The effects of aluminum and phosphorous on some of physiological characteristics of Brassica napus
Автор: Tohidi Zahra, Baghizadeh Amin, Enteshari Shekofeh
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.11, 2015 года.
Бесплатный доступ
The environmental toxicity of Aluminum causes nervous, lung and kidney disease in human body. Aluminum toxicity depends on different factors and these factors cause penetration of toxic element to environment soil. The acidity of environment and the root of plants cause the lack of tolerance in absorption of nutrients and makes abnormality in the growth of plants. In this study the effect of Aluminum (40 µmol) and different levels of Phosphorous (0, 40, 80, 320 µmol) in one cultivar of Brassica napus were investigated. Results showed in plants that treated with Aluminum, Shoot length, Root length and Chlorophyll a, b content significantly decreased but Malondialdehyde (MDA) and Reduced Sugars content significantly increased. On the other hand, in plants that treated with Phosphorous and Aluminum decreased in growth parameters and Chlorophylls content moderated against plants that treated just with Al and MDA content and reduced sugar content decreased. From these results we suggested that Phosphorous treatment reduced the harmful effect of Aluminum toxicity in this cultivar of canola.
Aluminum, phosphorous, brassica napus
Короткий адрес: https://sciup.org/14323916
IDR: 14323916
Текст научной статьи The effects of aluminum and phosphorous on some of physiological characteristics of Brassica napus
MATERIALS AND METHODS
The test plant in this research was Brassica napus. It belongs to the Brassicaceae family and it is economically important due to its oil seeds. At first, the selected seeds were disinfected by Sodium Hypochlorite 0.1%. Then they were washed by distilled water and were put in it for an hour. To grow the plant in a flower pot, seeding bed was chosen of prelate. This bed is almost lack of any ion and its action exchange capacity is ignorable. To be assure that prelate as seeding bed is lack of Al ion, it was washed with distilled water of pH=5.5, finally sterilized by autoclave. The pots of seeds were moved to greenhouse. Greenhouse conditions: light mixture of sodium halide lamp and halogen lamp with 800 Lux, 16 hours light period, 8 hours darkness, 23±1°C. During the first week after seeding in flower pot, irrigation was done by distilled water. Irrigation was twice a day and 80ml in each one. After a week of irrigation with distilled water, irrigation was started with Long Ashton perfect nutritional solution once a day for two weeks. Preparing the solution repeated for two weeks and used. PH of the prepared solution was regulated about 5.5 by Potash solution of 0.1 molar and sulphoric acid of 0.1 molar. Two weeks after irrigation with the nutrition solution lacking Al ion, treatment of the plants started as following: some of pots irrigated with nutrition solution contains 0, 20, 40 and 60 micro molar of AlCl3 and some other pots irrigated with nutrition solution contains 0, 40, 80 and 320 micro molar of KH2PO4 with AlCl3 fix concentration of 40 micro molar. This irrigation method was done daily for three weeks. After 3 weeks different parts of the plant removed and were kept in freeze liquid nitrogen of -16ºC in a fridge until the time of experiment. The following cases were studied in order to study the effect of Al on Brassica napus plantlet: Evaluation of chlorophyll and carotenoid (Lichtenthaler, 1987), Evaluation of flavonoids by spectrophotometer (-Krizek et al., 1981), Evaluation of peroxidation of membrane fats such as MDA (Heath et al.,
1969), And also other Aldehydes of the membrane (Meirs et al., 1992), Evaluation of phenol compounds (Sonald et al., 1999), Evaluation of reducing sugar (Somogy, 1952), Evaluation of solution carbohydrate (Fales, 1951), Evaluation of protein concentration (Bradford, 1976) and Evaluation of proline ( Bates et al., 1973 ).
RESULTS
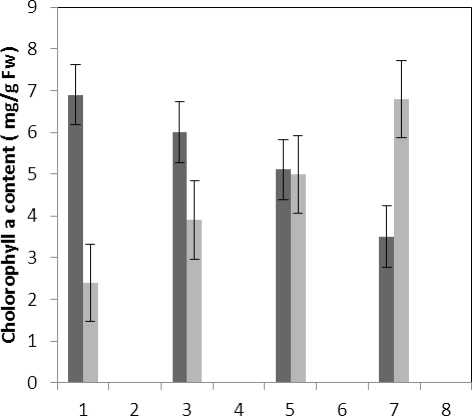
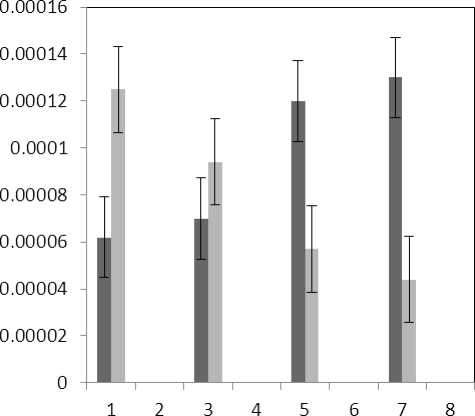
Treatment by both Al and P causes significant increase in carotenoid of the plant that this increase is significant (P≤0.05) in 80 micro molar concentration of P. Also treatment by Al causes decrease in carotenoid of the leaf which is not significant statistically (Fig. 1). Treatment by both Al and P causes significant increase of chlorophyll a just with 320-micromolar concentration of P. Treatment by Al also causes significant decrease (P≤0.05) of chlorophyll a (Fig. 2). Treatment by Al causes significant decrease (P≤0.05) of chlorophyll b in the plant leaf; this decrease is highly significant in 60-micromolar concentration. Treatment by both Al and P also causes an increase in chlorophyll b which is not a significant increase statistically (Fig. 3). Results of the effect of treatments on MDA are in Fig. 7. Treatment by Al causes significant increase (P≤0.05) of membrane MDA concentration; treatment by both Al and P also causes significant decrease
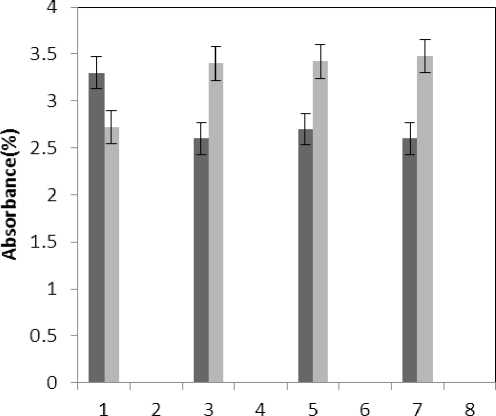
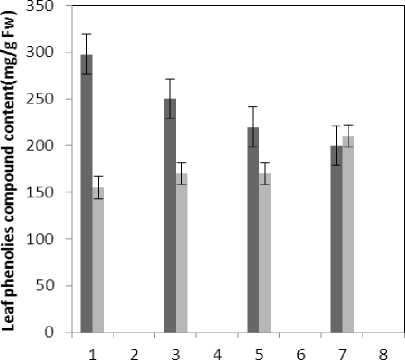
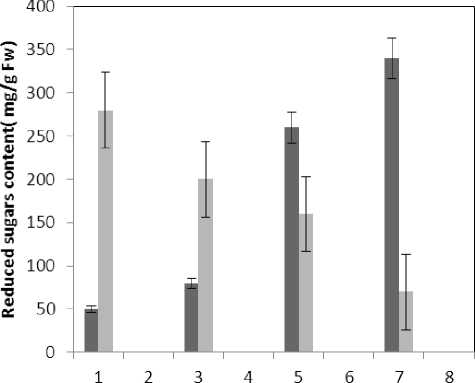
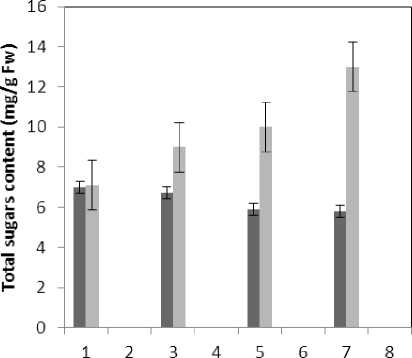
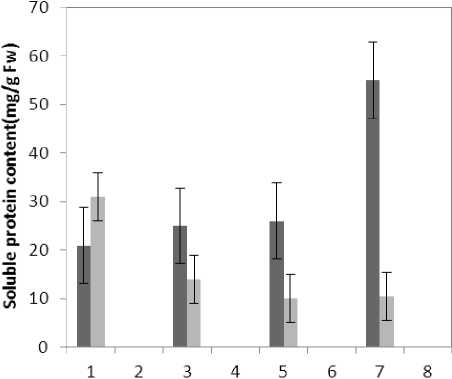
(P≤0.05) of membrane MDA concentration. Results of the effect of treatment by Al and treatment by both Al and P on other membrane Aldehydes delineated in Fig. 8. In marker plant the treatment by Al causes significant increase (P≤0.05) in concentration of other membrane MDAs. Treatment by both Al and P also causes significant decrease (P≤0.05) in concentration of other membrane lipids. Treatment by Al causes significant decrease (P≤0.05) in absorption of flavonoids in wavelength of 270nm (Fig. 4); treatment by both Al and P causes an increase in absorption of flavonoids in wavelength of 270nm. Treatment by both Al and P causes significant increase (P≤0.05) in absorption of flavonoids in wavelength of 300nm (Fig. 5), just in 320-micromolar concentration of P. Treatment by Al causes decrease in absorption of flavonoids in wavelength of 300nm which is not a significant decrease statistically. Treatment by both Al and P causes significant increase (P≤0.05) in absorption of flavonoids in wavelength of 330nm (Fig. 6), just in 320-micromolar concentration of P. Treatment by Al also causes a bit decrease in absorption of flavonoids in wavelength of 330nm which is not a significant decrease statistically. Treatment by both Al and P causes significant increase (P≤0.05) in phenol compounds of the plant in proportion to the marker plant, and the most influence observed in 320-micromolar concentration of P. Treatment by Al also causes decrease in phenol compounds of the plant which is not a significant decrease statistically. Treatment by Al causes significant increase (P≤0.05) in plant proline which is a very significant increase in 40- and 60-micromolar concentration of Al. Treatment by both Al and P also causes decrease in plant proline which is not a significant decrease statistically. Treatment by both Al and P causes a very significant decrease (P≤0.05) in plant protein. Treatment by Al also causes significant increase (P≤0.05) in plant protein which its most influence is in 60-micromolar concentration of Al. Treatment by Al causes a very significant increase (P≤0.05) in reducing sugars of the plant which its most influence observed in 40- and 60-micromolar concentration of the Al. Treatment by both Al and P also causes a very significant decrease (P≤0.05) in reducing sugars which its most influence observed in 80- and 320-micromolar concentration of P. Treatment by Al causes significant decrease (P≤0.05) in soluble carbohydrates of the plant which its most influence observed in 40- and 60-micromolar concentration of Al. Treatment by both Al and P also causes a very significant increase (P≤0.05) in soluble carbohydrates of the plant which its most influence observed in 80- and 320-micromolar concentration of P.
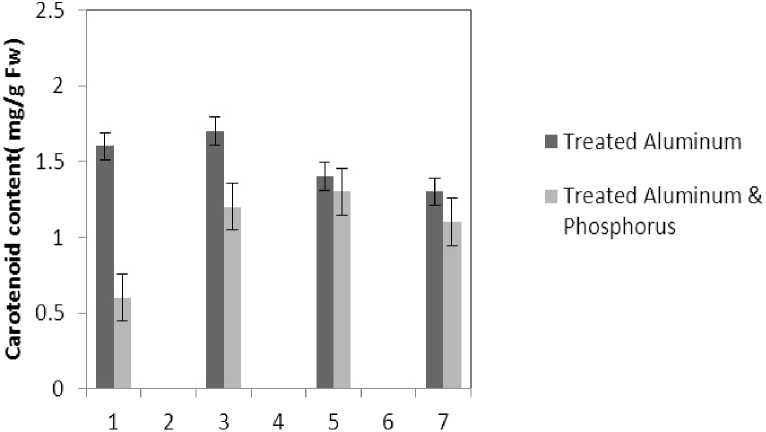
Figure 1 . The effect of Aluminum and Phosphorus concentration on carotenoids content
Al : 1 - 0; 3 - 20; 5 - 40; 7 - 60 µM AlCl 3
Al and P : 1 - 0; 3 - 40; 5 - 80; 7 - 320 µM of KH 2 PO 4 with fix concentration of 40 µM AlCl 3
■ Treated Aluminum
Figure 2 . The effect of Aluminum and Phosphorus concentration on chlorophyll a content
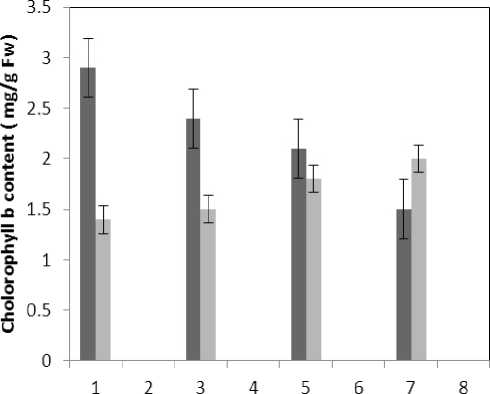
Figure 3 . The effect of Aluminum and Phosphorus on chlorophyll b content
■ Treated Aluminum
Treated Aluminum and Phosphorus
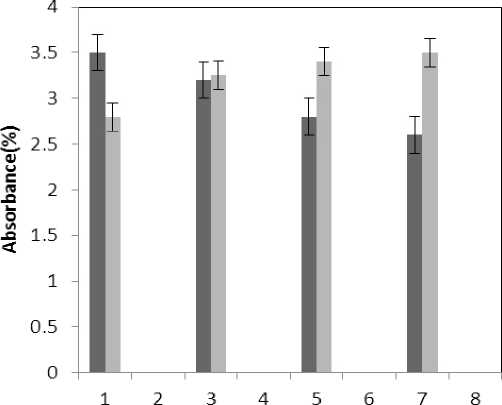
Figure 4 . The effect of treatments on flavonoids absorb ( 270=ג )
Treated Aluminum
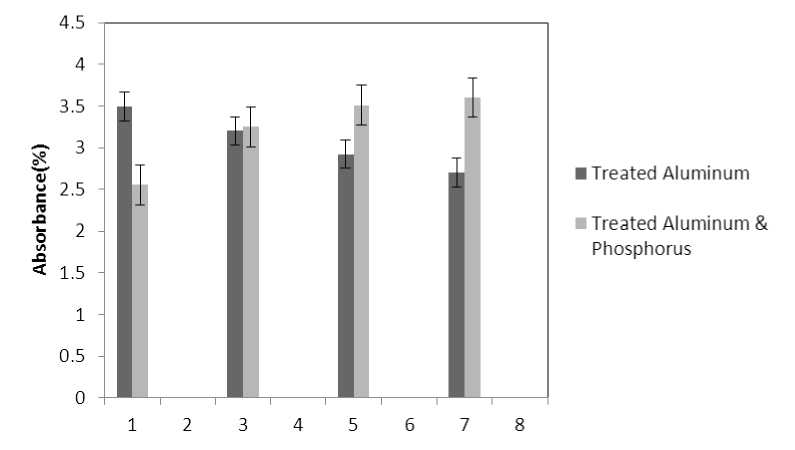
Figure 5 . The effect of treatments on flavonoids absorb ( 300=ג )
■ Treated Aluminum
Figure 6 . The effect of treatments on flavonoids absorb ( 330=ג )
■ Treated Aluminum
Treated Aluminum and Phosphorus
Figure 7 . The effect of treatments on membrane MDA content
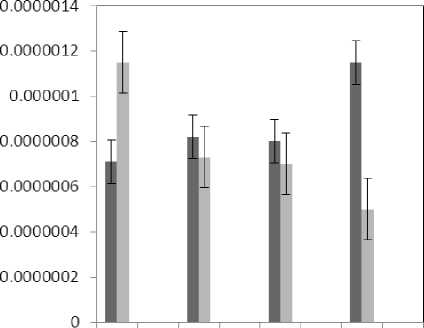
Figure 8 : The effect of treatments on the aldehyde content
■ Treated Aluminum
■ Treated Aluminum
Phosphorus
Figure 9 : The effect of treatments on the phenolies compound content
■ Treated Aluminum
Figure 10 : The effect of treatments on reduced sugars content
■ Treated Aluminum
Figure 11 : The effect of Treatments on Total sugars content
Phosphorus
■ Treated Aluminum
Figure 12 : The effect of Treatments on Soluble protein content
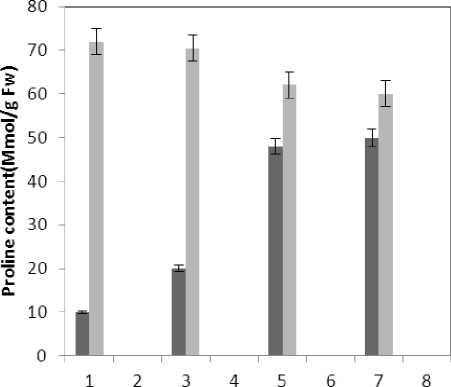
■ Treated Aluminum
Phosphorus
Figure 13 : The effect of Treatments on Proline content
DISCUSSION
There are many reports about the effect of Al on carotenoids content of the plants (Greger et al ., 1991). The results of this research also delineated that Al has significant effect on chlorophyll and carotenoids content of Brassica napus plantlet in its all concentrations. Intense effects of Al on photosynthesis have some aspects that one of them is defection in chlorophyll biosynthesis (Haag-kerwer et al., 1999). Al can control chlorophyll biosynthesis by means of controlling protochlorophyllide reductase enzyme or by controlling enzymes exist in water-break complex at place of photo-system II oxidation, it controls photosynthesis electron transmission and through this it prevents the chlorophyll stimulation effect and consequently prevents the transmission of electron reactions chain (Lagriffoul et al., 1998). Decrease in chlorophyll content in presence of heavy metals is probably due to oxidative induction. It was reported that decrease in carotenoid content can be due to non-photochemical collapse of carotenoids-stimulated chlorophylls and lead to break of carotenoid structure. Flavonoids are introduced as secondary metabolites of the plant based on phenyl benzopropen structure and also as bio-flavonoid. Biosynthesis of the flavonoids is complex and causes involvement of several enzyme phases. In initial phases of flavonoids synthesis, phenyl alanine which is derived from shikmic path, changes into coenzyme A by means of enzymes of methylpropanoid path PAL, C 4 H and 4CL. CH S is the first enzyme of synthesizing flavonoids and causes the combination of coenzyme A and three molecules of Malonyl-CoA
(made from Acetyl-CoA) which is necessary for formation of Naringenin. Then Naringenin change into other types by, Glycolysation and Metilation of its three rings. In this research also treatment by Al decreases the rate of flavonoids absorption in three wavelengths of 300, 270 and 330. Probably Al affecting on enzymes existing in flavonoids path such as Chalkone synthetize and phenyl ammonia leas cause decrease in aggregation of this pigment in plant. In treatment by both Al and P, Phosphorus also prevents the effect of Al on this group of enzymes and therefore this pigment is increasing in the plant (Jenkins, 1999). Peroxidation of fats is a free radicals-based process. Free radicals of oxygen, oxygen, radicals of hydroxyl and protonized superoxide anions attack unsaturated fat acids (i.e. linoleic acid). The result of this process is formation of analyzed products like Aldehydes. This process is used as an index to choose tolerant and sensitive plants against environmental stresses (Frankel, 1985). Increase of fats peroxidation usually considered as index of oxidative stress increase. Fats peroxidation Increase in treatment by Al shows insufficiency of not tolerance mechanisms emerged in plant against oxidative stress. Phenolic compounds especially hydroxyl-cinamic acids exist too much in epidermis. Existence of these compounds in leaf makes it thicker, removing free radicals and changing the amount of chlorophyll and carotenoid. Aluminum effects on enzymes like phenyl alanine ammonia leas, CHS and other enzymes in phenyl propanoid path. The phenyl alanine ammonia leas enzyme causes phenyl alanine changing into trans-cinamic acid and formation of phenolic compounds like flavonoids, tannins and lignin (Gitz et al., 2004). Probably Al causes decrease of phenolic compounds by affecting on this enzyme (Gitz et al., 2004). Existence of phenolic compounds like esters of Hydroxy-Cinamic acid in Epidermis cells increases tolerance of plant cells against damages of environmental stresses. Therefore, in Brassica napus, formation of Phenolic compounds under the treatment of both Al and P increases the plant tolerance against Al and increasing the amount of theses compounds in Phosphorus-treated plants is probably due to effect of thus element on synthesizing enzymes of the compounds in Phenyl Propanone direction (Sonald et al., 1999).
Список литературы The effects of aluminum and phosphorous on some of physiological characteristics of Brassica napus
- Amangel K., Kerkby E. (1993) Principles of plant natrition. 1: 109
- Amami A. (1996). Methods of plant analysis. 972: 248
- Acreduany P. (1997). Soil conservation 1: 177-196
- Aniol A. (1990). Genetics of tolerance to aluminum in wheat (Triticum aestivum L.) Plant Soil. 123: 223-227
- Bates L.S., Waldren R.P. and Teare I.D. (1973). Rapid determination of free proline for water stress studies. Plant and Soil. 29: 205-207
- Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248-254
- Delhaize E, Ryan P.R. (1995) Update on environmental stress: aluminum toxicity and tolerance in plants. Plant Physiol. 107: 315-321
- Demir Y. (2000) Growth and Proline Content on germinating wheat genotypes under ultraviolet light. Turkish Journal of Botany 24: 67-70
- Fales F.W. (1951) The assimilation and degradation of carbohydrates by yeast cells. Journal of Biological Chemistry 193: 113-124
- Frankel. E.N. (1985). Chemistry of free radical and singlet oxidation of lipids. Progress in lipid research 23: 197-221
- Gitz D.C., Liu-Gitz L., Mcclure J.W., Huerta A. (2004). Effects of a PAL. Inhibitor on Phenolic accumulation and UV-B tolerance in Spiodela intermedia (Koch). Journal of Experimental Botany, 55: 919-927
- Greger M. & Ogren E. (1991). Direct and indirect effects of Cd2+ on photosynthesis in sugar beet (Beta valgaris). Physiologia Plantarum. 43(1): 129
- Haag-kerwer A., Schafer H.J., Heiss S., Walter C. & Rausch T. (1999). Cadmium exposure in Brassica juncea cause a decline in transpiration rate and leaf expansion without effect on photosynthesis. Journal of Experimental Botany. 50(341): 1827-1835
- Heath R.L., and Pacher L. (1969). Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Biochem Biophys 125: 189-198
- Jenkins G.I. (1999) Regulation of phenylpropanoid and flavonoid biosynthesis genes by UV-B in Arabidopsis. In: plant responses to environmental stress. Ed by: Smallwood, Calvent and Bowles. Bios. Scientific Publishers. Oxford
- Khudsar T., Mahmooduzzafar & Iqbal M. (2001). Cadmium induced changes in leaf epidermis, photosynthetic rate and pigment concentrations in Cajanus cajan. Biologia Plantarum. 44(1): 59-64
- Koster K.L. and Leopold A.C. (1988). Sugars and desiccation tolerance in seeds. Plant Physiology 96: 302-304
- Krizek P.T., and Worley J.F., (1981). The influence of spectral quality on the internodal response of intact bean plants to brassins. Physiol Plant 51: 259-264
- Lagriffoul A., Mocquot B., Mench M. & Vangronsveld J. (1998) Cadmium toxicity effects on growth, mineral contents, and activities of related enzymes in young maize plants (zea mays). Plant and Soil. 200: 241-250
- Lichtenthaler H.K. (1987) Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods in Enzymology. 148: 350-382
- Luo M., Bi S. (2003). Solid phase extraction-spectrophotometric determination of dissolved aluminum in soil extracts and ground waters. Biochem. 97: 173-178
- Meirs S., Philosoph-Hadu S., and Aharoni N. (1992) Ethylene-increased Accumulation of Fluorescent Lipid-peroxidation Products Detected during Senescence of Parsley by a Newly Developed Method. Journal of the American Society for Horticultural Science. 17: 128-132
- Sonald S.F. and Laima S.K.(1999) Phenolics and cold tolerance of Brassica napus. Plant Agriculture. 1: 1-5
- Somogy M. (1952). Notes on sugar determination. Journal of Biological Chemistry. 195: 19-23
- Tangen G., Wickstr T.M., Lierhagen S., Vogt R. & Lund W. (2002) Fractionation and Determination of Aluminum and Iron in Soil Water Samples Using SPE Cartridges and ICP-AES. Environ. Sci. Technol. 36(24): 5421-5425
- Verma S. & Dubey R.S. (2001) Effect of cadmium on soluble sugars and enzymes of their metabolism in rice. Biologia Plantarum. 1: 117-123
- Wilkens B.J. and Loch J.P.G. (1997). Accumulation of cadmium and zinc from diffuse emission on acid sandy soils, as a function of soil composition. Water Air Soil Pollution. 96: 1-16
- Yokoi S., Bressan R.A. and Hasegawa P.M. (2002). Salt stress tolerance of plants. JIRCAS working Report. 25-33


