Synthesis and study by electron microsco-py of inverse opals from zirconium oxide
Автор: K. A. Shabanova, Y. Y. Loginov, O. V. Shabanova, D. Kokh, I. V. Nemtsev
Журнал: Siberian Aerospace Journal @vestnik-sibsau-en
Рубрика: Technological processes and material science
Статья в выпуске: 4 vol.23, 2022 года.
Бесплатный доступ
Zirconia has a high dielectric constant and high thermal stability. There are many methods for the syn-thesis of nanocrystalline materials from zirconium dioxide. These include hydrothermal synthesis, gas-phase chemical reactions, cryochemical synthesis, plasma chemistry methods - these methods are expen-sive and complex. In this work, we propose a relatively simple method for controlling the growth of zirco-nia nanocrystals by synthesis in polymer templates (template synthesis of inverse opals). Inverse opals have unique physical and chemical properties, so they can be widely used in optics, optoelectronics, biological research, catalysis, functional ceramics, which is also relevant in the rocket and space industry. As a start-ing material, we used a water-alcohol solution of zirconium oxychloride, with which we impregnated tem-plates of monodisperse submicron spherical particles of polymethyl methacrylate. After impregnation of these templates, the solution solidified in a limited pore space of 20–40 nm. After that, we annealed the result-ing templates to remove the polymer matrix. In this case, structures consisting of zirconium dioxide nano-crystals were formed. Using the methods of scanning and transmission electron microscopy, we assessed the morphology of the obtained materials, and showed that under conditions of limited diffusion, zirconia forms crystals with a size of 10–30 nm. Also, depending on the calcination temperature, materials with dif-ferent crystalline modifications are obtained. As a result, we have shown that water-alcoholic solutions of zirconium oxychloride are a convenient means for obtaining nanocrystalline materials, including inverse opals from zirconium dioxide, by template synthesis.
Zirconia, photonic crystal, inverse opal, template synthesis, electron microscopy
Короткий адрес: https://sciup.org/148329668
IDR: 148329668 | УДК: 544.77.023.5 | DOI: 10.31772/2712-8970-2022-23-4-763-770
Текст научной статьи Synthesis and study by electron microsco-py of inverse opals from zirconium oxide
Methods for the synthesis of nanocrystalline materials with controlled structure are extremely relevant at present [1-4]. Micro- and nanocrystalline materials based on zirconia are widely used in various fields of space-rocket industry. They include laser atomized films and ceramics doped with rare and rare-earth elements, including nanoporous ceramics. Zirconium oxide has high dielectric constant and high thermal stability, so the methods of obtaining materials with desired properties on its basis are very diverse [5-10]. They include hydrothermal synthesis, gas-phase chemical reactions, cryochemical synthesis, and plasma chemistry methods.
Precious opal is a system of spherical particles packed in a face-centered cubic lattice with a diameter on the scale of the wavelength of visible light. Inverse opal is a periodic system of submicron monoscale pores in an optically transparent inorganic framework. Like opal, it is a three-dimensional photonic crystal (PC) [11]. The distinctive feature of PC is the possibility to control the rate of optical radiation and localization of electromagnetic waves, for example, by changing the structure of the material of which opal is composed.
Depending on the synthesis conditions, opal-like PC structures have unique physical and chemical properties, so inverse opals can be widely used in optics, optoelectronics, biological research, and catalysis [12].
The aim of this work was to obtain samples of inverse opals from zirconium dioxide by template synthesis; different crystal modifications of zirconium oxide by calcination, and to study them by electron microscopy.
Template synthesis is the production of an inorganic material by impregnating a polymer template, such as solutions of metal salts or metal oxide sols, followed by removal of the organic material [13]. The organic template is removed by annealing, after which the inorganic material takes the form of the original template.
In this work, electron microscopy was used to evaluate the morphology of the obtained samples, the size and periodicity of the pores. It was also important to determine the size and configuration of zirconium dioxide crystallites.
Samples and methodology of the experiment
Precipitated in regular structures, spherical particles of polymethylmethacrylate (PMMA) were used as matrices (templates) for obtaining inverse opals [3]. Since the pores between the particles are very small, cracking of the sample can occur when liquid penetrates there due to capillary forces. The impregnating liquid must also be nonsolvent for PMMA and not cause chemical interaction with a matrix.
Colloidal solutions of substances that form a solid transparent material after gelling and calcination are commonly used, but true solutions can also be used, such as zirconium oxychloride (ZOC), an inorganic compound (zirconium and hydrochloric acid oxosol) that is readily soluble in water and alcohol and decomposes to an oxide at temperatures above 400 °C.
The matrices were impregnated with a water-alcohol solution of zirconium oxychloride: 10 ml of water and 50 ml of 96% alcohol were added to 25 g of ZrOCl 2 (Fig. 1).

Рис. 1. Пропитка ПММА-матриц водно-спиртовым раствором оксихлорида циркония
Pic. 1. Impregnation of matrices from PMMA with a water-alcohol solution of zirconium oxychloride
During impregnation, the templates cracked, but this problem was solved by pre-calcination of the matrices at 120 °C, i.e., close to the glass transition temperature, so that the particles would stick together more firmly. Fig. 2, a shows an electron micrograph of the template, Fig. 2, b - electron micrograph of the impregnated matrix.
After impregnation, the samples were covered with crucibles to avoid ignition of the polymer template and annealed at 450 °C at a heating rate of 2 °C/min for 6 h. The samples were similarly annealed at 550 °C. The purpose of the annealing was not only to remove the polymer matrix by pyrolysis, but also to sinter the inorganic part. Fig. 2, c shows an electron micrograph of the inverted opal obtained in this way.
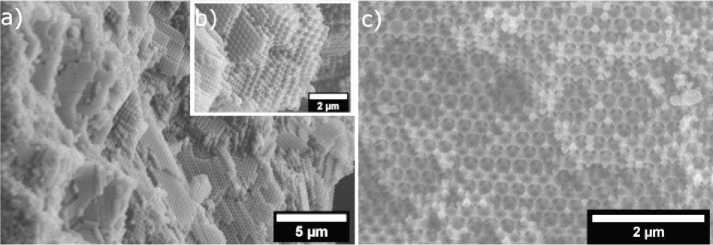
Рис. 1. Электронная микрофотография: а – темплата из ПММА; b – пропитанного образца; c – инверсного опала
Fig. 1. Electron micrograph: a – PMMA template; b – impregnated sample; c – inverse opal
To study the obtained samples of inverse zirconium oxide opals (Fig. 3) by electron microscopy, sample preparation was carried out.
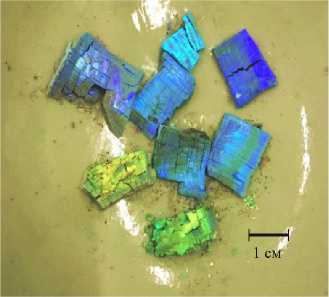
Рис. 3. Полученные инверсные опалы из оксида циркония
Pic. 3. Obtained zirconium oxide inverse opals
For scanning electron microscopy (SEM), we prepared small pieces of samples, attached them to a sample holder with a special adhesive, then sputtered platinum so that the surface was electrically conductive.
For transmission electron microscopy (TEM), small samples were crushed in beakers, diluted with alcohol, and sonicated for 2 min. Then, a drop of the resulting suspension was applied to a grid holder and placed in the working chamber of the microscope for examination.
Results and discussion
The images from the scanning electron microscope Hitachi S5500 (Japan) (Fig. 4) show that the structure of the obtained inverse zirconium oxide opal is highly organized and monodimensional, since the domain structure of the original template was preserved. The pore sizes are approximately 220 nm, and the walls are very thin (from units to tens of nanometers). The images are shown in different projections, and one of them (Fig. 4, a ) clearly shows that the walls consist of fine crystalline formations.
The study was also performed on a Hitachi HT7700 transmission electron microscope. The microphotograph (Fig. 5, a ) shows that the inorganic framework consists of nanosized zirconium oxide crystals - small crystalline formations that were observed in SEM.
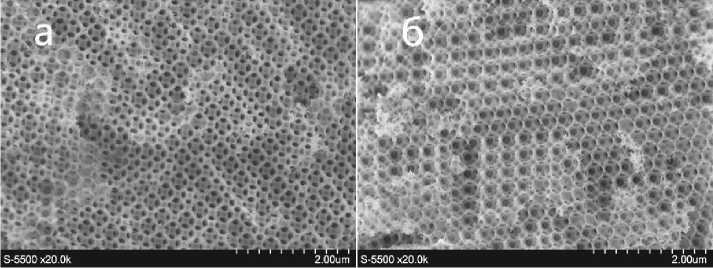
Рис. 4. РЭМ изображения инверсного опала из оксида циркония
Pic. 4. SEM images of an inverse zirconium oxide opal
Using the same equipment to determine the crystal structure of inverse opal from zirconium oxide annealed at 450 °C, an image of electron diffraction was obtained (Fig. 5, b ). The radii of the rings were measured and compared with the literature data [14]. The most probable crystal configuration of ZrO 2 in the obtained inverse opal is tetragonal (see table).
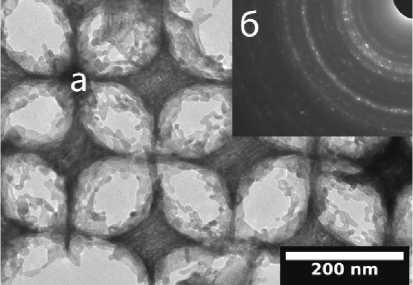
Рис. 5. ПЭМ изображения: а – микрофотография инверсного опала; б – дифракция электронов
Pic. 5. TEM images: a – micrograph of inverse opal; b – electron diffraction
Comparison of experimental and reference data on the diameter of electronographic rings for determination of crystalline phase
|
Experimental results |
Reference data |
||
|
The data are typical for the tetragonal configuration of ZrO 2 |
|||
|
Ring number |
1/Å |
Å |
Å |
|
1 |
0,197 |
5,076 |
5,10 |
|
2 |
0,276 |
3,623 |
3,69 |
|
6 |
0,394 |
2,538 |
2,55 |
|
8 |
0,451 |
2,217 |
2,21 |
|
ZrO 2 is close to the tetragonal configuration |
|||
|
3 |
0,321 |
3,115 |
3,19 |
|
4 |
0,342 |
2,924 |
2,85 |
|
7 |
0,412 |
2,427 |
2,34 |
|
The data are supposedly related to impurities |
|||
|
5 1 |
0,358 |
2,793 |
2,63 |
The same studies were performed for zirconium oxide inverse opals annealed at 550 °C. The crystal phase of such structure is also tetragonal, but monoclinic modification appears. The obtained data are confirmed by the results of X-ray phase analysis [15].
Conclusion
Inverse zirconium oxide opals based on templates of submicron particles of polymethylmethacrylate were obtained. The obtained samples were examined by the method of electron microscopy.
On the basis of the performed work the following conclusions can be made:
-
- water-alcohol solutions of zirconium oxychloride are applicable for obtaining inverse opals;
-
- depending on the temperature treatment, samples with tetragonal and partially tetragonal and monoclinic modification were obtained. It can be assumed that samples with cubic crystal modification can be obtained at higher processing temperatures (800-1000 °C);
-
- template synthesis allows to obtain nanocrystalline materials from relatively inexpensive components.
Работа выполнена в рамках проекта № FWES-2022-0012 «Оптические свойства и структурное упорядочение природных и природоподобных органических ламеллярных систем». Исследования были выполнены на оборудовании Красноярского регионального центра коллективного пользования ФИЦ КНЦ СО РАН.
Acknowledgment
The work was carried out within the project № FWES-2022-0012 “Optical properties and structural ordering of natural and nature-like organic lamellar systems”. The studies were performed on the equipment of the Krasnoyarsk Regional Center for Collective Use of the FRC KSC SB RAS.
Список литературы Synthesis and study by electron microsco-py of inverse opals from zirconium oxide
- Rusanov A. I. [The amazing world of nanostructures]. Zhurnal obshchey khimii. 2002, Vol. 72, No. 4, P. 532–549 (In Russ.).
- Sergeev G. B. [Size effects in nanochemistry]. Rossiyskiy khimicheskiy zhurnal. 2002, Vol. XLVI, No. 5, P. 22–29 (In Russ.).
- Glezer A. M. [Amorphous and nanocrystalline structures: similarities, differences, mutual transi-tions]. Rossiyskiy khimicheskiy zhurnal. 2002, Vol. XLVI, No. 5, P. 57–63 (In Russ.).
- Melikhov I. V. [Patterns of crystallization with the formation of nano-dispersed solid phases]. Neorganicheskiye materialy. 2000, Vol. 36, No. 3, P. 350–359 (In Russ.).
- Panova T. I., Glushkova V. B., Glushkova V. B. [Kinetics of grain growth in ceramics based on ZrO2 densified with the use of explosion energy] Neorganicheskiye materialy. 1999, Vol. 35, No. 2, P. 233–236 (In Russ.).
- Pozhidaeva O. V., Korytkova E. N., Romanov D. P., Gusarov V. V. [Formation of zirconium di-oxide nanocrystals in hydrothermal media of different chemical composition]. Journal of General Chemistry. 2002, Vol. 72, Iss. 6, P. 910–914 (In Russ.).
- Podbolotov K. B., Nort A., Isobello A. Yu. [Phase composition and structure of ceramic samples based on zirconium dioxide obtained by exothermic synthesis]. Neorganicheskiye materialy. 2021, Vol. 57, No. 10, P. 1128–1137 (In Russ.).
- Krivtsov I. V., Ustimenko A. V., Ilkaeva M. V., Avdin V. V. [Synthesis of zirconium dioxide nanoparticles by thermal decomposition of zirconium complex with citric acid]. Vestn. YUUrGU. Ser. Khimiya. 2013, Vol. 5, No. 4, P. 38–41 (In Russ.).
- Bosak N. A., Chumakov A. N., Shevchenok A. A. et al. [Structure and properties of zirconium oxide films doped with yttrium oxide obtained by laser deposition in vacuum]. Journal of the Belarus-ian State University. Physics. 2020, No. 2, P. 10 –18.
- Petrunin V. F., Popov V. V., Hongzhi Z., Timofeev A. A. [Synthesis of nanocrystalline high-temperature phases of zirconium dioxide]. Inorganic Materials. 2004, Vol. 40(3), P. 303–311 (In Russ.).
- Shabanov V. F., Zyryanov V. Ya. Fotonnyye kristally i nanokompozity: strukturoobrazovaniye, opticheskiye i dielektricheskiye svoystva [Photonic crystals and nanocomposites: structure formation, optical and dielectric properties]. Moscow, Izdatel'stvo SO RAN Publ., 2009, 257 p.
- Shabanov V. F., Zyryanov V. Ya. Metamaterialy i strukturno organizovannyye sredy dlya op-toelektroniki, SVCH-tekhniki i nanofotoniki [Metamaterials and structurally organized media for opto- electronics, microwave technology and nanophotonics]. Moscow, Izdatel'stvo SO RAN Publ., 2013, 369 p.
- Nemtsev I. V. et al. [Photonic crystal structures based on submicron particles of polymethyl methacrylate]. Proceedings based on the materials of the VI International Conference and Youth School (ITNT-2020). In 4 volumes. Ed. S. V. Karpeeva. 2020, P. 608–614.
- International Diffraction Database: The International Centre for Diffraction Data (ICDD – JCPDS) PDF2.
- Sychev V. V., Zaitseva Yu. N., Eremina A. O. et al. [Levulinic acid conversion to γ-valerolactone via transfer hydrogenation over Zr-containing catalysts in isopropanol]. J. Sib. Fed. Univ. Chem. 2022, Vol. 15(1), P. 137–155.


