Synthesis of carbon nanoparticles and study of bioaccumulation in seeds and its application in plant fortification
Автор: Challaraj Emmanuel E. S., Sabu Judith Ann, Baby Thekkinieth Ninsa, Feby George
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.19, 2023 года.
Бесплатный доступ
The aim of the study is to carry out green synthesis of carbon nanoparticles and to study its applications in plant fortification and to analyze the bioaccumulation of minerals in the seeds. The synthesis of carbon nanoparticles was by pyrolysis of coconut milk. The average particle size and surface morphology was determined by scanning electron microscope, UV spectroscopy was used to find the optical spectra, Raman spectroscopy was conducted for comparing Raman shift and the crystallite size was calculated by (XRD) analysis. The soil used for cultivation was also analyzed before and after addition of carbon nanoparticles and the changes in pH and amount of minerals and organic matter was studied and showed improved soil characteristics after addition of carbon nanoparticles in a 1:10 ratio. The growth of a monocot and dicot plant species were analyzed as control and test for up to 22 days to study the effect of carbon nanoparticles on physical and chemical parameters of the plant. The results did show a significant rate of increase in root and shoot length of the test plants. Chemical parameters such as concentration of IAA, amount of protein and estimation of chlorophyll was determined by Salkowski test, Lowry’s test and Chlorophyll estimation test respectively which also showed positive results to the addition of carbon nanoparticles. There was also a significant increase in magnesium content in the seeds which proved mineral bioaccumulation in the soil as a result of carbon nanoparticle addition.
Carbon nanoparticles, bioaccumulation, plant fortification
Короткий адрес: https://sciup.org/143180107
IDR: 143180107
Текст научной статьи Synthesis of carbon nanoparticles and study of bioaccumulation in seeds and its application in plant fortification
Nanotechnology has opened a wide spectrum of opportunity with multiple applications in the field of agriculture to increase crop yield and also play vital role in the growth and fortification of plants. Various types of nanoparticles play vital roles in the growth and fortification of plants. The most accepted nanostructures range from 100nm or less (Duhan et al., 2017).
Carbon is one of the most abundant organic compounds available in nature and is considered due to its high applications and ease of utilization and exhibit least amount of toxicity when compared to other metal oxide nanoparticles. Carbon nanoparticles are nanosized carbon elements created through various methods including carbonization, heating, activation, and grinding. Nanoparticles less than 100 nm in size are of great use. The pure carbon particles have a molar mass of 12.01 g/mol and an incredibly high melting and boiling point: both above 3500 ° C.
Among many synthesis methods present for nanoparticle production, carbon is easily available in many organic sources, being an organic compound. In the discussed work the carbon nanoparticles are obtained from pyrolysis of coconut milk, which is obtained in the form of black residue. The black residue considered as waste material is reused to produce carbon nanoparticles and introduced into agriculture to enhance seed germination and faster plant development by adding CNTs to any bio-fertilizer based substrate and takes advantage of porous membrane of plant and hence gets absorbed into the plants. Carbon nanoparticles with porous surface has a large surface area and provides the nanoparticles to binding, chemical reactions, or absorption functions.
A study suggested on a one-step thermal process with coconut shell using temperature variation above 400°C and obtains carbon chars (Mi et al., 2012). One of the most abundant source of carbon is coconut milk, which is usually used to obtain coconut oil, which is obtained from thermal pyrolysis (Roshni and Ottoor, 2015). When different metal oxide nanoparticles like CuO, TiO2, ZnO, CuZnFe2O4, Fe3O4, Fe2O3 and carbon nanoparticles were compared for their toxicity it was found that carbon-based particles exhibited least amount of toxicity. Carbon based nanoparticles showed lowest cytotoxic effects and caused DNA damage in lowest rate (Karlsson et al., 2008).
Additions of nanoparticles have proved to improve germination and growth in plants to increase their application in the field of agriculture (Karami et al., 2019) . The nutrient demand can also be fulfilled using nanotechnology and hence boost the farmers to adopt newer methods to sustainable agriculture and hence increase the quantity and quality of products in a faster rate and reach the growing demands. Due to high commercial value, CNTs are developed according to the requirement. Methods like arc discharge, laser ablation, plasma torch chemical water deposition, hydrothermal carbonization and pyrolysis are some of the techniques that is used to develop the nano-sized with required parameters like the diameter, number of layers and chirality. The use of most of the above mentioned methods utilizes large amount of temperature and catalyst and purification procedures to obtain the particles and so considering these factors thermal pyrolysis can be considered as a one-step approach to develop CNTs as its inexpensive and less time consuming method (Hakim et al., 2018). In order to exclude the toxicity issues associated with use of nanoparticles, green synthesized particles are used, which also reduces pollution (Elemike et al., 2019).
As per a review work, they have observed the increased growth in onion root and stem when give one-day treatment hydroponically (Mukherjee et al., 2016). The CNTs were seen adsorbed to root surface (Wild and Jones 2009). Nano-fertilizers are more advantageous to the conventional fertilizers because they can triple the efficiency of the nutrients, reduce the requirement of chemical fertilizers, make the crops drought and disease resistant and are less hazardous to the environment. They can easily get absorbed by plants due to their high surface area to volume ratio (Li et al., 2016). Several scientists have identified in their trials with different crops that certain concentrations of rare earth elements have a positive effect on the uptake of nutrients by roots and their movement to leaves; however, higher concentrations inhibit the uptake of plant nutrients, the growth of roots and above ground parts and may cause damage to leaves (Guo et al., 1988 and Diatloff et al., 1996).
The excellent capacity of high resolution inductively coupled plasma mass spectroscopy for measurement of the rare earth element is demonstrated by investigating different materials such as geological matrices (sediments, soils), plant tissues and marine animal tissues (Liang et al., 2005). Magnesium is least taken into interest when compared to other minerals, but many vital functioning of plant is aided by Mg which includes photo phosphorylation, photosynthetic carbon dioxide (CO2) fixation, etc. Also the activity of enzyme RuBP has a major role played by magnesium helping in photosynthesis (Cakmak and Yazici, 2010) .
MATERIALS AND METHODS
Coconut milk , seeds of maize and peas as dicot and monocot samples; Soil samples;
Reagents used: FeCl3, per chloric acid, ethyl alcohol, Na 2 CO 3 , NaOH, CuSO 4 , KNaC4H4O6*4H2O, FC reagent, working standards (BSA), MgCO3 and acetone.
Synthesis of Nano particles
A simple and one step procedure was used for the synthesis of CNPs from coconut milk by thermal pyrolysis at a temperature of 120–150°C for 2–5 min without using any carbonizing or passivating agent (Roshni and Ottoor, 2015). Coconut milk was obtained from freshly grated coconut and was used in its purest form. The method followed was of thermal pyrolysis of milk at 110-120 X for about 15-20 minutes and upon heating, a black residue was obtained which was then separated by filtration and then heated in hot air oven at 100° C for 3 hours. The residue was then powdered into fine particles and used for further experimentation.
Plant fortification
The plants were sowed for studying the effects of nanoparticles on soil as well as plants. A control set was planted with dicot seed as peas and monocot seeds as maize. The control set of soil was deprived of nanomaterial. The control was divided into three sets to obtain the growth parameters of the plants to compare. The test soil was prepared with nanomaterial in a ratio of 1:10 of nanomaterial and soil respectively. And as in control sets the seeds were also sown in test soil in the sets of three to carry out the studies (Li et al., 2016).
Physical parameters
The seed were examined on a daily basis and the first set of physical parameters was obtained on the 10th day of planting and then later 2 more sets were collected at a difference of 6 days. The parameters like root length and shoot length were obtained and an average were taken to study the comparison.
Chemical parameters
Estimation of IAA
To determine the amounts of Indole Acetic Acid (IAA) produced by the seeds, a colorimetric technique was performed with Van Urk Salkowski reagent using the Salkowski’s method (Ehmann, 1977). The seeds were sowed (with and without CNPs) and grown at 28°C for 4 days. The seeds were then collected, crushed and then was centrifuged after incubation. Supernatant was reserved and 1ml was mixed with 2ml of Salkowski’s reagent (2% 0.5 FeCl 3 in 35% HClO 4 solution) and kept in the dark. The optical density (OD) was recorded at 530 nm after 30 minutes and 120 minutes.
Estimation of protein
Total protein content in the leaves were analyzed by Lowry’s method. Phenols react with phosphomolybdic acid in Folin-Ciocalteau (FC) reagent in alkaline medium to produce blue coloured complex quantified at 650nm. 0.5gm of leaves were homogenized in 10X volume of 80% ethanol, and centrifuged at 10000 rpm for 20 minutes. The residue was re-extracted with 80% ethanol, the supernatant was evaporated to dryness and dissolved in distilled water with known volume (0.5 ml) of FC reagent. After 3 minutes 2ml of 20% sodium carbonate was added and the mixture was placed in a boiling water bath for 1 minute. The amount of phenol in the extract was then quantified using a spectrophotometer at 650nm. A standard catechol solution corresponding to 2-10g concentration was added to FC reagent and sodium carbonate. A standard curve was constructed using an electronic calculator on the linear regression mode using which, the concentration of phenol in samples were read. The values were expressed as milligrams of phenol per gram of leaf (Malik and Singh, 1980).
Chlorophyll estimation
Chlorophyll was extracted with 80% acetone and the absorption at 663nm and 645nm were read in spectrophotometer. Using the absorption co-efficient the amount of chlorophyll was calculated. One gram of leaf was extracted with 20 ml of 80% acetone and centrifuged at 5000 rpm for 5 minutes. The supernatant was transferred to a fresh flask until the residue was colourless. The supernatant was made up to 100 ml with 80% acetone. The absorbance of the solution was read at 645nm and 663nm against 80% acetone blank and the amount of chlorophyll was estimated (Witham et al., 1971).
Soil analysis
The soil samples were collected from agricultural (field side). The pH of the soil was measured by using the pH meter with a range of 0-14 pH (Ananthalakshmi and Sathyaprabha, 2016). The sample for analysis was done by collecting the soil samples and was set as control, and test was prepared using a ratio 1:10 of nanomaterial and soil respectively. Soil was analyzed for various parameters for quantitative analysis using agricoop 2011, IS 3025,IS 6092 (Part 6) 1985, Black Wet Combustion method by BCX Bio-organics.
Fractionation and accumulation of Minerals in seeds
The samples were also subjected to bioaccumulation study for analysing the heavy metal accumulated in the seed, that can have greater effect on the plant growth. All samples were analysed in triplicates by ICP-OES Perkin-Elmer; model Optima™ 2000 DV, using winLab32 software for the analysis. The analytical measurements were made with a simultaneous Perkin– Elmer ICP OES, model optima 2000DV, winLab32™, version 7.0 software. ICP multi element standard solution (10 to 50 mg/) containing nine elements such as (Hg, Zn, P, Fe, Cr, Mg, Cu, Na and K) were used in the preparation of calibration solutions.
Four duplicate fresh plants were harvested, cleaned, and washed with deionized water three times. The whole plant was separated into root, stem, and leaf in the pot experiment and into root, stem, leaf, flower, and grain in the plot experiment. These sub plant samples were dried in a microwave oven and ground in a 1-mm sieve. Each 0.5g plant or sub plant sample was treated with 8cm3 of a mixed oxidizing solution (15 mol L-1 HNO3 and 9 mol L-1 H2O2, v/v) and digested for 30 min at 2600 kPa (80 psi) in a MDS-2000 microwave oven. The sample was diluted to a final volume of 25ml with de-ionized water before analysis. Concentrations of REs in each samples were determined by a Plasma spectrometer operated at a sampling rate of 1.0 mL min-1 with a measuring time of 40 sec. The standard solution of mixed REs was prepared by diluting the stock solutions in two steps with 1% concentrated HNO3. REE concentrations in the samples were determined by ICP-OES (Optima 5300 DV). The presence of REEs was measured in diverse parts of wheat and corn plants (leaf, root and shoot). Fractionation of plants was done and acid digestion was carried out. Accumulation in plant samples was analyzed using ICP-OES (Optima 5300 DV ICP- OES).
Characterization of nanoparticles
SEM micrographs of carbon particles at 25,000 to 200,000 times magnification were performed. The elemental analysis of prepared spherical nanoparticles was performed using the energy dispersive X-ray (EDX) spectroscopy arranged on the SEM. SEM-EDS was performed to obtain the physical structures of the CNPs (Shooto and Dikio, 2011). The operating voltage for SEM-EDS was 5.00KV. The standards for EDS were KCl, MgCO 3 , SiO 2 . XRD was analyzed using Rigaku, smartlab x-ray defractometer.
UV-visible spectra were recorded in the range between 200 and 800nm using a UV-Vis spectrophotometer. Optical spectra where obtained immediately after the synthesis and after 6-7 days by measuring the absorption of the prepared as in a quartz cuvette with a 1cm optical path (Li et al., 2008). The samples were then subjected to UV analysis to obtain the absorbance of the CNPs by using Labman –UV1200 series, and where subjected to absorbance range from 200nm to 450nm.
Along with the above studies the electron microscopy study was also done by Raman spectroscopy. Raman measurements were performed with a Renishaw InVia spectrometer using the 532nm wavelength of an Ar+ laser. The instrument was calibrated using an internal silicon standard (521 cm–1). The spectrum was obtained using a dynamic scan for 5 accumulations at 1s exposure time each at 10% laser intensity (23mW). The Raman spectrum of pure Si was subtracted from the spectrum of collected carbon nanoparticles (Wu et al., 2013).
RESULTS AND DISCUSSION
The physical parameters were observed as shoot and the root length of the plants of control and the test samples were compared. Shoot and root lengths were measured on different days as 10th day,16th day and day 22nd day. A set of 3 values was obtained and average was calculated. As per results obtained, the test plants for maize as well peas the test set showed a faster growth and had an increased root and shoot and root length. The plants were observed to have healthy shoot and have root length (Table 1 and 2).
The soil used for the experiment was characterized by BCX Bio Organics and parameters such as pH, CEC, available amount of nitrogen, phosphorus, potassium, magnesium and calcium (in ppm) and the percentage of organic matter and carbon present in the soil before and after addition of carbon nanoparticles were compared. The table below shows that the soil characteristics have been improved by the addition of carbon nanoparticles and hence in turn promote the growth of plants at a faster rate (Table-3 and 4). In the same way, for peas, the concentration of IAA in the test was calculated to be 90.3μg/ml which is much higher than the concentration of IAA in the control, which was 44.42μg/ml. These results show that the addition of carbon nanoparticles to soil has increased the concentration of IAA, which is an essential growth factor in plants.
Lowry’s method was used to compare the protein content present in the plants (both maize and peas) before and after the addition of carbon nanoparticles. In both the plants the amount of protein present was found to be more in the test samples (containing carbon nanoparticles) than the control samples (without carbon nanoparticles). The concentration of protein for test sample in maize was calculated to be 188μg/ml which was higher than for the control which was 140.38μg/ml. In the same way for peas, the concentration of protein in test was found to be 183.88μg/ml, higher than control, which was 130.03μg/ml. The addition of carbon nanoparticles has proved to increase protein accumulation in plants and hence improve their growth larger leaf size than the control set of plants.
An estimation of chlorophyll was performed to identify the rate of increase in chlorophyll in the leaves. Plants were tested for chlorophyll in control as well as test. According to the optical density from standard graph, the chlorophyll content in the peas were estimated as 0.136 at 645nm for control and 0.157 for the test and for maize the Optical density was obtained as 0.062 in control and 0.07 in test. And the chlorophyll estimates at 663nm the optical density obtained was 0.297 for control peas and 0.43 for the test. Similarly, for maize the at 663nm the optical density was 0.109 for control and 0.122 for test.
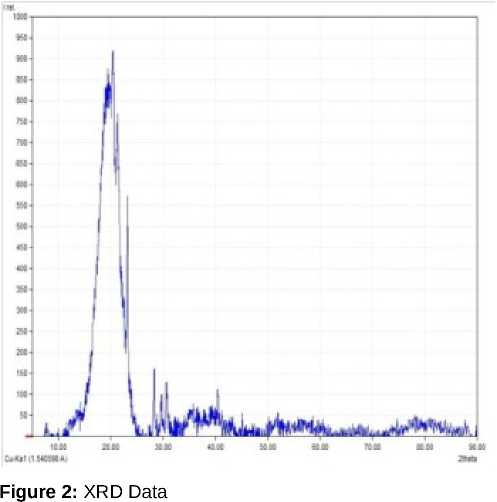
As per the obtained results the chlorophyll estimates in the leaves showed increased content for those in test samples that is with nanoparticles. The scanning electron microscopy for the particles was performed that was used to obtain the surface images of the particles. A variety of images can be produced under different magnifications (Figure 1). Through the use of an energy-dispersive x-ray spectroscopy (EDS) as well, the elements predominant in the sample can be identified. In learning the elemental and microstructural composition of the sample, one can compare the properties with those of known specimens. The atomic and weight percentage of minerals were obtained, which showed higher weight and atomic percentage for carbon. The particles were irregularly shaped and had aggregate structure. The particles size of carbon nanoparticles was obtained as 12.30nm form Debyer Scherrer formula D= 0.9λ/βcosӨ. From the XRD data, we can calculate the 3dimensional order of powdered sample (Kempinski et al., 2014). K is Scherrer constant (0.94), is X-ray wavelength 1.54, is half-intensity width of the diffraction peak. The reflecting peak at 2 = 24 chosen for calculation, and the beta is 0.14 (Figure 2).
Table 1. Physical parameters obtained for maize and peas for its shoot length for test and control.
|
DAY |
MAIZE |
PEAS |
||
|
C |
T |
C |
T |
|
|
10 |
1.1 cm |
5.5cm |
1.1 |
2.3 |
|
16 |
2.83cm |
12.4cm |
4.1 |
6.4 |
|
22 |
11.6cm |
26.6cm |
7.5 |
15 |
Table 2. Physical parameters obtained for maize and peas for its root length for test and control.
|
DAY |
MAIZE |
PEAS |
||
|
C |
T |
C |
T |
|
|
10 |
3.8cm |
4.5cm |
4 |
7.7 |
|
16 |
6.2cm |
12.5cm |
6.3 |
10.8 |
|
22 |
11.3cm |
26.3cm |
10.7 |
21.9 |
Table 3. Quantitative measurement of soil parameters of Control and Test soil
|
PARAMETER |
LIMITS |
CONTROL |
TEST |
|
pH |
6.5-8.5 |
6.75 |
6.93 |
|
CEC |
Greater than 15 |
12 |
12 |
|
Available Nitrogen, ppm |
110-200 |
142 |
158 |
|
Available Phosphorus, ppm |
5-10 |
6 |
4 |
|
Available Potassium, ppm |
50-125 |
41 |
67 |
|
Extractable Calcium, ppm |
800-1500 |
990 |
823 |
|
Extractable Magnesium, ppm |
150-250 |
155 |
185 |
|
Organic Matter, % |
1-4 |
1.37 |
2.06 |
|
Organic Carbon, % |
0.5-1.0 |
0.80 |
1.27 |
Table 4. Estimation of bioaccumulation of minerals in the seeds of the samples
|
ELEMENT |
WAVELENGTH λ(nm) |
MINERAL ACCUMULATION(ppm) |
DETECTION LIMIT |
|
|
CONTROL |
TEST |
|||
|
Magnesium (Mg) |
280.271 285.213 |
71.48 ± 3.9 |
92.32 ± 1.4 |
<0.1 |
|
Potassium (K) |
766.490 |
119.8 ± 1.6 |
108 ± 1.3 |
0.1-1 |
|
Sodium (Na) |
330.237 |
120.10 ± 2.4 |
114 ± 1.8 |
0.1-1 |
|
Phosphorus (P) |
213.617 214.914 |
106.0 ± 1.2 |
102 ± 2.5 |
0.1-1 |
|
Mercury (Hg) |
194.168 |
BDL |
BDL |
1-10 |
|
Copper (Cu) |
327.393 |
BDL |
BDL |
0.1-1 |
|
Iron (Fe) |
238.204 |
BDL |
BDL |
<0.1 |
|
Zinc (Zn) |
206.200 |
BDL |
BDL |
0.1-1 |
|
Chromium (Cr) |
267.716 |
BDL |
BDL |
0.1-1 |

Figure 1. SEM images for carbon nanoparticles. A) Typical SEM image of the carbon nanoparticles obtained. B) Closer view of SEM image of nanoparticles.

200 400 600 800 1000 1200 1400 1600 1800 2000
Raman shift (cm1)
Figure 4: Raman Shift
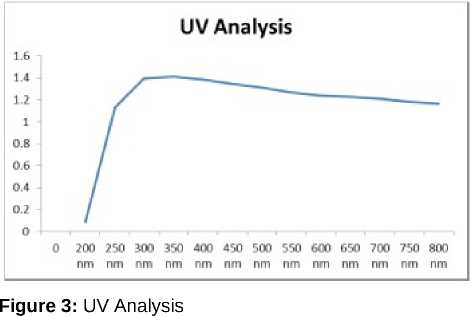
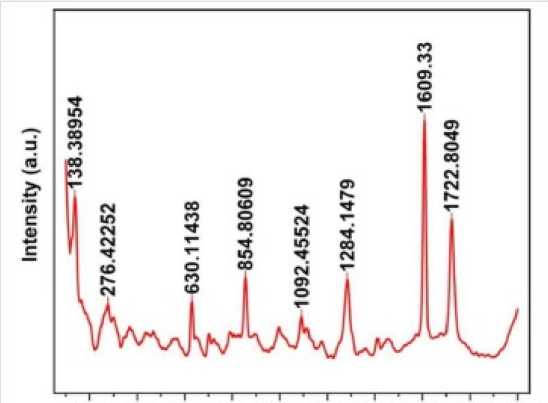
The nanoparticles were subjected to UV absorption Spectroscopy; the absorbance range was obtained from 300nm to 350nm (Figure 3). The range appeared as such due to the mineral impurities present in the sample (Roshni and Ottoor, 2015). The Raman spectroscopy graph obtained showed the Raman shifts at 1284.14 & 1609.33 matching with carbon nanoparticles published previously (Bystrzejewski et al., 2013). The above performed study has signified a new knowledge of CNPs. And its application for crop development and also an advantageous to minimize the waste production form the oil milling factories. The test plant samples were seen to have more growth than the control sample. They exhibited an elevated level of plant chlorophyll, protein and IAA contents which is essential for plant growth. The plants also showed a change in the physical characters like root and shoot length, which was observed to be more in test plants as compared to the control set of the plants.
The Mg2+ was found to be accumulated in the seed that serves as co-factor in plant photosynthesis process, which will significantly benefit dicot as compared to monocot. The size obtained after characterization was 12.30nm which was easily absorbed by the roots. The test such as Raman Spectroscopy (Figure 4), UV confirmed the presence of carbon nanoparticle. As per the above performed study CNPs can prove as a great source of plant fortification and increased crop yield and enhancing the proportion of soil minerals and elevating soil fertility.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Synthesis of carbon nanoparticles and study of bioaccumulation in seeds and its application in plant fortification
- Ananthalakshmi S. and Sathyaprabha. M. (2016). Analysis of Soil Properties, Nutrients and Structure of the Soil Samples From Agriculture and Non-Agriculture Area. Indian Journal of Applied Research, 6(3), 20-22.
- Bystrzejewski, M., tab^dz, O., Kaszuwara, W., Huczko, A. and Lange, H., (2013). Controlling the diameter and magnetic properties of carbon-encapsulated iron nanoparticles produced by carbon arc discharge. Powder technology, 246, pp.7-15.
- Cakmak, I. and Yazici, A.M., (2010). Magnesium: a forgotten element in crop production. Better crops, 94(2), pp.23-25.
- Diatloff, E., Asher, C.J. and Smith, F.W., (1996), January. Rare earth elements and plant growth. In Proc 8th Aust Agron Conf Toowoomba, Queensland, Aust (Vol. 30, pp. 3-6).
- Duhan, J.S., Kumar, R., Kumar, N., Kaur, P., Nehra, K. and Duhan, S., (2017). Nanotechnology: The new perspective in precision agriculture. Biotechnology Reports, 15, pp.11-23.
- Ehmann, A., (1977). The Van Urk-Salkowski reagent—a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. Journal of Chromatography A, 132(2), pp.267-276.
- Elemike, E.E., Uzoh, I.M., Onwudiwe, D.C. and Babalola, O.O. (2019). The role of anotechnology in the fortification of plant nutrients and improvement of crop production. Applied Sciences, 9(3), p.499.
- Guo, B.S., Zhu, W.M., Xiong, B.K., Ji, Y.J., Liu, Z. and Wu, Z.M., (1988). Rare earths in agriculture. Agricultural scientific technological press, Beijing, China, pp.23-208.
- Hakim, Y.Z., Yulizar, Y., Nurcahyo, A. and Surya, M., (2018). Green synthesis of carbon nanotubes from coconut shell waste for the adsorption of Pb (II) ions. Acta Chimica Asiana, 1(1), pp.6-10.
- Karami, P., Khasraghi, S.S., Hashemi, M., Rabiei, S. and Shojaei, A., (2019). Polymer/nanodiamond composites-a comprehensive review from synthesis and fabrication to properties and applications. Advances in colloid and interface science, 269, pp.122-151.
- Karlsson, H.L., Cronholm, P., Gustafsson, J. and Moller, L., (2008). Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chemical research in toxicology, 21(9), pp.1726-1732.
- Kempinski, W., Los, S., Kempinski, M. and Markowski, D., (2014). Experimental techniques for the characterization of carbon nanoparticles-a brief overview. Beilstein journal of nanotechnology, 5(1), pp.1760-1766.
- Li, D., Müller, M.B., Gilje, S., Kaner, R.B. and Wallace, G.G., (2008). Processable aqueous dispersions of graphene nanosheets. Nature nanotechnology, 3(2), pp.101-105.
- Li, H., Shan, C., Zhang, Y., Cai, J., Zhang, W. and Pan, B., (2016). Arsenate adsorption by hydrous ferric oxide nanoparticles embedded in cross-linked anion exchanger: effect of the host pore structure. ACS Applied Materials & Interfaces, 8(5), pp.30123020.
- Li, Y., Chen, H., Dong, Y., Li, K., Li, L. and Li, J., (2016). Carbon nanoparticles/soy protein isolate bio-films with excellent mechanical and water barrier properties. Industrial Crops and Products, 82, pp.133-140.
- Liang, T., Zhang, S., Wang, L., Kung, H.T., Wang, Y., Hu, A. and Ding, S., (2005). Environmental biogeochemical behaviors of rare earth elements in soil-plant systems. Environmental Geochemistry and Health, 27(4), pp.301-311.
- Malik, C.P. and Singh, M.B., (1980). Plant enzymology and histo-enzymology. Kalyani Publishers, New Delhi, p. 286.
- Mi, J., Wang, X.R., Fan, R.J., Qu, W.H. and Li, W.C., (2012). Coconut-shell-based porous carbons with a tunable micro/mesopore ratio for high-performance supercapacitors. Energy & Fuels, 26(8), pp.5321-5329.
- Mukherjee, A., Majumdar, S., Servin, A.D., Pagano, L., Dhankher, O.P. and White, J.C., (2016). Carbon nanomaterials in agriculture: a critical review. Frontiers in plant science, 7, p.172.
- Roshni, V. and Ottoor, D., (2015). Synthesis of carbon nanoparticles using one step green approach and their application as mercuric ion sensor. Journal of Luminescence, 161, pp.117-122.
- Shooto, D.N. and Dikio, E.D., (2011). Morphological characterization of soot from the combustion of candle wax. Int. J. Electrochem. Sci, 6, pp.12691276.
- Wild, E. and Jones, K.C., (2009). Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants. Environmental science & technology, 43(14), pp.5290-5294.
- Witham, F.H., Blaydes, D.F. and Devlin, R.M., (1971). Chlorophyll absorption spectrum and quantitative determination. Experiments in plant physiology, 1971, pp.167-2.
- Wu, L., Cai, X., Nelson, K., Xing, W., Xia, J., Zhang, R., Stacy, A.J., Luderer, M., Lanza, G.M., Wang, L.V. and Shen, B., (2013). A green synthesis of carbon nanoparticles from honey and their use in real-time photoacoustic imaging. Nano research, 6(5), pp.312-325.


