The immune system contributes to the effectiveness of vaccine therapy in patients with metastatic melanoma
Автор: Mikhaylova I.N., Stakheyeva M.N., Shubina I.Zh., Chkadua G.Z., Borunova A.A., Zukov R.A., Bogdashin I.V., Choynzonov E.L., Cherdyntseva N.V.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Клинические исследования
Статья в выпуске: 2 т.22, 2023 года.
Бесплатный доступ
The aim of the study was to identify differences in the immune system parameters between metastatic melanoma patients who responded and did not respond to dendritic cell vaccination. Material and Methods. The study group included 20 patients with stage III-IV metastatic melanoma, who received vaccine therapy with dendritic cells (DC) in a prophylactic mode. The control groups included 13 patients who had symptoms of disease progression at the time of starting vaccine therapy, and 5 healthy donors. The DC-vaccine was prepared in the form of a suspension of the patient's autologous dendritic cells loaded with tumor antigens in vitro . A single dose had 2 million dendritic cells in 1 ml of phosphate buffer solution, which was administered intradermally in the nearest site to the regional lymphatic collectors. The immune system status was assessed before starting vaccination. The immune system status was evaluated according to the indexes of 25 peripheral blood cell populations using multicolor flow cytometry and integral characteristic in the form of the visual image generated by the visualization method of multidimensional data (NovoSpark, Canada). Results. The immune status in patients with metastatic melanoma at the start of DC-vaccination differed and was associated with the effectiveness of subsequent vaccine therapy. The response to vaccination was observed in patients whose immune system status was similar to that of healthy individuals. Low efficacy of DC-vaccine therapy was shown in patients whose immune system status corresponded to that of patients with disease progression. Alterations of the immune system in patients with metastatic melanoma were registered both at the level of individual immunological parameters and at the level of visualized integral characteristics. The integral characteristics of the immune system associated with the patient's immunocompromised status can be considered as a criterion for stratification of patients with metastatic melanoma for the effective DC-vaccine therapy. Conclusion. The effectiveness of vaccine therapy with dendritic cells in patients with metastatic melanoma is associated with the immune system state before starting this therapy.
Immune system, melanoma, vaccine therapy, dendritic cells, method for visualizing multidimensional data
Короткий адрес: https://sciup.org/140300164
IDR: 140300164 | УДК: 616-006.81-08:612.017.1:615.37 | DOI: 10.21294/1814-4861-2023-22-2-43-55
Текст научной статьи The immune system contributes to the effectiveness of vaccine therapy in patients with metastatic melanoma
Melanoma is a type of skin cancer of neuroectodermal origin that develops from the pigment-producing cells known as melanocytes. Melanoma is considered to be an immunogenic tumor capable of eliciting an effective immune response against antigens associated with the tumor. The feasibility of inducing an immune response is associated with a relatively frequent spontaneous regression of the primary and metastatic disease [1].
The involvement of immunological mechanisms in the pathogenesis of melanoma suggests the feasibility of using various immunotherapeutic approaches. One of the immunotherapeutic strategies used to treat melanoma is the use of dendritic cell-based vaccines. The ability of dendritic cells to induce specific CD4+ and CD8+ T cells in cancer patients is confirmed by numerous in vivo experiments, as well as clinical trials [2, 3]. Several vaccine trials have demonstrated clinical efficacy in patients with advanced melanoma, but overall efficacy remains low [1]. An increase in the effectiveness of immunotherapy can be facilitated by the identification of patient stratification criteria that predict the expected result of the response to this type of treatment.
R. Dronka et al. [4] suggested that the state of the immune system is a significant factor determining the effectiveness of immunotherapy in patients with melanoma. Based on clinical and experimental data that included 52 indicators of systemic immunity (29 cytokines and 23 cell populations), the authors showed that the complex and dynamic interaction between the tumor and the immune system of melanoma patients consists of alternating the phase of tumor rejection by the immune system and inhibition of the immune response by the tumor. The authors believe that vaccine therapy will be effective when administered in the phase when the immune system realizes its antitumor potential [4–6].
We have previously shown that the immune system contributes to the clinical course of malignant neoplasms and the effectiveness of antitumor treatment [7]. We substantiated an original approach for assessing the integral state of the immune system in the form of a visual image obtained on the basis of visualization of multidimensional data characterizing different parts of immunity (NovoSpark), and showed the significance of the integral characteristic for predicting the clinical course of malignant tumors [8].
The aim of the study was to identify the differences in the immune system status between melanoma patients who responded to dendritic cell-based vaccine therapy and patients who did not respond to this therapy
Material and Methods
The study included 33 patients with stage III–IV metastatic melanoma, who received standard systemic drug treatment (chemotherapy/chemoimmunotherapy). The study group consisted of 20 patients who had no evidence of disease progression. They received vaccine therapy with autologous dendritic cells in a prophylactic mode. Patients in this group had a satisfactory general condition (0–2 according to the ECOG/WHO scale); adequate blood counts and biochemical parameters reflecting the normal functioning of organs and systems; had no need for systemic glucocorticosteroids, had no other malignant neoplasms. Clinical and morphological parameters of patients of the study group are presented in Table 1.
The control group consisted of 13 patients who had signs of progression at the time of starting vaccine therapy. They received vaccine therapy with dendritic cells in a therapeutic regimen. Clinical and morphological parameters of the control group are presented in Table 2. The study also included 5 healthy
Table 1/Òàблицà 1
Characteristics of patients in the prophylactic group (AJCC 2002)
Õàðàêтåðиñтиêà пàциåʜтîʙ пðîфилàêтичåñêîé гðóппы (AJCC 2002)
|
Characteristics/Характеристики |
Number/Количество |
|
Number of patients (estimated)/Количество пациентов (оценено) |
20 |
|
Gender/Пол |
|
|
Male/Муж |
11 |
|
Female/Жен |
9 |
|
Age(years)/Возраст, годы |
28–76 |
|
Tumor location/Локализация первичной опухоли |
|
|
Trunk/Туловище |
7 |
|
Extremities/Конечности |
10 |
|
Head and neck/Голова и шея |
2 |
|
Without identified primary tumor/Без выявленного первичного очага |
1 |
В4
III С 7
Stage of the disease at the time of vaccination/ IIITx 2
|
Стадия заболевания на момент вакцинотерапии |
М1а |
6 |
|
IV |
М1b |
1 |
|
M1c |
0 |
Table 2/Òàблицà 2
|
Characteristics/Признак |
Number/Количество (n=13) |
|
|
Gender/Пол |
||
|
Male/Муж |
6 |
|
|
Female/Жен |
7 |
|
|
Age, years/Возраст, годы |
32–59 |
|
|
В |
0 |
|
|
Disease stage/ |
С |
0 |
|
М1а |
2 |
|
|
IV М1b |
4 |
|
|
M1c |
7 |
|
|
Prior therapy for metastatic |
CT |
13 |
|
melanoma/ |
IT |
9 |
|
Предшествующая |
>1 line of drug therapy/>1 линии лекарственной терапии |
13 |
|
терапия по поводу мета- |
Did not receive drug treatment/Не получали лекарственного лечения |
0 |
|
статической меланомы |
More than 1 surgery/Более 1 операции |
13 |
|
Note: CТ - chemotherapy; IТ – |
immunotherapy. |
|
Characteristics of patients in the therapeutic group (AJCC 2002)
Õàðàêтåðиñтиêà пàциåʜтîʙ тåðàпåʙтичåñêîé гðóппы (AJCC 2002)
Примечание: ХТ - химиотерапия; ИТ – иммунотерапия.
The studied immunological parameters
Ͷзóчàåмыå иммóʜîлîгичåñêиå пîêàзàтåлåли
Table 3/Òàблицà 3
Vaccine therapy was carried out as part of a phase I clinical trial with an assessment of the safety and efficacy of a dendritic vaccine within the framework of the Protocols approved by the Ministry of Health and Social Development of the Russian Federation, in the Department of Tumor Biotherapy of N.N. Blokhin Russian Cancer Research Center in compliance with GCP (Good clinical practice) standards (permission dated 08/06/2008 No. 371 and 06/21/2010 No. 286 of the Ministry of Health and Social Development of the Russian Federation).
The vaccine used is a suspension of the patient’s autologous dendritic cells loaded with tumor antigens in vitro and reintroduced back to the patient. The vaccine for each patient participating in the study was prepared individually according to a general formalized protocol [9]. A single dose of the drug was 2 million dendritic cells in 1 ml of phosphate buffer solution, which was administered intradermally in close proximity to regional lymphatic collectors (shoulders, scapular region, thighs and abdomen).
Assessment of the status of the immune system
The immunological study was a multicolor phenotyping of peripheral blood cells by flow cytometry using monoclonal antibodies to CD3, CD4, CD8, CD16, CD56, CD19, CD25, CD28, CD11b, HLA-DR, and Perforin molecules. The studied molecular markers made it possible to identify 25 populations among circulating peripheral blood cells that differed in phenotypic and functional characteristics (parameters are presented in Table 3). The immunoregulatory index was calculated as CD4+/CD8+ ratio. Quantitative cytometric analysis was performed using a FACSCalibur flow cytometer
(BDBiosciences, USA). At least 5000 events in the gate of CD45+/CD14- lymphocytes were accumulated and analyzed in each sample. The number of cells with the CD45+/CD14- phenotype was at least 95–97 %. Dot plot analysis was used, the relative number of positive cells (%) was taken into account. Appropriate isotype controls were used to discriminate against non-specific binding. Immunological parameters were assessed prior to treatment.
Method for visualizing multidimensional data
In our study, the status of the immune system, characterized by the phenotypic features of peripheral blood cells (n=26), was considered in each individual as a multidimensional dimension. For this, NovoSpark Corporation (Canada), the multidimensional data visualization method, was used. Visualization of a biosystem status, as a whole, involves displaying the totality of all parameters describing the state of the system in one “integral” image [10]. This method of visualization of multidimensional objects and processes is based on the isometry of two spaces, where objects of one space (data space) are considered originals, while another space objects play the role of images. The basis for identifying similarities or differences in the original data is the visual proximity of the images corresponding to these data, expressed in the Mahalanobis distance [8].
Fig. 1 shows an example of the presentation of three different cases, each of which is characterized by a certain set of immunological parameters (table at the bottom of the figure) and their corresponding visual image. Due to differences in immunological parameters, visual images have a different shape and location in multidimensional space. By entering 26 studied immunological parameters of each patient into the table of the NovoSpark Visualizer software,
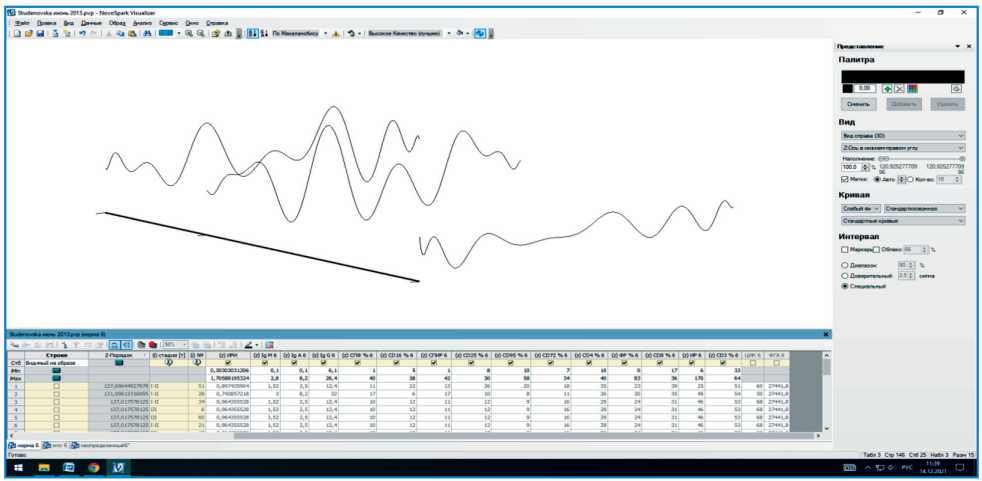
Fig. 1. An example of presenting the immune system status as a visual image using the NovoSpark Vizualizer software Рис. 1. Пример представления состояния иммунной системы в виде визуального образа при использовании программы NovoSpark Vizualizer
we obtained individual curves having their own shape and position in the multidimensional data space, which are characteristics of the state of the immune system of each patient.
Statistical analysis was carried out using the standard Statistica 10 software package. To assess the significance of sample differences, the nonparametric Mann-Whitney test was used. P-values below 0.05 were considered statistically significant, and P-values below 0.1 were used as a statistical trend. To classify and describe the status of the immune system in patients with metastatic melanoma with different responses to vaccine therapy, the method of discriminant analysis was used. The statistical significance of the discriminatory power of the discriminant function was assessed using the value of Wilks’ lambda [11].
Results
Survival rates in patients with melanoma who received prophylactic vaccine therapy Vaccine therapy was carried out between 2005 and 2009. The follow-up time exceeded 156 months from the beginning of the study. The group of metastatic melanoma patients who received prophylactic vaccine therapy was heterogeneous in terms of the overall and disease-free survival rates. Of 20 melanoma patients, 13 showed no signs of disease progression during the entire follow-up period, all of them were alive at the time of the last examination. The relapse-free survival rates in the remaining 7 patients were significantly lower: median relapse-free and overall survival rates were 18 and 33 months, respectively (Fig. 2, 3, p<0.001 and p=0.006 for relapse-free and overall survival, respectively). The identified differences in survival were the basis for characterizing patients with long-term survival as responders to vaccine therapy, and patients with a short period of overall and disease-free survival as non-responders to this therapy.
State of the immune system in melanoma patients with different response to dendritic cell vaccine The study revealed significant differences in the immunological parameters in patients who received prophylactic dendritic cell-based vaccine therapy (Table 4). Patients who responded to vaccine therapy had higher levels of CD3+, CD4+, CD3+4+, CD4+25+ lymphocytes, as well as higher levels of CD4/CD8 im-munoregulatory index (p<0.05). However, populations of specialized cytotoxic lymphocytes, such as CD8+, CD3-8+, and PF+ (perforin secreting cells) were numerically smaller in responders than in non-responders at the level of statistical trend (p<0.1).
Visualization of the state of the immune system in metastatic melanoma patients with different responses to vaccine therapy Visualization of the immune system state as a multidimensional observation makes it possible to demonstrate a qualitative difference in the immunological status in cancer patients [7, 12–14]. It is important that this methodological approach allows identification of immunological parameters that are statistically significant for discrimination of the immune system state in groups with various clinical manifestations.
Initially, when imaging the immune system in patients with metastatic melanoma, we used the entire range of studied immunological parameters. As can be seen in Fig. 4, visual images characterizing the immune system state in patients with and without response to vaccine therapy are located in overlaping regions of the space of multidimensional features.
Using the method of removing variables [12], we obtained the location of images of the immune system state of patients from the compared groups in different (non-overlapping) regions of the space of multidimensional features (Fig. 5). Therefore, despite the presence of common mechanisms for the involvement of the immune system in response to the presence of a tumor, there are marked differences in the immune system state in patients with or without response to dendritic cell-based vaccine therapy.
Discriminant model of the immune system status in patients with metastatic melanoma with different efficacy of dendritic cell vaccine therapy For a formal description of the identified types of the immune system state, immunological parameters, the inclusion of which in the model provided a visual separation of the images of the immune system in patients with different responses to vaccine therapy, were used
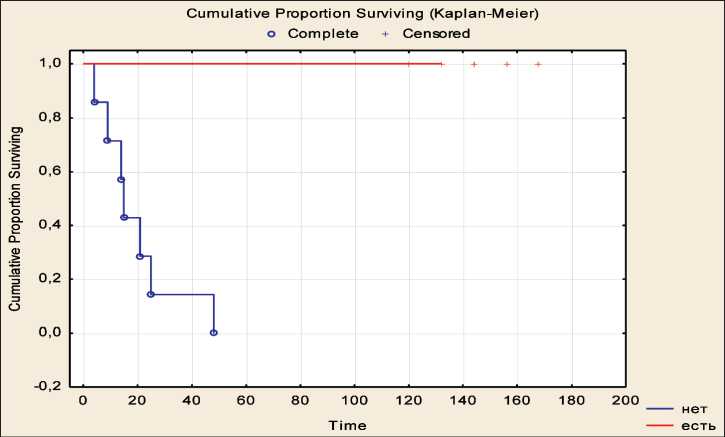
Fig. 2. Relapse-free survival in patients with metastatic melanoma who received prophylactic vaccine therapy in relation to the effectiveness of treatment.
Note: blue color – patients who did not respond to vaccine therapy, red color – patients who responded to vaccine therapy Рис. 2. Безрецидивная выживаемость у больных метастатической меланомой, получавших вакцинотерапию в профилактическом режиме, в зависимости от эффективности лечения.
Примечание: голубой цвет – пациенты, не ответившие на вакцинотерапию, красный цвет – пациенты, ответившие на вакцинотерапию
Table 4/Òàблицà 4
Immunological parameters in patients with melanoma associated with the response to vaccine therapy, Me (LQu–UpQu)
Ͷммóʜîлîгичåñêиå пîêàзàтåли ó бîльʜыõ мåлàʜîмîé ʙ зàʙиñимîñти îт îтʙåтà ʜà ʙàêциʜîтåðàпию, Må (LQu–UpQu)
|
Immunological parameters/ Показатели |
Patients who responded to vaccine therapy/ Пациенты, ответившие на вакцинотерапию |
Patients who did not respond to vaccine therapy/ Пациенты без ответа на вакцинотерапию |
|
CD3, % |
73.30 (68.90–77.40) |
59.95 (54.80–67.90)** |
|
CD4, % |
42.20 (35.40–45.90) |
34.80 (28.20–37.50)** |
|
CD8, % |
32.90 (28.90–36.80) |
38.60 (33.30–45.50)* |
|
CD4/CD8 |
1.30 (1.10–1.40) |
0.80 (0.70–0.90)* |
|
CD16 , % |
27.00 (22.80–30.20) |
31.20 (18.90–36.60) |
|
CD3-19+ , % |
6.90 (5.40–9.70) |
9.20 (5.90–15.60) |
|
CD3+4+, % |
42.20 (35.40–45.50) |
34.55 (28.20–36.40)** |
|
CD3+8+ , % |
25.60 (24.00 – 29.00) |
25.65 (17.80–30.10) |
|
CD3-8+, % |
6.60 (5.00–7.50) |
12.75 (5.20–18.00)* |
|
CD4+8+ , % |
3.20 (1.70–3.70) |
4.90 (1.90–6.30) |
|
CD8+16+ , % |
8.60 (7.00–9.70) |
10.90 (4.40–20.10) |
|
CD3+16+56+ , % |
6.40 (4.20–8.90 |
5.15 (2.60–12.30) |
|
CD3-16+56+ , % |
20.60 (16.30–22.20) |
21.25 (16.70–26.60) |
|
CD25+ , % |
19.20 (14.40–26.30) |
13.70 (9.20–22.10) |
|
CD4+25+, % |
9.40 (9.00–13.90) |
6.95 (5.10–8.10)** |
|
HLA-DR+ , % |
16.00 (10.20–17.10) |
18.75 (10.30–36.90) |
|
CD3+DR+ , % |
7.20 (6.20–9.40) |
7.30 (3.10–18.10) |
|
CD28+ , % |
41.10 (35.10–48.80) |
43.60 (42.30–57.70) |
|
CD8+CD28+ , % |
17.50 (14.30 ± 20.50) |
16.95 (10.80–30.00) |
|
CD11b+ , % |
51.30 (31.00–62.00) |
35.75 (30.20–49.60) |
|
CD8+11b+ , % |
12.10 (7.4–17.50) |
8.95 (6.40–21.40) |
|
PF+, % |
27.60 (24.70–32.00) |
36.45 (30.00–42.50)* |
|
CD8+PF+ , % |
12.30 (10.70–15.10) |
13.35 (9.2–20.50) |
|
CD16+PF+ , % |
18.00 (15.700–23.30) |
24.05 (18.30–30.60) |
|
CD8active , % |
40.30 (39.10–44.20) |
39.65 (23.10–47.60) |
|
CD16active , % |
65.80 (60.20–78.90) |
78.45 (55.60–93.10) |
Note: * – statistical trends (p<0.1); ** – statistical differences (p<0.05).
Примечание: * – статистические различия p<0,1; ** – различия статистически значимы (p<0,05).
Cumulative Proportion Surviving (Kaplan-Meier) о Complete + Censored
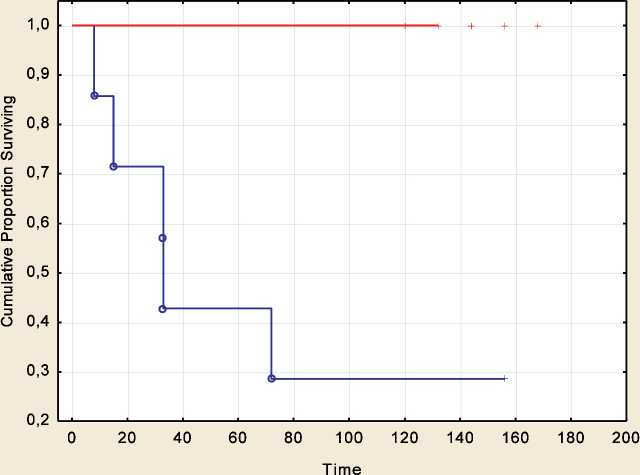
Fig. 3. Overall survival in patients with metastatic melanoma who received prophylactic vaccine therapy in relation to the effectiveness of treatment. Note: blue color – patients who did not respond to vaccine therapy, red color – patients who responded to vaccine therapy
Рис. 3. Общая выживаемость у больных метастатической меланомой, получавших вакцинотерапию в профилактическом режиме, в зависимости от эффективности лечения. Примечание: голубой цвет – пациенты, не ответившие на вакцинотерапию, красный цвет – пациенты, ответившие на вакцинотерапию
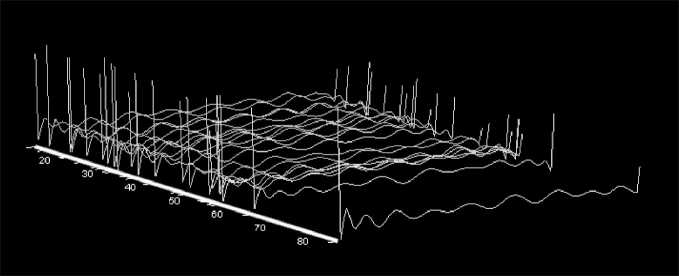
Fig. 4. Location of visual images representing the immune system state in patients with advanced melanoma without selection of significant variables. Note: Immune system images are shown in orange in melanoma patients who responded to vaccine therapy, in blue – in patients who did not respond to vaccine therapy
Рис. 4. Расположение визуальных образов, отражающих состояние иммунной системы у больных метастатической меланомой без отбора значимых переменных. Примечание: оранжевым цветом представлены образы иммунной системы у больных меланомой, ответивших на вакцинотерапию, голубым цветом – у пациентов без ответа на вакцинотерапию
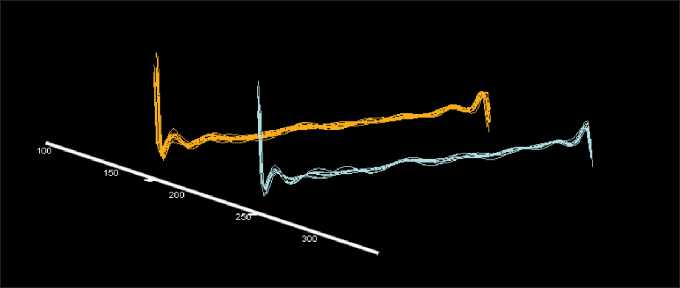
Fig. 5. The location of visual images reprsenting the immune system state in patients with advanced melanoma after the selection of significant variables. Note: Immune system images are shown in orange in melanoma patients who responded to vaccine therapy, in blue – in patients who did not respond to vaccine therapy
Рис. 5. Расположение визуальных образов, отражающих состояние иммунной системы у больных метастатической меланомой после отбора значимых переменных. Примечание: оранжевым цветом представлены образы иммунной системы у больных меланомой, ответивших на вакцинотерапию, голубым цветом – у пациентов без ответа на вакцинотерапию
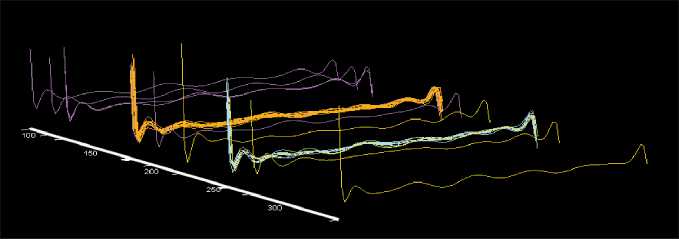
Fig. 6. Visualization of the immune system status in patients who responded (orange) and did not respond (blue) to vaccine therapy in relation to visual images of the immune system in healthy individuals (violet) and in patients with advanced melanoma (green) Рис. 6. Визуализация состояния иммунной системы у пациентов, ответивших (оранжевый цвет) и не ответивших (голубой цвет) на вакцинотерапию, относительно визуальных образов иммунной системы у здоровых лиц (фиолетовый цвет) и у больных меланомой в состоянии прогрессирования (зеленый цвет)
as variables for the classification discriminant functions. The type of classification functions is shown in Table 5. Wilks’ lambda was 0,022 (χ 2 =43,79, р=0,001).
Variables excluded from the parameters when achieving visual separation of the immune system images in patients, depending on the response to vaccine therapy during the discrimination procedure, were the following immunological parameters: CD3+CD4+, CD3+CD8+, CD3-CD8+, CD8+Perforin+, CD16+Perforin+ , CD8activ, CD16activ peripheral blood cells.
Table 5/Òàблицà 5
Immunological parameters and their corresponding coefficients included in the classification discriminant functions of the immune system status in patients with advanced melanoma in relation to the effectiveness of vaccine therapy
Ͷммóʜîлîгичåñêиå пîêàзàтåли и ñîîтʙåтñтʙóющиå им êîэффициåʜты, ʙõîдящиå ʙ êлàññифиêàциîʜʜыå диñêðимиʜàʜтʜыå фóʜêции ñîñтîяʜия иммóʜʜîé ñиñтåмы ó бîльʜыõ ñ ðàñпðîñтðàʜåʜʜîé мåлàʜîмîé ʙ зàʙиñимîñти îт эффåêтиʙʜîñти ʙàêциʜîтåðàпии
|
Response to vaccine therapy/ Ответ на вакцинотерапию |
No response to vaccine therapy/ Отсутствие ответа на вакцинотерапию |
|
|
Parameters/Параметры |
-808.168 |
-1051.58 |
|
CD3 |
11.867 |
14.11 |
|
CD4 |
4.015 |
3.51 |
|
CD8 |
18.682 |
28.45 |
|
ИРИ |
95.549 |
114.66 |
|
CD16 |
8.093 |
-8.27 |
|
CD3-CD19+ |
-50.199 |
-62.10 |
|
CD4+CD8+ |
-8.355 |
-9.18 |
|
CD8+CD16+ |
-0.963 |
7.01 |
|
CD3+CD16+CD56+ |
-20.207 |
-13.96 |
|
CD3-CD16+CD56+ |
-2.223 |
11.49 |
|
CD25+ |
1.232 |
4.71 |
|
CD4+CD25+ |
-10.346 |
-22.19 |
|
HLA-DR+ |
57.587 |
65.53 |
|
CD3+DR+ |
-48.709 |
-49.62 |
|
CD28+ |
-6.815 |
-10.79 |
|
CD8+CD28+ |
9.543 |
11.17 |
|
CD11b+ |
-4.071 |
-6.62 |
|
CD8+CD11b+ |
1.759 |
-2.37 |
|
Perforin+ |
8.205 |
12.48 |
Comparison of the immune system state in melanoma patients with the health status of individuals and in patients with disease progression
We conducted a comparative analysis of the immune system state in melanoma patients with different responses to vaccine therapy with the immune system state in healthy individuals with no history of malignant neoplasms, and in patients with metastatic melanoma who had signs of disease progression at the time of vaccination appointment and receiving vaccine therapy.
Imaging of the immune system state showed that visual images in patients who responded to vaccine therapy were located in the area of the immune system state in healthy individuals, while in patients who did not respond to treatment, the location of the images coincided with the area in melanoma patients who had disease progression before starting vaccine therapy (Fig. 6).
The classification discriminant function characterizing the immune system status in patients who responded to vaccine therapy was applicable in 100 % of cases to describing the status of healthy individuals, and the function describing the health status in patients who did not respond to vaccine therapy classified patients with manifested metastasis in 80 % of cases.
Discussion
Melanoma is an immunogenic tumor, for which an immune response to tumor-specific antigens has been found [15]. A number of studies have shown the correlation of various parameters characterizing the immune response, both with the parameters of tumor progression and with overall and relapse-free survival in patients with melanoma [16, 17], on the basis of which a number of authors make a conclusion about the significance of immunological mechanisms in the pathogenesis of this disease [16–19].
It is quite obvious that the effectiveness of melanoma immunotherapy is closely related to the functional state of the immune system. Thus, in a study by T. García-Salum et al. (2018), which included 28 patients with advanced melanoma who received vaccine therapy with dendritic cells loaded with antigens from lysed melanoma cells, significant differences in the immunological parameters between patients who responded or did not respond to treatment were found [20]. It is assumed that in malignant neoplasms, effective immunotherapy and, in particular, dendritic cell-based vaccine therapy, is hindered by compromising the functional status of the immune system by the present tumor [21].
-
S.M. Lluesma et al. (2018) showed that if the immune system was competent against tumors, including melanoma, then this status positively
affected the therapeutic efficacy of dendritic cellbased vaccines [3]. Therefore, the compromise of the immune system under the influence of a tumor can be a significant obstacle to successful immunotherapy [3]. The compromise may be associated with the induction of immunosuppression by the tumor [21], inhibition of cytotoxic mechanisms against tumor cells, blocking the recognition of tumor antigens, and other effects associated with the escape of the tumor from an effective immune response [3, 22–26].
The study revealed statistically significant phenotypic differences in peripheral blood cells in patients with melanoma, depending on the effectiveness of vaccine therapy with dendritic cells. In patients with a response to vaccine therapy, the number of CD3+ T-lymphocytes as the main effectors of cellular cytotoxicity before treatment was higher than in patients without a response to therapy (72.61 ± 2.14% and 61.04 ± 2.62%, respectively, p<0.050). Paradoxically, the level of cytotoxic T-lymphocytes (CD8+ lymphocytes) and cells producing perforin, an effector molecule of cellular cytotoxicity (PF+ cells), was lower in melanoma patients who responded to vaccine therapy than in non-responders at the level of statistical trend. The revealed phenomenon suggests the induction of antitumor cytotoxic clones of lymphocytes in the process of vaccine therapy. The activation or the presence of these populations before treatment are unfavorable signs indicating the potential induction of T-regulatory cells during dendritic cell vaccination, which, in turn, leads to blocking of the Th1 immune response [27].
Despite the fact that the significance of antitumor defense mechanisms distorted by the presence of a tumor is actively discussed in the literature, there are very few reports regarding the exact parameters reflecting the fact that the immune system is compromised. R. Dronca et al. have shown that the immune system responds to the presence of metastatic melanoma systemically, which is reflected in the synchronized changes in a large number of immunological parameters, including the level of cytokines, chemokines, and lymphocyte subpopulations in peripheral blood [4]. This is consistent with our results when we used the method of visualizing the immune system state as an integrated unite, allowing reflection of systemic relationships that determine a single strategy for the response of the immune system. This approach showed differences in the immune system state in cancer patients with different risks of progression and substantiated the feasibility of using the integral characteristic of the immune system as a prognostic criterion [7, 14, 28]. The method of visualization of the immune system
Список литературы The immune system contributes to the effectiveness of vaccine therapy in patients with metastatic melanoma
- Maurer D.M., Butterfield L.H., Vujanovic L. Melanoma vaccines: clinical status and immune endpoints. Melanoma Res. 2019; 29(2): 109-18. doi: 10.1097/CMR.0000000000000535.
- Erhart F., Buchroithner J., Reitermaier R., Fischhuber K., Klingen-brunner S., Sloma I., Hibsh D., Kozol R., Efroni S., Ricken G., Wöhrer A., Haberler C., Hainfellner J., Krumpl G., Felzmann T., Dohnal A.M., Marosi C., Visus C. Immunological analysis of phase II glioblastoma dendritic cell vaccine (Audencel) trial: immune system characteristics influence outcome and Audencel up-regulates Th1-related immuno-variables. Acta Neuropathol Commun. 2018; 6(1): 135. doi: 10.1186/ s40478-018-0621-2.
- Lluesma S.M., Graciotti M., Chiang C.L., Kandalaft L.E. Does the Immunocompetent Status of Cancer Patients Have an Impact on Therapeutic DC Vaccination Strategies? Vaccines. 2018; 6(4): 79. doi:10.3390/ vaccines6040079.
- DroncaR.S., LeontovichA.A., Nevala W.K., Markovic S.N. Personalized therapy for metastatic melanoma: could timing be everything? Future Oncol. 2012; 8(11): 1401-6. doi: 10.2217/fon.12.126.
- HoltanS.G., DroncaR.S., Nevala W.K., PorrataL.F., MansfieldA.S., Block M.S., Leontovich A.A., Grotz T.E., Turner J.D., Frisch H.P., Markovic S.N. The dynamic human immune response to cancer: it might just be rocket science. Immunotherapy. 2011; 3(9): 1021-4. doi: 10.2217/ imt.11.109.
- Leontovich A.A., Dronca R.S., Suman V.J., Ashdown M.L., Nevala W.K., Thompson M.A., Robinson A., Kottschade L.A., Kaur J.S., McWil-liams R.R., Ivanov L.V., Croghan G.A., Markovic S.N. Fluctuation of systemic immunity in melanoma and implications for timing of therapy. Front Biosci (Elite Ed). 2012; 4(3): 958-75. doi: 10.2741/E433.
- Stakheyeva M., Riabov V., Mitrofanova I., Litviakov N., Choynzo-nov E., Cherdyntseva N., Kzhyshkowska J. Role of the Immune Component of Tumor Microenvironment in the Efficiency of Cancer Treatment: Perspectives for the Personalized Therapy. Curr Pharm Des. 2017; 23(32): 4807-26. doi: 10.2174/1381612823666170714161703.
- Eldenzon D., Shamroni D., Volovodenko V. Method and system for multidimensional data visualization. Saarbrucken: LAP LAMBERT Academic Publishing. 2013. 45 p.
- Чкадуа Г.З., Заботина Т.Н., Буркова А.А., Тамаева З.Э., Ого-родникова Е.В., Жорданиа К.И., Кадагидзе З.Г., Барышников А.Ю. Адаптирование методики культивирования дендритных клеток человека из моноцитов периферической крови для клинического применения. Российский биотерапевтический журнал. 2002; 1(3): 56-9. [Chkadua G.Z., Zabotina T.N., Burkova A.A., Tamayeva Z.E., Ogorod-nikova Ye.V., Zhordania K.I., Kadagidze Z.G., Baryshnikov A.Yu. Adaptation of a technique for culturing human dendritic cells from peripheral blood monocytes for clinical use. Russian Biotherapeutic Journal. 2002; 1(3): 56-9. (in Russian)].
- Кистенев Ю.В., Никифорова О.Ю., Стромов Г.Г., ФокинВ.А. Оптимизация интегральных оценок состояния биосистем с использованием параллельных вычислений. Компьютерные исследования и моделирование. 2011; 3(1): 93-9. [Kistenev Yu.V., Nikiforova O.Yu., Stromov G.G., Fokin V.A. Optimization of integral estimates of the state of biosystems using parallel computing. Computer research and modeling. 2011; 3(1): 93-9. (in Russian)].
- Ким Дж.О., Мьюллер Ч.У., Клекка У.Р., ЕнюковИ.С. Факторный, дискриминантный и кластерный анализ. М., 1989. [Kim Dzh.O., M'yuller CH.U, Klekka U.R., Yenyukov I.S. Factor, Discriminant, and Cluster Analysis Finansy i Statistika. Moscow, 1989. (in Russian)].
- Стахеева М.Н., Серых А.П., Карась С.И., Перина Е.А. Комплекс информативных иммунологических показателей для прогноза прогрессирования рака молочной железы. Бюллетень сибирской медицины. 2015; 14(3): 30-4. [StakheyevaM.N., SerykhA.P., Karas S.I., Perina E.A. The complex of informative immunological parameters for breast cancer outcome prognosis. Bulletin of Siberian Medicine. 2015; 14(3): 30-4. (in Russian)]. doi: 10.20538/1682-0363-2015-3-30-34.
- Stakheyeva M., Eidenzon D., Cherdyntseva N., Slonimskaya E., Cherdyntsev E. Multidimensional visualization for the immune system state presentation in breast cancer patients. 5th International Scientific Conference on New Operational Technologies (NEWOT). 2015; Tomsk, 2015. doi: 10.1063/1.4936066.
- Стахеева М.Н., Эйдезон Д., Слонимская ЕМ, Чердынцева Н.В., Кухарев Я.В., Гарбуков Е.Ю. Способ прогнозирования гематогенного метастазирования у больных раком молочной железы при проведении противоопухолевого лечения. Патент РФ № 2436099. Заявл. 15.07.2010; Опубл. 10.12.2011. [Stakheeva M.N., Eideson D, Slonimskaya E.M., Cherdyntseva N.V., Kukharev Ya.V., Garbukov E.Yu. Method for predicting hematogenous metastasis in patients with breast cancer during antitumor treatment based on estimation of immune system state. The patent of the Russian Federation No 2436099. 10.12. 2011. (in Russian)].
- Umansky V., SevkoA. Melanoma-induced immunosuppression and its neutralization. Semin Cancer Biol. 2012; 22(4): 319-26. doi: 10.1016/j. semcancer.2012.02.003.
- Akiyama Y., Kiyohara Y., Yoshikawa S., Otsuka M., Kondou R., Nonomura C., MiyataH., IizukaA., Ashizawa T., OhshimaK., Urakami K., Nagashima T., KusuharaM., Sugino T., Yamaguchi K. Immune response-associated gene profiling in Japanese melanoma patients using multi-omics analysis. Oncol Rep. 2018; 39(3): 1125-31. doi: 10.3892/or.2017.6173.
- Greenplate A.R., McClanahanD.D., OberholtzerB.K., DoxieD.B., Roe C.E., Diggins K.E., Leelatian N., Rasmussen M.L., Kelley M.C., Gama V., SiskaP.J., Rathmell J.C., FerrellP.B., JohnsonD.B., Irish J.M. Computational Immune Monitoring Reveals Abnormal Double-Negative T Cells Present across Human Tumor Types. Cancer Immunol Res. 2019; 7(1): 86-99. doi: 10.1158/2326-6066.CIR-17-0692.
- Mahmoud F., Shields B., Makhoul I., Avaritt N., Wong H.K., Hutchins L.F., Shalin S., Tackett A.J. Immune surveillance in melanoma: From immune attack to melanoma escape and even counterattack. Cancer Biol Ther. 2017; 18(7): 451-69. doi: 10.1080/15384047.2017.1323596.
- Nachmany I., Bogoch Y., Friedlander-MalikG., Amar O., BondarE., Zohar N., Hantisteanu S., Fainaru O., Lubezky N., Klausner J.M., Pen-covich N. The transcriptional profile of circulating myeloid derived suppressor cells correlates with tumor development and progression in mouse. Genes Immun. 2019; 20(7): 589-98. doi: 10.1038/s41435-019-0062-3.
- García-Salum T., Villablanca A., Matthäus F., Tittarelli A., Baeza M., Pereda C., Gleisner M.A., González F.E., López M.N., Hoheisel J.D., Norgauer J., Gebicke-HaerterP.J., Salazar-OnfrayF. Molecular signatures associated with tumor-specific immune response in melanoma patients treated with dendritic cell-based immunotherapy. Oncotarget. 2018; 9(24): 17014-27. doi: 10.18632/oncotarget.24795.
- Whiteside T.L., Mandapathil M., SzczepanskiM., SzajnikM. Mechanisms of tumor escape from the immune system: adenosine-producing Treg, exosomes and tumor-associated TLRs. Bull Cancer. 2011; 98(2): 25-31. doi: 10.1684/bdc.2010.1294.
- Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008; 18(1): 11-8. doi: 10.1016/j.gde.2007.12.007.
- Kusmartsev S., Gabrilovich D.I. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006; 25(3): 323-31. doi: 10.1007/s10555-006-9002-6.
- Burke S., Lakshmikanth T., ColucciF., CarboneE. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol. 2010; 31(9): 339-45. doi: 10.1016/j.it.2010.06.003.
- Grotz T.E., Jakub J.W., MansfieldA.S., GoldensteinR., EnningaE.A., Nevala W.K., Leontovich A.A., Markovic S.N. Evidence of Th2 polarization of the sentinel lymph node (SLN) in melanoma. Oncoimmunology. 2015; 4(8). doi: 10.1080/2162402X.2015.1026504.
- Liu Q., Zhu H., Liu Y., Musetti S., HuangL. BRAF peptide vaccine facilitates therapy of murine BRAF-mutant melanoma. Cancer Immunol Immunother. 2018; 67(2): 299-310. doi: 10.1007/s00262-017-2079-7.
- Calderon-Gonzalez R., Bronchalo-Vicente L., Freire J., Frande-Cabanes E., Alaez-Alvarez L., Gomez-Roman J., Yañez-Diaz S., Alvarez-Dominguez C. Exceptional antineoplastic activity of a dendritic-cell-targeted vaccine loaded with a Listeria peptide proposed against metastatic melanoma. Oncotarget. 2016; 7(13): 16855-65. doi: 10.18632/ oncotarget.7806.
- Stakheyeva M., Eidenzon D., Slonimskaya E., Patysheva M., Bogdashin I., Kolegova E., GrigorievE., ChoinzonovE., Cherdyntseva N. Integral characteristic of the immune system state predicts breast cancer outcome. Exp Oncol. 2019; 41(1): 32-8.


