The Influence Manganese on the Kinetics of Oxidation of the Zn5Al and Zn22Al Alloys
Автор: Rahimov F.A., Obidov Z.R.
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Рубрика: Исследования. Проектирование. Опыт эксплуатации
Статья в выпуске: 5 т.18, 2025 года.
Бесплатный доступ
The article presents the results of thermogravimetric studies on the influence of alloying additions of manganese on the oxidation kinetics of zinc-aluminium alloys Zn5Al and Zn22Al. The values of the true oxidation rate of the zinc-aluminium alloys Zn5Al and Zn22Al, alloyed with manganese, calculated from the kinetic curves, are given as a function of temperature and the composition of the studied alloys. It is observed that small additions of manganese (0.01–0.05 wt.%) significantly reduce the true oxidation rate of the zinc-aluminium alloys Zn5Al and Zn22Al, while simultaneously increasing the apparent activation energy of this process. Larger additions of manganese (0.1 and 0.5 wt.%) also reduce the oxidability of the base alloy.
Zn5Al and Zn22Al alloys, manganese, thermogravimetrical method, oxidation rate, activation energy
Короткий адрес: https://sciup.org/146283149
IDR: 146283149 | УДК: 669.76+542.943
Текст научной статьи The Influence Manganese on the Kinetics of Oxidation of the Zn5Al and Zn22Al Alloys
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License (CC BY-NC 4.0).
Влияние марганца на кинетику окисления сплавовZn5Al и Zn22Al
Ф. А. Рахимов, З. Р. Обидов
Центр по исследованию инновационных технологий Национальной академии наук Таджикистана Таджикистан, Душанбе
Цитирование: Рахимов Ф. А. Влияние марганца на кинетику окисления сплавов Zn5Al и Zn22Al / Ф. А. Рахимов, З. Р. Обидов // Журн. Сиб. федер. ун-та. Техника и технологии, 2025, 18(5). С. 606–612. EDN: ECUJCU platinum-platinum rhodium thermocouple was used, with its hot end touching the surface of the investigated alloy. The thermocouple was placed in a sheath made of aluminum oxide. To maintain the specified temperature with an accuracy of ±2 °C, the furnace load was regulated by a thyristor. A PP-63 potentiometer was used to register the temperature [26–28]. After the experiment, the system was cooled, and the reactive surface was determined by weighing the crucible with its contents. Then, the oxide film formed on the sample’s surface was removed, and its structure was studied using X-ray phase analysis [29].
Results and discussion
Thermogravimetric studies of the oxidation kinetics of Zn5Al and Zn22Al – Mn alloys were carried out at temperatures of 523, 573 and 623 K. The interaction of the Zn5Al and Zn22Al alloy with various concentrations of manganese with oxygen in the gas phase at the temperatures is significantly different from the oxidation of the initial Zn5Al and Zn22Al alloy. The linear dependence is maintained for 12–15 min, further, as the oxide film is formed, the nature of the oxidation process becomes hyperbolic and the formation of the protective oxide surface ends by 30 minutes. Doping of the Zn5Al and Zn22Al alloy with manganese (in the range of 0.01–0.5 wt%) contributes to a certain decrease in the true oxidation rate (Fig. 1, 2). A significant effect on the oxidizability of alloys is their chemical composition. Among the alloys, the Zn5Al and Zn22Al alloy with 0.01 wt% manganese has a minimum oxidation rate and maximum activation energy of 160.5 kJ/mol Zn5Al and 147.5 kJ/mol Zn22Al alloy (Table 1).
Doping manganese from 0.1 to 0.5 wt% to the alloy is impractical, since it leads to an increase in the oxidation rate and, accordingly, decreases the activation energy of oxidation of alloys. The effective activation energy of the oxidation process of these alloys varies from 128.4 to 125.2 and 122.5 kJ / mol Zn5Al and from 151.2 to 141.3 and 137.1 kJ / mol Zn22Al (Table 1).
When alloying the base alloys Zn5Al and Zn22Al with manganese from 0.01 to 0.05 wt.%, the oxidation rate decreases. However, an increase in the specific weight of the samples is observed after raising the temperature. Comparing the zinc alloys Zn5Al and Zn22Al with manganese-alloyed alloys, it can be noted that both the unalloyed alloy and the one alloyed with the third component have the highest value of effective activation energy and a slightly lower true oxidation rate (Table 2).
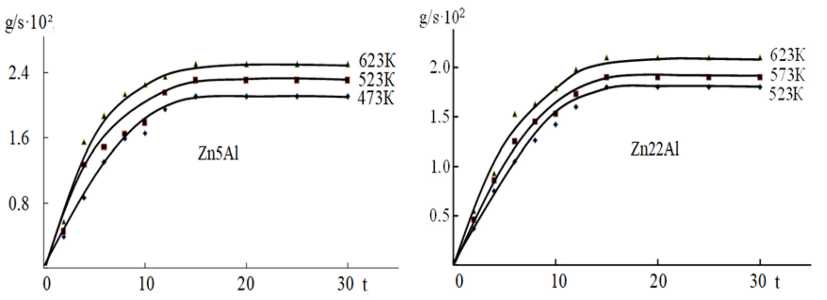
Fig. 1. Kinetic curves of the oxidation process of zinc Zn5Al and Zn22Al alloys
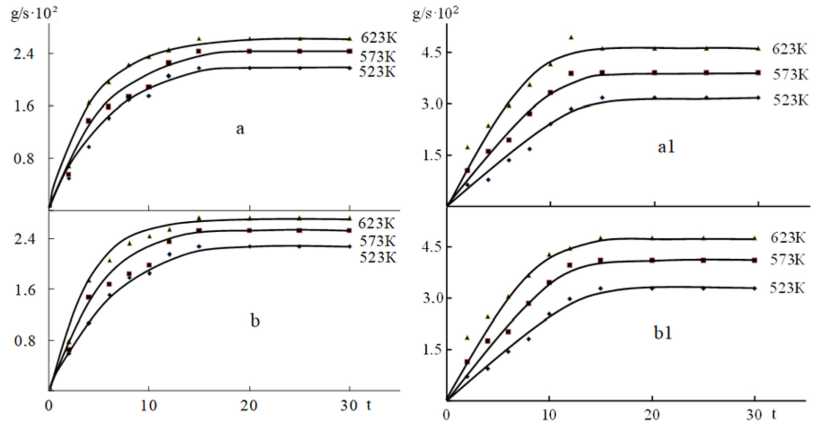
Fig. 2. Kinetic curves of the oxidation process of zinc alloys containing manganese, wt.%: 0.1 (a, a1); 0.5 (b, b1)
Table 1. Kinetic and energetic parameters of the oxidation process of zinc alloys Zn5Al and Zn22Al, alloyed with manganese, in the solid state
|
Content Mn in the alloy, wt% |
Temperature of oxidation, К |
The true oxidation rate K·104, kg/m2·s |
Effective activation energy, kJ/mol |
||
|
Zn5Al и Zn22Al |
Zn5Al |
Zn22Al |
Zn5Al |
Zn22Al |
|
|
- |
523 |
3.07 |
3.00 |
128.4 |
151.2 |
|
573 |
3.55 |
3.44 |
|||
|
623 |
3.91 |
3.79 |
|||
|
0.01 |
523 |
2.90 |
4.36 |
160.5 |
147.5 |
|
573 |
2.98 |
4.68 |
|||
|
623 |
3.06 |
5.15 |
|||
|
0.05 |
523 |
2.99 |
4.42 |
158.0 |
145.0 |
|
573 |
3.05 |
4.75 |
|||
|
623 |
3.14 |
5.21 |
|||
|
0.1 |
523 |
3.25 |
4.65 |
125.2 |
141.3 |
|
573 |
3.66 |
4.98 |
|||
|
623 |
3.07 |
5.44 |
|||
|
0.5 |
523 |
3.55 |
4.88 |
122.5 |
137.1 |
|
573 |
3.91 |
5.21 |
|||
|
623 |
2.90 |
5.67 |
|||
The oxidation products of the studied alloys were analyzed using X-ray phase analysis on the DRON-3.0 instrument. For example, the X-ray diffraction patterns of the products formed on the surface of the Zn22Al alloys with 0.5 % manganese during oxidation show the formation of the following protective oxides: ZnO, Al 2 O 3 , and Mn 2 O 3 (Fig. 3).
Table 2. Quadratic polynomials of the kinetic curves of the oxidation of zinc alloys Zn5Al and Zn22Al, alloyed with manganese, in the solid state
|
Manganese content in the alloy, wt.%. |
Oxidation temperature, K |
Quadratic polynomials of the kinetic oxidation curves of the alloys |
Regression coefficient, R |
|
Zn5Al |
|||
|
523 |
y = –2E – 06x4 + 0.000x3–0.017x2 + 0.337x |
0.993 |
|
|
- |
573 |
y = –3E – 06x4+ 0.000x3–0.022x2 + 0.371x |
0.989 |
|
623 |
y = –2E – 05x4+ 0.001x3–0.050x2 + 0.618x |
0.995 |
|
|
523 |
y = –2E – 05x4–0.004х3 + 0.053х2 + 0.235х |
0.991 |
|
|
0.5 |
573 |
y = –3E – 05x4–0.002х3–0.025х2 + 0.391х |
0.986 |
|
623 |
y = –2E – 05x4+ 0.002х3–0.089х2 + 0.402х |
0.994 |
|
|
Zn22Al |
|||
|
523 |
y = 0.002x4 + 0.005x3–0.066x2 + 0.334x |
0.987 |
|
|
- |
573 |
y = 0.002x4+ 0.003x3–0.053x2 + 0.369x |
0.990 |
|
623 |
y = 0.001x4+ 0.002x3–0.069x2 + 0.615x |
0.993 |
|
|
523 |
y = 0.002x4+ 0.004х3–0.048х2 + 0.291х |
0.986 |
|
|
0.5 |
573 |
y = 0.002x4+ 0.003х3–0.066х2 + 0.303х |
0.989 |
|
623 |
y = 0.001x4+ 0.002х3–0.073х2 + 0.311х |
0.994 |
|
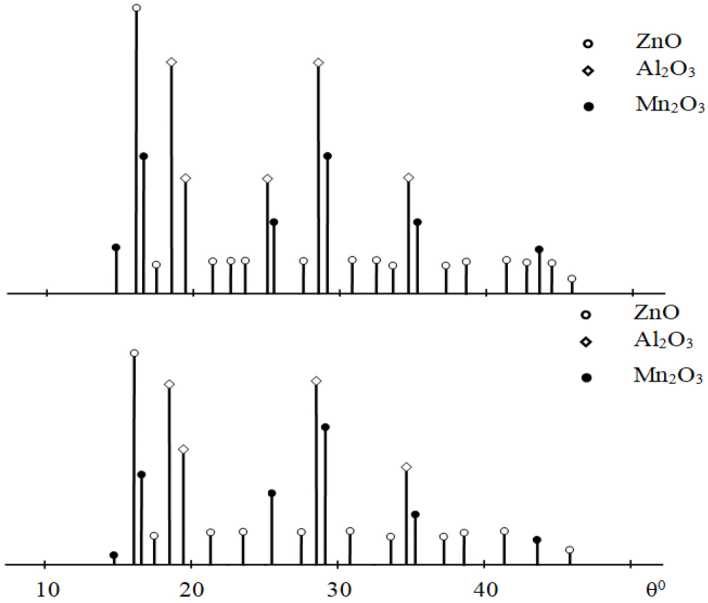
Fig. 3. X-ray diffraction patterns of the oxidation products of the Zn22Al-0.01Mn
Conclusions
As a result of the experimental study, a hyperbolic mechanism for the oxidation of Zn5Al and Zn22Al-Mn alloys was established. The kinetic and diffusion regimes of the oxidation of the alloys by atmospheric oxygen were identified. The possibility of increasing the anodic resistance of Zn5Al and Zn22Al alloys to oxidation by doping with manganese (0.01–1.0 %) was demonstrated.


