The diagnostic value of IL-6 and thymidine phosphorylase in the progression of osteosarcoma
Автор: Abdul Azeez B.A., Abd Alhussain G.S., Salman R.S., Al-fahham A.A.
Журнал: Гений ортопедии @geniy-ortopedii
Рубрика: Оригинальные статьи
Статья в выпуске: 2 т.31, 2025 года.
Бесплатный доступ
Introduction Thymidine phosphorylase (TP) is known to be correlated with the pathogenesis of solid tumors. IL-6 is overexpressed in osteosarcoma, and data exist showing that high concentrations of IL-6 are linked to a poor prognosis.The aim of this study is to investigate the diagnostic role of thymidine phosphorylase and IL-6 in the pathogenesis and progression in patients with osteosarcoma.Materials and methods Thirty patients diagnosed with osteosarcoma (with age ranging between 15-44 years) were included in the current study. Those patients were distributed as 6, 15, 5 and 4 subjects for stages AI, BI, II and III respectively.Results Statistical analysis pointed out that IL-6 tends to be increased patients in stage III (3.89 ± 0.34 ng/ml) compared to stage AI, BI and II: 1.48 ± 0.22 ng/ml, 1.55 ± 0.24 ng/ml and 2.45 ± 0.45 ng/ml respectively. Regarding thymidine phosphorylase, the current study also found that it tends be increased patients in stage III 8.3 ± 0.33 ng/ml comparing to stage AI, BI and II: 7.2 ± 0.92 ng/ml, 6.82 ± 1.14 ng/ml and 7.8 ± 0.22 ng/ml, respectively. The area under the curve (AUC) for thymidine phosphorylase was 0.87, with high significant difference p function show_abstract() { $('#abstract1').hide(); $('#abstract2').show(); $('#abstract_expand').hide(); }
Thymidine phosphorylase, il-6, diagnosis power, osteosarcoma
Короткий адрес: https://sciup.org/142245092
IDR: 142245092 | УДК: [616.15-07: | DOI: 10.18019/1028-4427-2025-31-2-245-251
Текст научной статьи The diagnostic value of IL-6 and thymidine phosphorylase in the progression of osteosarcoma
Введение . Тимидинфосфорилаза (ТФ) коррелирует с патогенезом солидных опухолей. Интерлейкин-6 (ИЛ-6) имеет повышенную экспрессию при остеосаркоме. Высокие концентрации ИЛ-6 дают основание предполагать плохой прогноз и агрессивный характер опухоли.
Цель работы — изучение диагностической роли ТФ и ИЛ-6 в патогенезе и прогрессировании остеосаркомы.
Материалы и методы . В текущее исследование включены 30 пациентов с диагнозом остеосаркома в возрасте от 15 до 44 лет. Пациенты распределены по стадиям AI ( n = 6), BI ( n = 15), II ( n = 5) и III ( n = 4).
Результаты . Статистический анализ показал, что ИЛ-6 имеет тенденцию к повышению у пациентов на стадии III, (3,89 ± 0,34) нг/мл, по сравнению со стадиями AI, BI и II: (1,48 ± 0,22) нг/мл, (1,55 ± 0,24) нг/мл и (2,45 ± 0,45) нг/мл. Что касается ТФ, данное исследование также показало, что она имеет тенденцию к повышению у пациентов на стадии III, (8,3 ± 0,33) нг/мл, по сравнению со стадиями AI, BI и II: (7,2 ± 0,92) нг/мл, (6,82 ± 1,14) нг/мл и (7,8 ± 0,22) нг/мл соответственно. Площадь под кривой (AUC) для ТФ составила 0,87 с высокой значимой разницей p < 0,001 при точке отсечения 2,44, в то время как коэффициенты чувствительности и специфичности составили 0,85 и 0,71. Что касается ИЛ-6, площадь под кривой (AUC) составила 0,75, со значительной разницей p < 0,038, при точке отсечения 6,32, в то время как коэффициенты чувствительности и специфичности составили 0,81 и 0,69 соответственно. Эти биомаркеры также можно использовать в диагностике и прогрессировании остеосаркомы. Обсуждение . Высокий уровень ТФ является показателем агрессивности опухоли и плохим прогностическим фактором. Отдельные исследования посвящены экспрессии TФ на разных стадиях остеосаркомы, но их число невелико. В последнее время ТФ привлекает значительное внимание своим участием в биологии рака, особенно своим влиянием на патогенез и прогноз заболевания. Согласно исследованиям, TФ играет важную роль в развитии большинства злокачественных заболеваний, и особенно злокачественных новообразований костей. ТФ способствует ангиогенезу, экспрессируя в опухоль-ас-социированных макрофагах в строме опухоли. Экспрессия ТФ изучена при различных типах рака в качестве прогностического фактора. На основе данных литературы могут быть разработаны новые прогностические модели для пациентов с раком костей.
Заключение . Активность ТФ и ИЛ-6 имеет значительную диагностическую ценность при прогрессировании остеосаркомы.
INTRODUCTION
Osteosarcoma (OS), the most common primary bone malignancy, has a worldwide annual incidence of 3.4 per million population. The main pathophysiological mechanism includes multiple possible genetic drivers of the disorder associated with bone formation, leading to a progressive and metastatic malignant transformation. Although significant steps have been made towards the prognosis of patients with localized forms having the event-free survival rate above 60 % it generally remains grim in cases of those with metastatic versions and stays below 30 % [1]. The pathogenesis of osteosarcoma is polygenic and compiles genetic, molecular, and cellular aspects. While these sources do not straightly talk about osteosarcoma, knowledge of cancer genetics from other cancers can help direct views, including the genetic foundation for myeloproliferative neoplasms commented on by W. Vainchenker et R. Kralovics to the landscape of osteosarcoma at the molecular level [2]. Mutation of specific genes acting as tumor suppressors, for instance tumor protein p53 (TP53) and retinoblastoma 1 (RB1), defines most of the tumorigenic pathways in osteosarcoma. Experience has also shown that two to three driver gene mutations can transform osteoblasts to become osteosarcomas. This event likely follows loss of the p53 and Rb pathways based on what transformation has been shown to use across tumor types. Loss of differentiation may help explain why normal differentiation is blocked and sarcomas have primitive-appearing cells. The findings here are consistent with the strong genetic evidence indicative of the critical role of TP53 as a major tumor suppressor gene in human tumorigenesis and sarcoma genesis specifically [2]. Osteosarcoma is the third most common cancer in adolescence, after lymphomas and brain tumors, with an annual incidence of 5.6 cases per million children below 15 years of age. Osteosarcoma incidence is bimodal, peaking at 18 and 60 years of age, and is slightly more common in males. Peak incidence is in the second decade of life. Before the age of five, OS is rare. OS arises sporadically, with few cases related to known inherited defects in cell cycle regulation, but about 70 % of tumor specimens show a chromosomal abnormality. Some key mutated genes drive the initiation and progression of osteosarcoma [3]. Thymidine phosphorylase (TP) is a rather interesting enzyme that performs functions in nucleosides metabolism that executes salvage of pyrimidine with its functional activities in the pathway. First described in 1953, stimulated by TP is the reversible bioconversion of thymidine (TdR) into thymine and 2-deoxy- α -D-ribose-1-phosphate (dRib-1-P), as well as deoxy uridine phosphorolysis into uracil and 2-deoxy- α -D-ribose-1-phosphate. However, this reaction is reversible; TP's major function is catabolic. There are two different names for one molecule: angiogenic factor platelet-derived endothelial-cell growth (PD-ECGF) and TP. That metabolic role aside, TP plays an essential role in angiogenesis. The reaction is catalyzed by TP expressed as: TdR + H 2 O ↔ thymine + 2-deoxy- α -D-ribose [4, 5]. The reversible nature of the reaction has arisen some debate as to the reality of TP catalyzing that reaction to any appreciable extent, such a view seems to dismiss a good body of consistent evidence, which is becoming available, that supports a catalytic function for this enzyme in pyrimidine degradation both in vitro and in vivo within the large majority of cells that TP is the primarily source of this activity [6–7]. Furthermore, several human cancers overexpress TP. The human TP gene is located on chromosome 22q13. TP is a dimer protein composed of two identical subunits non-covalently associated with a dimeric molecular mass of 51 kD [8]. Thymidine phosphorylase (TP) levels are expressed in some epithelial, glial cells, macrophages, and stromal cells. It is manifested in the nucleus. Here it regulates the supply of pyrimidine nucleosides that are necessary for DNA synthesis as well as in the cytoplasm, where it exhibits enzymatic activities [9]. IL-6 is a pleiotropic cytokine, synthesizing from various cells, including endothelial cells, keratinocytes, fibroblasts, and tumor cells; it is considered as the most important cytokine responsible for induction of events-ranging over many biological processes. IL-6 is a key inflammatory mediator implicated in the procession and advancement of osteosarcoma. IL-6 binds with a receptor complex having α -gp80 (or IL-6R) and then further association with gp 130 [10]. IL-6 exerts inflammatory responses and has been impugned in the pathogenesis of a variety of cancers. High levels of this cytokine within the tumor microenvironment are also well documented to underlie the poor prognosis and aggressive nature of the tumor; D.T. Fisher et al., in 2014, focus their assertions that IL-6 may have paradoxical effects within the tumor microenvironment, dichotomously acting not only as a growth promoter but also as an apoptosis inhibitor. This duality perfectly underscores the complexity surrounding attempts to target this cytokine in cancer therapy [11]. In 2016, T. Tanaka et al. expound on how IL-6 contributes to inflammation-driven carcinogenesis, a process that takes place in colorectal cancer as basic mechanisms work similarly in osteosarcoma. This leads to expression in the tumors’ proximity of factors like IL-6 that further enhance chronic signaling through pathways like JAK/STAT to advance and support tumorigenetic processes [12].
The aim of this study is to find out the diagnostic role of thymidine phosphorylase and IL-6 in the pathogenesis and stages progression in patients with osteosarcoma.
MATERIAL AND METHODS
Patients and data collection
A prospective study was performed at Al-Forat Al-Awsat Oncology Center in Najaf and Oncology Center in Baghdad/Iraq in the period from September 2023 to June 2024. Thirty patients diagnosed as having osteosarcoma, aged between 15–44 years were included in the present study. The general information (age, gender, and stage) was collected from the records of the patients in the Oncology Center. An amount of 5 milliliters venous blood samples were collected. The blood samples were allowed to coagulate in the gel tube at room temperature for 20 minutes. After coagulation, the samples were centrifuged at 2000xg for 20 min to obtain the serum, which was stored at (–20 °C) until assayed. IL-6 and thymidine phosphorylase were estimated in the serum by ELISA kit (My BioSource, USA) for serum thymidine phosphorylase and IL-6 determination. The Enneking system for staging classifies according to grade (I or II), intra- or extracompartmental location (A or B), and the presence of metastases (III). So, stage IA is a low-grade intra-cortical tumor, IB is a low-grade extra-cortical tumor, IIA is a high-grade intra-cortical tumor, and IIB is a high-grade extra-cortical tumor. Metastatic disease instantly catapults to III stage [3].
Statistical Analysis
The SPSS software version 25.0 (SPSS, Chicago) was used for all the appropriate statistical analyses, the normality test which was used to test who want to continuous data for Shapiro Wilk test. All normally distributed data are acted as mean ± standard deviation. ANOVA test was used to find out the statistical difference in TP and Il-6 with patients' groups classified by tumor stages. The receiver operating characteristic curve (ROC) was used to test the predictive value of thymidine phosphorylase and IL-6 in predicting relapse among osteosarcoma patients. A p -value less than .05 was considered to indicate a statistically significant difference.
RESULTS
Patients who participated in the current study have a wide range of age (15–44 years) with a mean of (26.25 ± 6.22) years; the majority of them (43.33 %) within the interval (15–24) years, while those within (24–34) years constitute (40 %) of the sample. It seems that osteosarcoma is more common in male (60 %) compared to female (40 %). The distribution of patients according to the stage of osteosarcoma was as follows 6, 15, 5 and 4 individuals for stages AI, BI, II and III respectively (Table 1).
Data regarding thymidine phosphorylase and IL-6 were found to follow normal distribution. Accordingly, these data were expressed as mean and standard deviation. The statistical analysis pointed out that thymidine phosphorylase tends to be increased in patients in stage III (3.89 ± 0.34 ng/ml) compared to stage AI, BI and II: (1.48 ± 0.22 ng/ml), (1.55 ± 0.24 ng/ml)
Table 1
Statistical distribution (frequency and percentage) of patients by their general characteristics
|
Items |
Sub-group |
Patients N = 30 |
|
|
Freq. |
% |
||
|
Age |
15–24 |
13 |
43.33 |
|
25–34 |
12 |
40 |
|
|
35–44 |
10 |
33.33 |
|
|
Mean ± SD 26.25 ± 6.22 |
|||
|
Sex |
Male |
18 |
60 |
|
Female |
12 |
40 |
|
|
Stage |
AI |
6 |
20 |
|
BI |
15 |
50 |
|
|
II |
5 |
16.67 |
|
|
III |
4 |
13.33 |
|
and (2.45 ± 0.45 ng/ml) respectively (Fig. 1). Regarding IL-6, the current study also found that IL-6 tends to be increased in patients in stage III (8.3 ± 0.33 ng/ml) compared to stage AI, BI and II: (7.2 ± 0.92 ng/ml), (6.82 ± 1.14 ng/ml) and (7.8 ± 0.22 ng/ml) respectively (Fig.
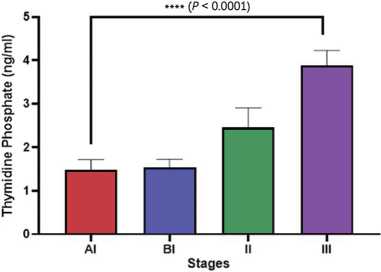
Fig. 1. Differences in serum thymidine phosphorylase in different stages of osteosarcoma
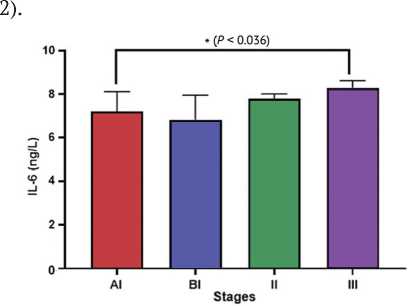
Fig. 2. Differences in serum IL-6 in different stages of osteosarcoma
The receiver-operating characteristic (ROC) curve analysis of thymidine phosphorylase and IL-6 was used in predicting relapse in patients with osteosarcoma. The area under the curve (AUC) for thymidine phosphorylase was 0.87, with high significant difference p < 0.001, at a cut-off point 2.44, while the sensitivity and specificity ratios were 0.85, and 0.71 respectively. Regarding IL-6, the area under the curve (AUC) was 0.75, with significant difference p < 0.038, at a cut-off point 6.32, while the sensitivity and specificity ratios were 0.81 and 0.69 respectively, as shown in Table 2 and Fig. 3.
Table 2
The areas under the curve (AUC), sensitivity and specificity of the biomarkers for the diagnosis of osteosarcoma
|
Biomarkers |
(AUC) |
Sig. p-value |
Cut-off Point |
Sensitivity (%) |
Specificity (%) |
|
TP |
0.87 |
0.01 |
2.44 |
0.84 |
0.71 |
|
IL-6 |
0.75 |
0.038 |
6.32 |
0.81 |
0.69 |
TP: Thymidine phosphorylase; AUC: Area Under the Curve
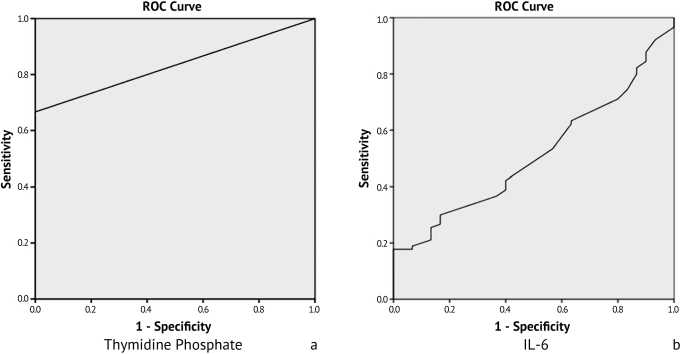
Fig. 3. Diagonal segment of the Receiver operating characteristic (ROC) curve for (a) thymidine phosphorylase and (b) IL-6 in for the diagnosis of osteosarcoma
DISCUSSION
Thymidine phosphorylase activity in 30 cases of osteosarcoma was analyzed by ELISA, including (6 cases at Stage AI, 15 at Stage BI, 5 at Stage II, 4 at Stage III). Thymidine phosphorylase is upregulated in a broad set of solid tumors and enhances progression of tumor via anti-apoptotic activity. High levels of TP are expressions of tumor aggressiveness and poor prognostic factors. The presence of high intracellular levels of this enzyme indicates increased chemosensitivity to pyrimidine antimetabolites [13–14]. This study exhibited that thymidine phosphorylase is present in high concentration in patients with higher stages of osteosarcoma. Although some separate studies focus on TP expression at different osteosarcoma stages, their numbers are few, arguing that there is a gap in the current available literature. Thymidine phosphorylase is an enzyme that acts in thymidine metabolism and more recently has attracted significant attention for its involvement in cancer biology, especially with regard to its influence on disease pathogenesis and prognosis [15]. Indeed, according to available studies, TYMP plays a major part in the development of most malignant diseases, and particularly bone-related malignancies [16]. Thymidine phosphorylase significantly promotes bone lesion formation by inhibiting osteoblast activity and stimulating osteoclast activity. This reaction models TYMP as a facilitator of myeloma-induced bone lesions and thus argues for its potential contribution to other inflammatory events accompanying bone cancer [16]. The other striking feature is TYMP's contribution to angiogenesis. It has been observed that TYMP, it promotes angiogenesis, is expressed in tumor-associated macrophages within the tumor stroma. This correlation between TYMP expression, angiogenesis, and poor prognosis underscores its potential as a key player in the tumor microenvironment: influencing both tumor growth and metastasis [17]. TYMP expression has been studied in different types of cancer as a predictive body. Thus, TYMP expression was found to be higher with a high systemic immune-inflammation index, suggesting that TYMP levels are related to patient outcomes [18]. This relationship is especially significant in the sensitivity of TYMP as a biomarker to postoperative patients with gastric cancer, which means that it has the potential to be developed as a clinical prognostic biomarker. The expression in tumor tissues with high SII values was much higher compared with those with low SII values, indicating their association with poor prognostic factors in various cancers other than bone cancer [19]. J.F. Xu et al. successfully isolated exosomes related to chemotherapy responses in osteosarcoma containing differentially expressed microRNA and mRNA. In view of this trend, highly increased TYMP may indicate a more aggressive tumor phenotype and subsequently become a prospective aim for therapeutic intervention [20]. The present study highlighted that IL-6 is found in high concentration in patients with higher stages of osteosarcoma. Specific research on IL-6 in osteosarcoma is scanty, though several inferences can be made from those done in other malignancies. Increased levels of IL-6 have been documented in several inflammatory conditions as observations by P. Conti et al. (2020), who reported a significant increase of IL-6 in patients critically ill. In line with this, the author observed that proinflammatory cytokines like IL-6 could contribute to an immunosuppressive tumor microenvironment — a situation not suitable for effective anti-tumor immunity [21]. This association provided further insights into the role of IL-6 as a mediator of systemic inflammatory responses in disease severity, as might be relevant with osteosarcoma. There is accumulating evidence that IL-6 plays an essential role in regulating immune responses in cancer [22]. Data by A. Mazzoni et al. (2020) show the defect of immune cell cytotoxicity in severe COVID-19 patients that depends on IL-6. Secretion of such high levels of IL-6 would, therefore, skew the immune response to a more tolerogenic state. This leads to questioning whether analogously it could do so in immunotherapeutically treated osteosarcoma patients by providing room for immune response, thus lowering effectiveness of the strategy [23]. On the one hand, IL-6 is one of the pleiotropic cytokines that are surely critical in many physiological processes and simultaneously steers pathogenetic processes, especially inflammation and cancer. CAF-derived IL-6 is indeed strongly pro-tumorigenic and contributes significantly to stroma-dense growth in several cancer entities, including pancreatic ductal adenocarcinoma. Reports show recurring interplay between IL-6 and CAFs to create a pro-tumorigenic environment that supports further tumor growth and metastasis [24]. In line with this, it seems that IL-6 is not just a byproduct of the disease but takes on the form of an active player in the tumor microenvironment that per se influences the behavior of malignant cells. Through this, dysregulated IL-6 signaling in bone cancer may result in increased tumor growth and, therefore, increased survival [25]. The prolonged activation of the STAT3 pathway, which is driven by IL-6, has been implicative of cancer progression and chronic inflammatory diseases. This persistent STAT3 activation underscores, directly, the importance of IL-6 maintaining an inflammatory state that is conducive to cancer development. One of the critical prognostic values of IL-6 in cancer is its well-known relationship to poor prognosis and the growth of many tumors, such as prostate, which leads to a negative correlation with regards to survivals and responses to chemotherapy [26–27]. The negative correlation of IL-6 might make it a good biomarker for the progression of the disease, which in turn would give clues about the likelihood of response to treatment and general outcome in patients. In this regard, whether similar relationships exist between IL-6 levels and clinical outcomes in bone cancer will underpin further attempts. IL-6, by promoting cancer cell growth and blocking apoptosis, seems to be essential for the switch from tumor types to more aggressive phenotypes [27]. New prognostic models for bone cancer patients could be developed based on that information.
CONCLUSION
It is suggested that thymidine phosphorylase activity and IL-6 have a significant high diagnostic power in the diagnosis and progression of osteosarcoma.
Conflict of interest Not declared.
Funding The authors rely only on their own financial support.
Ethics approval The project in this study was approved by the ethical committee of the Medical College in the University of Kufa (No. 135 in 2024).
Consent to participate Prior to collecting samples, all patients involved in the study were required to provide written consent for their participation.


