The influence of duration of high-temperature exposure on the properties of carbon nitride obtained in molten salts
Автор: Golovin M.S., Trotsenko D.I., Morozov R.S.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Физическая химия
Статья в выпуске: 2 т.13, 2021 года.
Бесплатный доступ
In the present work the influence of duration of thermal treatment on the properties and photocatalytic performance of carbon nitride materials is investigated. Preparation of photocatalytic material proceeded in the eutectic molten salts KCl/LiCl mixture. This way of thermal treatment results in crystalline material with ordered structure, improved in comparison to convenient preparation methods. Duration was altered in range from 2 to 10 hours. Materials were studied by the methods of X-ray diffraction patterns, scanning electron microscopy. It was found that most ordered materials with the maximal degree of crystallinity are formed after 2-6 hours of high-temperature treatment. Elongation of thermal treatment up to 8 and 10 hours leads to less ordered material with the enhanced share o amorphous phase. Carbon nitride materials were used as photocatalysts for the selective oxidation of benzyl alcohol to benzaldehyde and demonstrate high selectivities to target product. 2 hours of thermal treatment leads to formation of photocatalyst with the highest conversion (79.2 %) and selectivity (92.4 %) values. Less effective material is formed after 10 hours of treatment, where selecivity level retained, while conversion drops to 48.6 %. Morphology of materials has maximal effect on the photocatalytic properties - high crystallinity is the main feature of catalytically effective materials.
Carbon nitride, hydrothermal, photocatalysis
Короткий адрес: https://sciup.org/147234261
IDR: 147234261 | УДК: 544.723 | DOI: 10.14529/chem210210
Текст научной статьи The influence of duration of high-temperature exposure on the properties of carbon nitride obtained in molten salts
Graphtic carbon nitride (GCN) is one of the most stable carbon nitride polymorph. It has semiconductor properties, which allows to apply it as photocatalyst for organic matter degradation [1–3], synthetic reactions [4], carbon dioxide reduction [5], nitrogen fixation reactions [6], hydrogen evolution [7–9]. One of most frequently studied applications is wastewaters treatment where organic contaminants are being degraded by highly active hydroxyl radicals. Meanwhile, more sophisticated utilization may be performed in non-aqueous solutions, where formation of hydroxyl radicals is restricted. It is possible to selectively oxidize organic compounds or keep C-C bonds formation in mild, non-oxidative conditions [10].
Convenient ways of carbon nitride preparation by the means of powders calcinations are known to result weakly ordered materials [11]. Through this procedure linear heptazine polymer forms, which are connected to each other by hydrogen bonds [4]. This material has high hydrogen amount of about 2 % – this fact points to the incomplete condensation of functional groups. Low extent of carbon nitride polymerization leads to incomplete formation of conjugated electronic structure. Carbon nitride, prepared through this way represented by particles of irregular shape with significant share of amorphous phase– this lowers its photocatalytic efficiency due to hindered charge transport, easiness of charges recombination [12].
The efficiency of C3N4 photocatalytic application may be enhanced via the improvement or its structure [13]. Highly ordered material facilitatates charges migration and their accessibility to substrates [14]. High degree if charge migration is the result of π orbital conjugation, therefore highly ordered structure is necessary [15]. Ordered structure may be the result of templates application, but nevertheless that templates needed to be excluded from the structure of material after synthesis – this needs additional chemicals etc. [16], therefore template-free procedure is desired. Previously developed alternative route for improvement of carbon nitride crystallinity include ionothermal synthetic process, preformed through 2 steps of thermal treatment in molten salts with the subsequent heating in the sealed ampule. It allows to get uniform nanoparticles of C 3 N 4 semiconductor [17].
Additional way to improve carbon nitride performance is to dope it. Doping include: formation of nitrogen vacancies [18], carbon vacancies [19], doping with metal- or non-metal heteroatom [20], copolymerization with different monomers [21], molecular doping [22]. One of recently developed way is doping with cyano groups [23]. Cyano group boosts visible light absorption, hinders charge recombination and makes process of exfoliation easier due to the enhancement of distance between single layers of carbon nitride [24].
In this work highly crystalline carbon nitride, doped with cyano groups, was prepared via 1-step process in molten eutectic KCl/LiCl mixtures from 5 different precursors: melamine, urea, thiourea, me-lamine/urea and melamine/thiourea mixtures. This process does not utilize any templates to prepare uniform hexagonal particles of carbon nitride. Transmission electron microscopy reveal ordered structure of hexagonal particles without amorphous inclusions. Crystalline structure and uniform shape of C3N4 particles facilitate non-recombinational delivery of charges to substrates.
Efficiency of photocatalytic performance was evaluated with the model reaction of selective oxidation of benzyl alcohol to benzaldehyde – industrially relevant substrate utilized for dyes synthesis, cosmetic, pefume industries as denaturant, flavouring agent, fragrance, as well as in the food industry [25]. Convenient ways of benzaldehyde preparation are not environmentally friendly because they exploit tough reaction conditions and strong oxidants such as permanganates and chromates. Proposed process of oxidation of benzyl alcohol to benzaldehyde is activated by solar irradiation, does not utilize harmful metal based oxidants, and has no toxic byproducts. It matches to strict requirements of «green» chemistry. The enhanced efficiency of eutectic processed materials roots from their improved structure with uniform particles and less share of amorphous phase.
Experimental
Preparation of carbon nitride materials
5 samples of carbon nitride were prepared, differing by the exposure time in the muffle furnace. In each case, 9 g of LiCl + 11 g of KCl + 4 g of melamine were mixed. The mixture was placed in a crucible with a lid and heated in a muffle furnace to 600°C with various holding times at this temperature. Characteristics of the samples and mass yield are shown in Table 1.
Table 1 Temperature program and mass yield for carbon nitride samples
|
Name |
Program |
Mass yield, % |
|
C 3 N 4 _2 |
10hours ↑600 °С, 2 → 600 °С |
51.3 |
|
C 3 N 4 _4 |
10hours ↑600 °С, 4 → 600 °С |
48.2 |
|
C 3 N 4 _6 |
10hours ↑600 °С, 6 → 600 °С |
60.3 |
|
C 3 N 4 _8 |
10hours ↑600 °С, 8 → 600 °С |
41.5 |
|
C 3 N 4 _10 |
10hours ↑600 °С, 10 → 600 °С |
39.4 |
Photocatalytic experiment
The activity of carbon nitride as a photocatalyst for the selective oxidation of benzyl alcohol to benzaldehyde was investigated. The photocatalytic process was carried out in a quartz reactor transparent to the UV spectra, the reactor has a water cooling jacket into which water was supplied – the temperature of the reaction mixture was 20 °C. The initial concentration of benzyl alcohol equals to 2 mmol/L, the volume of the reaction mixture was 30 ml, the matrix of 18 UV LEDs with a power of 1 W each served as an irradiation source, their luminosity spectrum represented one narrow peak with a maximum at 365 nm. Length of exposure time was equal to 5 hours. The concentrations of benzyl alcohol, benzaldehyde, and benzoic acid were determined by the means of high performance liquid chromatograph with a UV detector.
Characterization
Morphological investigation of the prepared composite silica-titania spheres was performed by a field emission scanning electron microscope (SEM) JEOL JSM 7001F. XRD patterns were recorded on a Rigaku Ultima IV diffractometer operating at Cu Kα radiation (λ = 0.154 nm). Products of photocatalytic experiment were studied with high performance liquid chromatograph Shimadzu Prominence LC-20 with UV detector.
Results and discussion
The maximum mass yield of carbon nitride (60.3 %) was detected for sample C 3 N 4 _6, which had an exposure time of 6 hours in the furnace. When increasing or decreasing the high-temperature exposure time, the mass yield decreases, the minimum value is reached at 10 hours of high-temperature exposure.
Morphology characteristics was studied by the means of scanning electron, samples C 3 N 4 _2, C3N4_6, C3N4_10 are presented on Figs. 1–3 respectively. It is clearly seen that carbon nitride materials, subjected to short time of high thermal treatment demonstrate higher rate of order in comparison to 10-hours treated. C 3 N 4 _2 sample exhibit cubic morphology and C 3 N 4 _6 has hexagonal morphology of particles, while carbon nitride, treated during 10 hours reveal irregular shape of its particles.
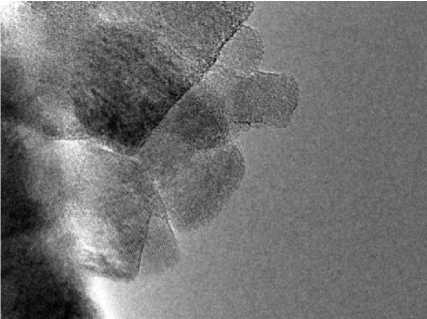
Fig. 1. C 3 N 4 _2 sample
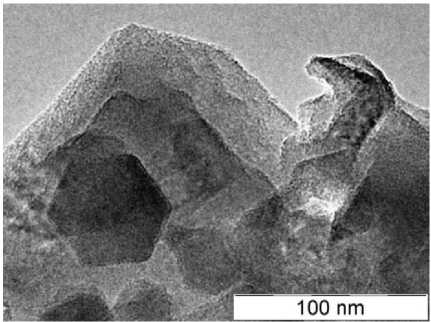
Fig. 2. C 3 N 4 _6 sample

Fig. 3. C 3 N 4 _10 sample
Duration of heat treatment
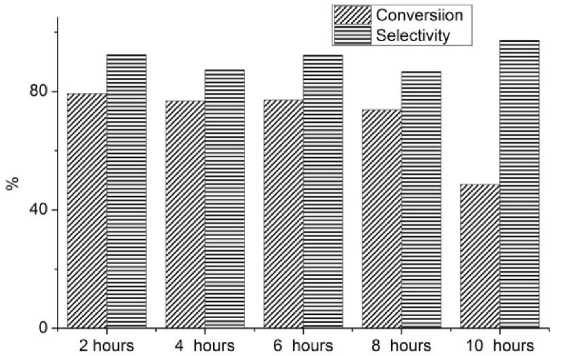
Fig. 4. Conversion and selectivity of photocatalytic experiment of benzyl alcohol oxidation
The values of benzyl alcohol conversion and selectivity for benzaldehyde are shown in Fig. 4 and Table 2. A significant decrease in the reaction rate catalyzed by carbon nitride, which is subjected to maximum exposure in the furnace for 10 hours, can be noted. The selectivity with respect to the formation of benzaldehyde for all samples is at a high level exceeding 86 %.
Table 2
Conversion and selectivity of photocatalytic experiment of benzyl alcohol oxidation
|
Name |
Conversion, % |
Selectivity, % |
|
C 3 N 4 _2 |
79.2 |
92.4 |
|
C 3 N 4 _4 |
76.8 |
87.3 |
|
C 3 N 4 _6 |
77.1 |
92.3 |
|
C 3 N 4 _8 |
73.8 |
86.7 |
|
C 3 N 4 _10 |
48.6 |
97.3 |
In all cases, the amount of benzoic acid formed is negligible. When summing the amounts of substances contained in the reaction mixture after 5 hours of irradiation, a value of less than 2 mmol/L is obtained – this indicates that part of the benzyl alcohol undergoes complete oxidation to undetectable products (carbon dioxide, water).
2 theta
!0h
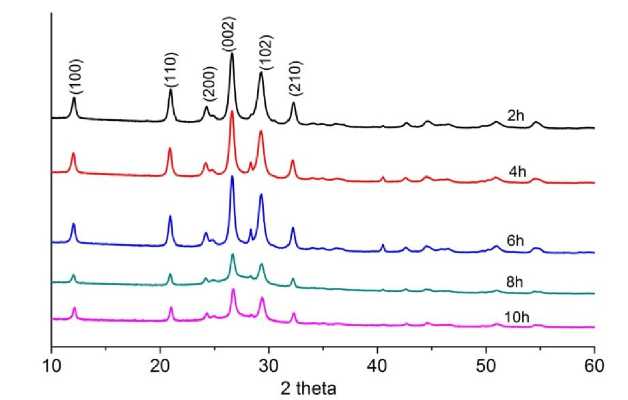
Fig. 5. XRD graph of carbon nitride materials
Powder X-ray diffractometry demonstrates the presence of the same crystalline phase of carbon nitride (Fig. 5). The degree of crystallinity of the materials is different; it reaches its maximum values for materials exposed to exposure at 600 °C for 2–6 hours. With a longer time of high-temperature exposure, the sizes of the ordering regions fall, as indicated by a decrease in the peak intensity and an increase in the width of the peaks at half maximum. A decrease in the degree of crystallinity established by powder x-ray diffractometry correlates with a decrease in the photocatalytic activity of the materials.
Conclusions
It was found that the optimal duration of high-temperature exposure can be called 2 to 6 hours. At a given exposure time, the formation of the most ordered material occurs, with a further increase in the exposure time, crystallinity decreases according to powder x-ray diffractometry and transmission electron microscopy. Materials held at high temperature for a given time demonstrate the best conversion characteristics of benzyl alcohol and selectivity for benzaldehyde in a photocatalytic experiment.
An increase in the high-temperature exposure time to 8 and, in particular, up to 10 hours leads to a decrease in the ordering sites determined by the method of powder x-ray diffractometry and transmission electron microscopy. Photocatalytic activity also decreases.
Список литературы The influence of duration of high-temperature exposure on the properties of carbon nitride obtained in molten salts
- Cui Y., Tang Y., Wang X. Template-Free Synthesis of Graphitic Carbon Nitride Hollow Spheres for Photocatalytic Degradation of Organic Pollutants. Mater. Lett. 2015, no. 161, pp. 197-200.
- Zhang R., Yu Y., Wang H., Du J. Mesoporous TiO2/g-C3N4 Composites with O-Ti-N Bridge for Improved Visible-Light Photodegradation of Enrofloxacin. Sci. Total Environ. 2020, no. 724.
- Hu C., Lei E., Hu K., Lai L., Zhao D., Zhao W., Rong H. Simple Synthesis of 3D Flower-like g-C3N4/TiO2 Composite Microspheres for Enhanced Visible-Light Photocatalytic Activity. J. Mater. Sci. 2020, no.55, pp. 151-162.
- Dong G., Zhang Y., Pan Q., Qiu J. A Fantastic Graphitic Carbon Nitride (g-C3N4) Material: Electronic Structure, Photocatalytic and Photoelectronic Properties. J. Photochem. Photobiol. C: Photochem. Rev. 2014, no. 20, pp. 33-50.
- Akhundi A., Habibi-Yangjeh A., Abitorabi M., Rahim Pouran S. Review on Photocatalytic Conversion of Carbon Dioxide to Value-Added Compounds and Renewable Fuels by Graphitic Carbon Nitride-Based Photocatalysts. Catal. Rev. - Sci. Eng. 2019, no. 61, pp. 595-628.
- Kong L., Wang J., Ma F., Sun M., Quan J. Graphitic Carbon Nitride Nanostructures: Catalysis, Appl. Mater. Today, 2019, no. 16. pp. 388-424.
- Yang J., Liang Y., Li K., Yang G., Wang K., Xu R., Xie X. One-Step Synthesis of Novel K+ and Cyano Groups Decorated Triazine-/Heptazine-Based g-C3N4 Tubular Homojunctions for Boosting Photocatalytic H2 Evolution. Appl. Catal. B: Environ. 2020, no. 262, p. 118252.
- Yan H., Yang H. TiO2 -g-C3N4 Composite Materials for Photocatalytic H2 Evolution Under Visible Light Irradiation. J. of Alloys and Compounds. 2011, no. 509, pp. 26-29.
- Volokh M., Peng G., Barrio J., Shalom M. Carbon Nitride Materials for Water Splitting Photoelectrochemical Cells. Angewandte Chemie - Int. Ed. 2019. no. 58, pp. 6138-6151.
- Savateev A., Antonietti M. Heterogeneous Organocatalysis for Photoredox Chemistry. ACS Catalysis, 2018, no. 8. pp. 9790-9808.
- Baca M., Kukulka W., Cendrowski K., Mijowska E., Kalenczuk R. J., Zielinska B. Graphitic Carbon Nitride and Titanium Dioxide Modified with 1 D and 2 D Carbon Structures for Photocatalysis. Chem. Sus. Chem. 2019, no. 12. pp. 612-620.
- Wang Y., Phua S. Z. F., Dong G., Liu X., He B., Zhai Q., Li Y., Zheng C., Quan H., Li Z., Zhao Y. Structure Tuning of Polymeric Carbon Nitride for Solar Energy Conversion: From Nano to Molecular Scale. Chem. 2019, no. 5. pp. 2775-2813.
- Li J., Zhang M., Li X., Li Q., Yang J. Effect of the Calcination Temperature on the Visible Light Photocatalytic Activity of Direct Contact Z-scheme g-C3N4-TiO2 Heterojunction. Appl. Catal. B: Environ. 2017, no. 212, pp. 106-114.
- Acharya R., Parida K. A Review on TiO2/g-C3N4 Visible-Light-Responsive Photocatalysts for Sustainable Energy Generation and Environmental Remediation. J. Environ. Chem. Eng. 2020, no. 8, pp. 103896.
- Zhang G., Li G., Lan Z. A., Lin L., Savateev A., Heil T., Zafeiratos S., Wang X., Antonietti M. Optimizing Optical Absorption, Exciton Dissociation, and Charge Transfer of a Polymeric Carbon Nitride with Ultrahigh Solar Hydrogen Production Activity. Angewandte Chemie - Int. Ed., 2017, no.56, pp.13445-13449.
- Thomas A., Fischer A., Goettmann F., Antonietti M., Müller J.O., Schlögl R., Carlsson J.M. Graphitic Carbon Nitride Materials: Variation of Structure and Morphology and Their Use as MetalFree Catalysts. J. Mater. Chem. 2008, no. 18, pp. 4893-4908.
- Bojdys M. J., Müller J. O., Antonietti M., Thomas A. Ionothermal Synthesis of Crystalline, Condensed, Graphitic Carbon Nitride. Chem. - A Eur. J. 2008, no. 14, pp. 8177-8182.
- Ding J., Xu W., Wan H., Yuan D., Chen C., Wang L., Guan G., Dai W.L. Nitrogen Vacancy Engineered Graphitic C3N4-Based Polymers for Photocatalytic Oxidation of Aromatic Alcohols to Aldehydes. Appl. Catal. B: Environ. 2018, no. 221, pp. 626-634.
- Yang P., Zhuzhang H., Wang R., Lin W., Wang X. Carbon Vacancies in a Melon Polymeric Matrix Promote Photocatalytic Carbon Dioxide Conversion. Angewandte Chemie - Int. Ed. 2019, no. 58, pp. 1134-1137.
- Zhao Y., Liu Z., Chu W., Song L., Zhang Z., Yu D., Tian Y., Xie S., Sun L. Large-Scale Synthesis of Nitrogen-Rich Carbon Nitride Microfibers by Using Graphitic Carbon Nitride as Precursor. Adv. Mater. 2008, no. 20, pp. 1777-1781.
- Gaddam S.K., Pothu R., Boddula R. Graphitic Carbon Nitride (g-C3N4) Reinforced Polymer Nanocomposite Systems - A Review. Polym. Compos. 2020, no. 41, pp 430-442.
- Zhang N., Wen L., Yan J., Liu Y. Dye-Sensitized Graphitic Carbon Ntride (g-C3N4) for Photocatalysis: a Brief Review. Chem. Pap. 2020, no. 74, pp. 389-406.
- Liu G., Zhao G., Zhou W., Liu Y., Pang H., Zhang H., Hao D., Meng X., Li P., Kako T., Ye J. In Situ Bond Modulation of Graphitic Carbon Nitride to Construct p-n Homojunctions for Enhanced Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2016, no. 26, pp. 6822-6829.
- Tan H., Gu X., Kong P., Lian Z., Li B., Zheng Z. Cyano Group Modified Carbon Nitride with Enhanced Photoactivity for Selective Oxidation of Benzylamine. Appl. Catal. B: Environ. 2019, no. 242, pp.67-75.
- Final Report on the Safety Assessment of Benzaldehyde. Int. J. Toxicol. 2006, no. 25, pp. 1127.


