 36. -№ 12. -С. 3015-3037.

![Synthesis and structure of tri- and tetraphenylantimony aroxides: Ph 3Sb[OC 6H 3(Br 2-2,6)(Me-4)] 2 and Ph 4SbOC 6H 3Br 2-2,6, Me-4 Synthesis and structure of tri- and tetraphenylantimony aroxides: Ph 3Sb[OC 6H 3(Br 2-2,6)(Me-4)] 2 and Ph 4SbOC 6H 3Br 2-2,6, Me-4](/file/cover/147160335/synthesis-and-structure-of-triand-tetraphenylantimony-aroxidesph-3sb-oc-6h-3.png)
