Synthesis of amides and salts of oxalic acid and their use as plant growth regulators
Автор: Sultanova Ja.
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Химические науки
Статья в выпуске: 3 т.10, 2024 года.
Бесплатный доступ
Oxalic acid plays a very important role in the life of plant organisms. In addition, its salts and amide derivatives also have growth-stimulating properties. In this work, we show the results of research in the field of synthesis of amide derivatives of oxalic acid, and also investigated their growth properties using the example of some agricultural crops. Have found that the mono amide of oxalic acid has a stronger effect on the growth of pea seeds compared to the disubstituted amide of this acid. Thus, we can conclude that salts and amide derivatives of oxalic acid have a pronounced regulatory property during the ripening of pea and corn seeds. In this regard, they have been recommended as growth stimulants for these plants in agricultural practice.
Oxalic acid, oxalates, acid amides, growth substances, growth stimulants, phytohormones, plant growth regulators
Короткий адрес: https://sciup.org/14129891
IDR: 14129891 | УДК: 547.541.2 | DOI: 10.33619/2414-2948/100/05
Текст научной статьи Synthesis of amides and salts of oxalic acid and their use as plant growth regulators
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 547.541.2.
Oxalic acid is the simplest representative of dibasic aliphatic acids and has all the properties of the latter.
но
он
oxalic acid
This acid easily forms amides and salts, both partial and complete salts and amides:
COOH-COOH + 2 Na → NaOOC-COONa + H 2
Oxalic acid is capable of reacting with ammonia when heated in an acidic environment to form oxamide and water:
COOH-COOH + 2 NH 3 → NH 2 – CO – CO –NH 2 + 2H 2 O.
At insufficient temperature, the reaction may proceed differently and leads to the formation of ammonium oxalate:
COOH-COOH + 2 NH 3 → NH 4 – CO – CO – NH 4
In this work, we review the results of research in the field of synthesis of amide derivatives of oxalic acid, and also show the main areas of application of these compounds. Thus, in works [1, 2] a new method for the preparation of oxalic acid esters and amides with a substituent of various chemical natures is described and it is shown that these compounds have many industrial applications, mainly as intermediates and stabilizers in the field of polymers.
Another patent [3] proposes a method for producing oxalic acid amide esters and their salts, which includes the following stages: introducing a certain amount of ammonia gas into low-temperature anhydrous ethanol to obtain an ethanol solution of anhydrous ammonia; and reacting an ethanol solution of ammonia used as an aminolysis agent with diethyl oxalate (or dimethyl oxalate) to produce oxalic acid amide ethyl ester (or oxalic acid amide methyl ester). This method has great economic benefits, can greatly improve product quality, and meet stringent product quality requirements in fields such as biochemical reagents and the like.
Patent [4] describes a method for producing amidoesters of oxalic acid, and patents [5, 6] propose a composition suitable for use as a friction modifier for an automatic transmission, containing a bis-amide or amide ester of oxalic acid containing at least two hydrocarbyl groups with a number of carbon atoms from 12 to 22.
Oxalic acid monoamides are of interest as bioisosteric replacements of phosphate groups in the creation of new enzyme inhibitors [7]. In this work, the authors demonstrated the use of oxalic acid as a Wang resin linker for the synthesis of single or series of phosphate biosters. The highly reactive acid chloride bound to the resin reacts with arylamines to form resin-bound N-aryloxamic acids (oxanilic acids). This methodology is particularly useful for the rapid synthesis of 2-(oxalilamino)benzoic acids (OBAs) as it can be used to synthesize libraries and eliminates the intermediate purification step required in solution-phase reactions. The products are cleaved from the resin with trifluoroacetic acid in dichloromethane in good yields.
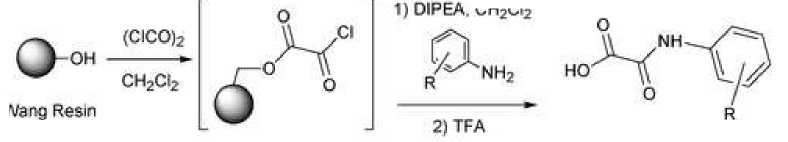
Wang Resin
CH2CI2
(CICO)2
1) DIPEA. CH2Ch
The synthesis and study of the areas of application of oxalic acid amides were also considered in [8, 9].
In [10] presents a stable new synthesis of oxalamides by the acceptorless dehydrogenation of ethylene glycol with amines to form H2, homogeneously catalyzed by a ruthenium chelate complex. The reverse hydrogenation reaction is also carried out using the same catalyst. A probable reaction mechanism is proposed based on stoichiometric reactions, NMR studies, X-ray crystallography, and the observation of probable intermediates.
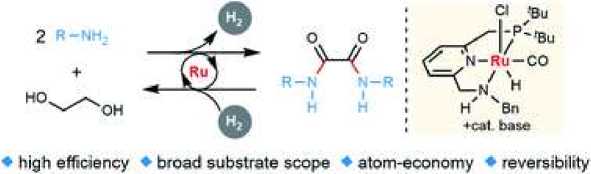
Research has also been devoted to the synthesis of oxalic acid amides [11, 12].
The patent [13] proposes a method for protecting organic materials that can be damaged by ultraviolet light from exposure to ultraviolet rays, characterized in that an oxalic acid amide ester is included in or applied to the surface of the protected materials. or a filter layer containing oxalic acid amide esters is placed in front of these materials.
Crystalline salts of oxalic acid stearamide, oleamide and elaidamide, consisting of 2 mol amide per 1 mol oxalic acid, were prepared and characterized using melting points, as well as IR and X-ray diffraction measurements [14]. Their IR spectra are compared with the spectra of amides. Long distances are reported for crystalline salts, as well as long and short distances for these amides and linoleamide.
It has been reported [15] that α-hydroxyamides are an important class of compounds found in natural products and bioactive molecules of drug candidates. In this work, the authors report a simple and direct approach to these compounds through the decarbonylation/decarboxylation of oxalic acid during a three-component Passerini reaction under solvent-free conditions under microwave heating. This very convenient procedure provides the title compounds via a possible concerted intramolecular decarbonylation/decarboxylation from an α-acyloxyamide intermediate.
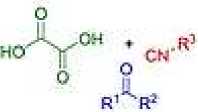
P-3CR sqlvnnlJroo
MW, JO =C, 5 mn
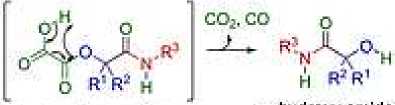
О mild roadie*! conditions
О sheet reaction Unes
Q solvent* nnt arfdilnui-trcn
О broad scope a^cylaxy amides и-liydroxy amide'S
63 examples
In [16], amides of succinic and oxalic acids were synthesized based on their reaction with monoethanolamine in molar ratios of 1:1 and 1:2. IR spectra of the resulting substances were obtained, their structure was determined and confirmed, and physicochemical properties were studied. 10% aqueous solutions of these substances were prepared, their physicochemical properties were determined, and their bactericidal properties were studied. The bactericidal effectiveness of succinic acid N-monoethanolamide was 82.9% at a concentration of 25 mg/l, 88% at a concentration of 50 mg/l and 95% at a concentration of 100 mg/l. The bactericidal effectiveness of N1,N2-succinic acid bis-monoethanolamide was 69% at a concentration of 25 mg/L, 83.2% at a concentration of 50 mg/L and 90% at a concentration of 100 mg/L. The bactericidal effect of oxalic acid N-monoethanolamide was also studied: 82% at a concentration of 25 mg/l, 91% at a concentration of 50 mg/l and 97% at a concentration of 100 mg/l.
Thus, from the above analysis of research results, we can conclude that amide derivatives of oxalic acid have a wide range of applications.
Among these areas, special mention should be made of the use of oxalic acid and its derivatives as plant growth regulators. In this direction, results were previously shown that described the stimulating properties of this acid and its functionally substituted derivatives. Thus, in works [17,18] it is noted that oxalic acid and its derivatives play an important role in the regulation of plant growth and development and participate in reactions that affect both biotic and abiotic stresses.
-
[19 ] collected and developed data indicating that calcium oxalate plays a significant and previously unanticipated role in the biochemistry of the extracellular matrix (ECM) of higher plants. Germine, long known as a protein marker of early growth in germinating wheat and later known as ECM protein, has been shown to be an oxalate oxidase. Dissolution of calcium oxalate and germin-induced degradation of the resulting soluble oxalate can lead to the release of Ca2+ and H 2 O 2 , which are known to play a central role in ECM biochemistry in higher plants. The discoveries about oxalate as a source of H 2 O 2 complement Warner's current understanding of the central role of H 2 O 2 in the development, differentiation, vascularization and signaling processes of higher plants.
I/ ин о
germin
It has been shown [20] that oxalic acid is widely distributed in plants and performs various physiological functions. It has been suggested that oxalic acid is biosynthesized in plants through three pathways, namely the glyoxylate-glycolate, ascorbate and oxaloacetate pathways. Oxalate decomposition occurs through oxidation, decarboxylation and acetylation. In addition, plant varieties and agronomic practices influence the accumulation of oxalic acid.
The secretion of oxalates by fungi provides many benefits for their growth and colonization of substrates [21]. The role of oxalic acid in pathogenesis is to acidify host tissues and sequester calcium from host cell walls. The formation of calcium oxalate crystals weakens cell walls, thereby allowing polygalacturonase to degrade more quickly in a synergistic response. There is a good correlation between pathogenesis, virulence and oxalic acid secretion. Solubility of soil nutrients is achieved by soil species when cations liberated by oxalate diffuse into clay layers and increase the effective solubility of Al and Fe. Oxalate stored in the hyphal mats of mycorrhizal species increases the availability of phosphates and sulfates. The formation of calcium oxalate crystals provides a reservoir of calcium in the ecosystem. The ability of oxalate to bind divalent cations promotes the detoxification of copper, which is especially noticeable in wood preserved with copper salts. Oxalate plays a unique role in the degradation of lignocellulose by wood-decaying basidiomycetes, acting as a low-molecular-weight decay-initiating agent. In addition, in white rot fungi, oxalate acts as a potential electron donor for lignin peroxidase-catalyzed reduction and chelates manganese, allowing Mn 3+ to dissolve from the manganese-enzyme complex and thus stimulating extracellular manganese peroxidase activity. Biosynthesis and degradation of oxalates are discussed.
The patent [22] describes a method of controlling pathogens using an oxalate-producing enzyme, alone or in combination with a toxic protein, which can be applied directly to the plant or produced by microorganisms on it, or by genetically modifying the plant to produce the enzyme.
Research has also been devoted to studying the role of oxalic acid and its derivatives in plant organisms [23-29].
Considering the above, it was of interest to conduct research in the field of studying the regulatory properties of salts and amide derivatives of oxalic acid in relation to the seeds of some agricultural crops [30–32].
For this purpose, we synthesized mono- and disubstituted salts, as well as ethanolamine complexes of oxalic acid and prepared their solutions of various concentrations: 0.05, 0.01, 0.005, 0.001 and 0.0001% and tested them as growth regulators for corn and peas. For comparison, solutions of indolylacetic acid, which is often used as a plant growth regulator, were prepared in the same concentrations. The calculated amount of seeds of the indicated plants was placed in two Petri dishes, and distilled water was added to one of the dishes, and prepared solutions of the analyzed substances were added to the other. After several days of observations, it was noticed that 0.0010.0001% solutions of oxalic acid derivatives promote intensive growth of both types of seeds and growth of both the root and above-ground parts of plants is observed. It has been established that in the case of salts, complete salts of oxalic acid have a more pronounced growth property compared to acidic salts, but in both cases an increase in seed growth is observed.
In addition, we have found that the mono amide of oxalic acid has a stronger effect on the growth of pea seeds compared to the disubstituted amide of this acid.
Thus, we can conclude that salts and amide derivatives of oxalic acid have a pronounced regulatory property during the ripening of pea and corn seeds. In this regard, they have been recommended as growth stimulants for these plants in agricultural practice.
Список литературы Synthesis of amides and salts of oxalic acid and their use as plant growth regulators
- Messina G., Sechi G., Lorenzoni L., Chessa G. Pat. 4981963. US. 1988. Method of preparation of oxalic acid esters and amides.
- Messina G., Sechi G., Lorenzoni L., Chessa G. Pat. 2052800Т3. ES. 1989. Method of preparation of oxalic acid esters and amides.
- Pat. 102442925A. CN. 2010. Preparation method of oxalic acid amide esters and salts thereof.
- Goldstein H., Clary S. Pat. 2609380A. US. 1949. Amido-amide derivatives of oxalic acid and processes of preparing the same.
- Vickerman R., Sacsomando D. Pat. 8691740B2. US. 2010. Oxalic acid bis-amides or amide-ester as friction modifiers in lubricants.
- Vickerman R., Sacsomando D. Pat. 2010096321A1. WO. Oxalic acid bis-amides or amideester as friction modifiers in lubricants.
- Georgiadis T. M., Baindur N., Player M. R. Solid-phase synthesis of an oxalic acid amide library // Journal of combinatorial chemistry. 2004. V. 6. №2. P. 224-229. https://doi.org/10.1021/cc030012r
- Petiunin G. P., Bulgakov V. A. Amides and hydrazides of oxalic acid. XXVI. The synthesis and properties of NR-oxamoylanthranilic acids // Farmatsevtychnyi Zhurnal. 1973. V. 28. №6. P. 21-24.
- Chernykh V. P., Valyashko N. N., Dzhan-Temirova T. S., Petyunin P. A. Amides and hydrazides of oxalic acid: XX. Substituted amides and heterylidene hydrazides of 4-N-acyl (heteryl)-sulfamoyloxanilic acids // Pharmaceutical Chemistry Journal. 1972. V. 6. №7. P. 426-428. https://doi.org/10.1007/BF00771576
- Zou Y. Q., Zhou Q. Q., Diskin-Posner Y., Ben-David Y., Milstein, D. Synthesis of oxalamides by acceptorless dehydrogenative coupling of ethylene glycol and amines and the reverse hydrogenation catalyzed by ruthenium // Chemical Science. 2020. V. 11. №27. P. 7188-7193. https://doi.org/10.1039/d0sc02194f
- Petiunin P. A., Chernykh V. P., Banny I. P. Amides and hydrazides of oxalic acid. XXIX. Synthesis and properties of NR substituted amides of benzylsulfonyloxaminic acid (Ukrainian) // Pharmaceutical journal. 1975. V. 30. №2. P. 26-29.
- Razuvaeva V. P., Pastukhova T. P., Petiunin G. P. Amides and hydrazides of oxalic acid. XXV. Synthesis and analgesic activity of esters of N-(4-antipyril)-oxaminic acid // Farmatsevtychnyi Zhurnal. 1977. V. 32. №1. P. 49-51. EDN: XLXFLE
- Luethi Ch., Biland H.R., Duennenberger M. Pat. 3639249A. US. 1967. Bis-oxalic acid ester amides for use as ultraviolet stabilizers.
- Mod R. R., Magne F. C., Skau E. L. Preparation and properties of oxalic acid salts of C18 saturated and unsaturated fatty amides // Journal of the American Oil Chemists' Society. 1973. V. 50. №4. P. 126-127. https://doi.org/10.1007/BF02633565
- Martinho L. A., Rosalba T. P. F., Andrade C. K. Z. Passerini Reaction to Access α‐ Hydroxy Amides by Facile Decarbonylation/Decarboxylation of Oxalic Acid // European Journal of Organic Chemistry. 2022. V. 2022. №48. P. e202201199. https://doi.org/10.1002/ejoc.202201199
- Ismayilov T. A., Suleymanova S. S., Asadova S. B. Synthesis of amides with monoethanolamine of amber and oxalic acid and research of their bactericide properties // Nature and Science. 2021. V. 3. №8. P. 40-47. http://www.doi.org/10.36719/2707-1146/13/40-47
- Li P., Liu C., Luo Y., Shi H., Li Q., PinChu C., Fan W. Oxalate in Plants: Metabolism, Function, Regulation, and Application // Journal of Agricultural and Food Chemistry. 2022. V. 70. №51. P. 16037-16049. https://doi.org/10.1021/acs.jafc.2c04787
- Libert B., Franceschi V. R. Oxalate in crop plants // Journal of Agricultural and Food Chemistry. 1987. V. 35. №6. P. 926-938. https://doi.org/10.1021/jf00078a019
- Lane B. G. Oxalate, germin, and the extracellular matrix of higher plants // The FASEB journal. 1994. V. 8. №3. P. 294-301. https://doi.org/10.1096/fasebj.8.3.8143935
- Cai X. F., Xu C. X., Wang X. L., Ge C. H., Wang Q. H. The oxalic acid in plants: Biosynthesis, degradation and its accumulation regulation // Zhiwu Shengli Xuebao/Plant Physiology Journal. 2015. V. 51. №3. P. 267-72.
- Dutton M. V., Evans C. S. Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment // Canadian journal of microbiology. 1996. V. 42. №9. P. 881-895. https://doi.org/10.1139/m96-114
- Zaghmout Q. Pat. 20060014641A1. US. 2006. Pathogen control with an oxalate (oxalic acid).
- Kang K., Zhang M., Yue L., Chen W., Dai Y., Lin K., Zhang W. Oxalic Acid Inhibits Feeding Behavior of the Brown Planthopper via Binding to Gustatory Receptor Gr23a // Cells. 2023. V. 12. №5. P. 771. https://doi.org/10.3390/cells12050771
- Tran D., Kadono T., Molas M. L., Errakhi R., Briand J., Biligui B., Bouteau F. A role for oxalic acid generation in ozone‐induced signallization in Arabidopis cells // Plant, Cell & Environment. 2013. V. 36. №3. P. 569-578. https://doi.org/10.1111/j.1365-3040.2012.02596.x
- El-Shabrawi H. M., Bakry B. A., Ahmed M. A., Abou-El-Lail M. Humic and oxalic acid stimulates grain yield and induces accumulation of plastidial carbohydrate metabolism enzymes in wheat grown under sandy soil conditions // Agricultural Sciences. 2015. V. 6. №1. P. 175. http://dx.doi.org/10.4236/as.2015.61016
- Ma Y., Wang X. P., Zhang S. F., Shi D. C., Sheng L. X. Effects of salt and alkali stress on growth, accumulation of oxalic acid, and activity of oxalic acid-metabolizing enzymes in Kochia sieversiana // Biologia plantarum. 2016. V. 60. P. 774-782. https://doi.org/10.1007/s10535-016-0650-2
- Soltys A., Studnicki M., Zawadzki G., Aleksandrowicz-Trzcinska M. The effects of salicylic acid, oxalic acid and chitosan on damping-off control and growth in Scots pine in a forest nursery // iForest-Biogeosciences and Forestry. 2020. V. 13. №5. P. 441. https://doi.org/10.3832/ifor3244-013
- Xiaodong X., Brinker R., Reynolds T., Abraham W., Graham J. Pat 6992045B2. US. 2001. Pesticide compositions containing oxalic acid.
- Gouveia C. S., Ganança J. F., Lebot V., de Carvalho M. Â. P. Quantitation of oxalates in corms and shoots of Colocasia esculenta (L.) Schott under drought conditions // Acta physiologiae plantarum. 2018. V. 40. P. 1-11. https://doi.org/10.1007/s11738-018-2784-7
- Sultanova J. F., Aliyeva L. I., Mammadov D. Sh., Piraliyev A. G., Nabiyev F. A. Etanolaminlərin qeyri-üzvi komplekslərinin bitkilərin inkişafına stimullaşdırıcı rolunun tədqiqi // Actual Problems of Modern Chemistry. 2019. P. 299.
- Sultanova J. F., Nabiyev F. A., Aliyeva G. A. Plant nutrients as an important environmentally friendly tool in the development of agriculture // Actual problems of modern natural and economic sciences: International scientific conference. Ganja, 2023. P. 19-21.
- Mammadov J. Sh., Nabiyev F. A., Gambarova F. D., Aliyeva G. A., Sultanova J. F., Huseynova I. E., Shakhtakhtinskaya Z. I. Research of stimulatory action of some derivatives of malonic acid on the development of plants // Proceedings. Natural and technical sciences series. 2023. №1. P. 35-42.


