The influence of induced oxidative stress on the development of powdery mildew pathogen in soft wheat leaves
Автор: Avetisyan G.A.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.19, 2023 года.
Бесплатный доступ
Experiments for the study of symptoms of the powdery mildew pathogen on wheat leaves showed that induced oxidative stress caused changes in conidial germination and appressorial formation of the wheat powdery mildew fungus. The oxidative stress was brought about by treatment with hydrogen peroxide and 3-amino-1,2,4-triazole. It has been shown that prooxidants have a prominent role in regulating fungal development, leading to abnormal conidial germination, thus preventing the fungal penetration into plant cells. Treatment of wheat plants with 5 mM H2O2 and 4 mM 3-amino-1,2,4-triazole resulted in a significant reduction of powdery mildew disease severity compared to the control. In most cases, on samples of infected plant tissues there were anomalies in the elongation of germ tubes and globe-shaped appressoria. From the data which was obtained in this study, it can be concluded that the result of the interaction of powdery mildew fungus with wheat is affected by the increased generation of reactive oxygen species, leading to suppression or disruption of the pathological process.
Abnormal conidial germination, induced oxidative stress, powdery mildew, wheat
Короткий адрес: https://sciup.org/143180971
IDR: 143180971
Текст научной статьи The influence of induced oxidative stress on the development of powdery mildew pathogen in soft wheat leaves
During their development plants are constantly exposed to various kinds of environmental influences. Environmental stress conditions are major factors limiting plant growth and productivity (Chapin, 1991). One way to protect plants from fungal pathogens is to reduce the infectivity of the parasite (Deverall, 1995). Such an approach to the study of the relationship between the pathogen and the host plant makes it possible to regulate the individual stages of the spread of a parasitic fungus through the affected plant and direct the development of the pathogen along an abnormal path (Wilson et al ., 2003).
One of the common responses of plants to different environmental stresses is production of reactive oxygen species (ROS). The major members of the ROS family include hydrogen peroxide, superoxide anion and hydroxyl radical (Vanacker et al ., 2000; Huckelhoven et al ., 2003). There are some data in the literature that in the places of interaction of biotrophic pathogens with a plant, the accumulation of reactive oxygen species (ROS) occurs in the plant cell (Dumas et al ., 1995; Zhou et al ., 1998; Trujillo et al ., 2006). Such a local increase in the level of ROS in the loci of infection can prevent spore germination and stop the development of the disease (Kauss, Jeblick, 1996; Dat et al ., 1998). Many researchers have noted that induced oxidative stress causes biochemical changes in plant cells infected with fungi (Baek, Rajashekar, 2000; Orozco-Cárdenas et al ., 2001; Casano et al ., 2001; Aroca et al ., 2005; Wan, Liu, 2008). Feng and others observed that pre-treatment of wheat plants with exogenous hydrogen peroxide increase the generation of ROS, under the influence of which resistance to the pathogen was formed (Feng et al ., 2008). Okuda and others observed that hydrogen peroxide contents in leaves of winter wheat increased by 3-amino-1,2,4-triazole treatment (Okuda et al ., 1992). Induced oxidative stress is supposed to affect the germination of conidia of the phytopathogenic fungus and direct the subsequent development of the pathogen in the tissues of the host plant along an abnormal path.
The development of the infectious process caused by the phytopathogenic fungus Blumeria graminis is confined to the epidermal tissue of the plant. The initial stages of infection during the first 72 hours include spore germination, germ tube formation and appressoria, penetration through the epidermis into plant cells. In this case, the result of the development of a biotrophic fungus may not appear at once, because during germination the phytopathogen does not kill the cells into which it penetrates, but extracts nutrients from the living cells and tissues of the plant. This feature of the powdery mildew pathogen allows the fungus to increase the duration of its stay in the tissues of a living plant, stimulating the metabolism of the affected host plant (Yarwood, 1957). However, such a long-term containment of the growth of the disease affects the ability of the phytopathogen to maximize spore germination, differentiation of infectious structures, and an increase in the rate of inoculum reproduction. Nevertheless, in the end, the germination of spores of the parasitic fungus and penetration into the plant leads to an increase in pathological changes in the tissues of the diseased host plant, and the disease is morphologically clearly manifested (D'yakov et al., 2001).
This article reports on observations on the effect of induced oxidative stress on the development of the infectious process caused by the phytopathogen Blumeria graminis in order to study the reasons for the change in the nature of the germination of the powdery mildew pathogen on wheat leaves to diagnose and predict the course of the disease. The oxidative stress was modeled by treatment with hydrogen peroxide and 3-amino-1,2,4-triazole.
MATERIALS AND METHODS
The objects of study were the soft wheat Triticum aestivum L. and the agent of powdery mildew of wheat Blumeria graminis (DC.) Speer. Wheat plants were grown in pots under natural light. 15-day-old wheat sprouts were inoculated with conidia of B. graminis , whose culture was supported on susceptible wheat.
The oxidative stress was brought about by treatment with 5 mM hydrogen peroxide and 4 mM 3-amino-1,2,4-triazole (3-АТА). The concentrations of substances were selected as minimal concentrations with an expressed inhibition of development of powdery mildew fungus. Distilled water was used in the control.
Dynamics of development and of differentiation of infesting structures of the agent of powdery mildew was investigated using light and scanning electron microscopes. To do this, the plant material was selected on the third to ninth day after infestation. Fresh leaves were examined in a LEO-1430 VP scanning electron microscope (Carl Zeiss, Germany) at -30° C using freezing consoles by Deben UK (United Kingdom). The material for light microscopy is stained with 1% amide black solution in 7% acetic acid, and then washed with distilled water. Samples of leaf tissue were examined by means of an Axioplan 2 light microscope (Carl Zeiss, Germany).
The table shows the arithmetic means and standard errors of the mean, 15 -20 leaves were used for each variant. Differences between values of parameters were considered significant at р < 0.05.
RESULTS AND DISCUSSION
The study of the development of Blumeria graminis on the leaf surface of a soft wheat showed that most of the powdery mildew conidia germinated, forming a primary germ tube and appressorial germ tube forming the appressorium (Fig. 1 A, B and Fig. 2 A). Penetrating into the tissues of the plant leaf, the causative agent of powdery mildew formed infectious hyphae, spreading over the entire surface of the leaves. In the control variant, due to the increased development of the fungus, the disease proceeded very noticeably, as a rule, young colonies formed in large numbers within 3-4 days after inoculation and intensive sporulation occurred (Fig. 2 B).
Experiments with prooxidants have shown that these substances inhibit the growth and development of the infectious structures of the powdery mildew fungus, causing anomalies in the morphology of the germ tubes (Fig. 2 C–F). On samples of infected plant tissues there were anomalies in the elongation of germ tubes and globe-shaped appressoria. For example, when using 5 mM H2O2, the development of the studied fungus was suppressed both on the surface of the plant and at the penetration site. Nevertheless, normally formed germ tubes penetrated in epidermal cells of the infected plant and formed the mycelium. When using 4 mM 3-ATA conidia of the powdery mildew pathogen poorly germinated and protruding short germ tubes often did not reach the surface of the plant cell, but those germ tubes that managed to gain a foothold on the surface germinated, forming normal infectious structures.
According to the table, under the influence of 5 mM H 2 O 2 and 4 mM 3-ATA, anomalies were observed in the germinating conidia of the powdery mildew pathogen and there were formed fewer normal infectious structures than in the control (Table 1). The highest number of anomalies of infectious structures were in a variant with 5 mM H 2 O 2 in the incubation medium of leaves. Treatment of wheat leaves with 4 mM 3-АТА significantly increased the quantity of conidia unable to germinate. The results demonstrate that all variants with treatment show decreased intensity of conidia germination and inhibition of infection.
Experiments conducted to study changes in the nature of the germination of the powdery mildew pathogen on wheat leaves showed that under the influence of wheat plant treatment with prooxidants, the germinating conidia of the powdery mildew pathogen had an elongation of germ tubes, most of the conidia exfoliated from the leaf surface, and the number of conidia forming appressoria decreased. An increase in the number of anomalies of the infectious structures of the fungus contributes to the delay in the development of the disease. As a result, an infectious process that is not accompanied by the penetration of the hyphae of the fungus into plant tissues or accompanied by the introduction of a pathogen into individual plant cells ultimately leads to the death of the fungus.
The available literature data indicate an important role of oxidative processes in plant responses, in particular, the production of ROS in plant cells increases several times under the influence of stresses of various nature (Foyer et al ., 1994; Thordal-Christensen et al . 1997; Vanacker et al ., 1998; Neill et al ., 2002; Vranova et al ., 2002). This article showed that the abnormal differentiation of the infectious structures of the powdery mildew fungus, leading to the inhibition of pathogen’s growth, was caused by induced oxidative stress. Apparently, induced oxidative stress which leads to over-generation of ROS in wheat leaves affects the resistance of plants to other unfavorable factors, such as infection by infectious diseases.
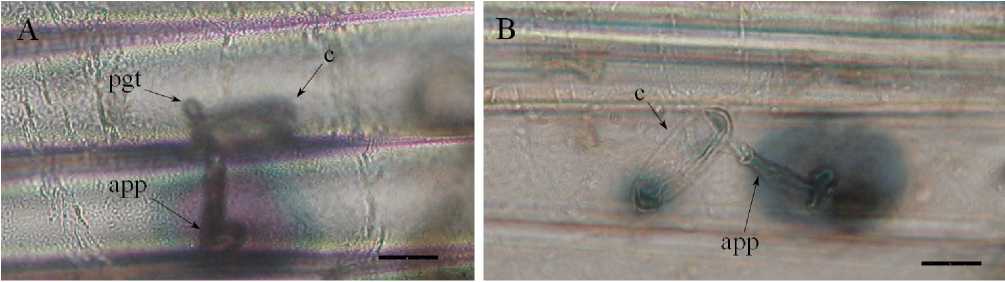
Figure. 1. Formation of the primary infectious structures of Blumeria graminis on surface of wheat leaves in the control variant (light microscope, 72 h after infection). Scale bars: 50 µm. Legend: app – appressorium, c – conidia, pgt – primary germ tube.
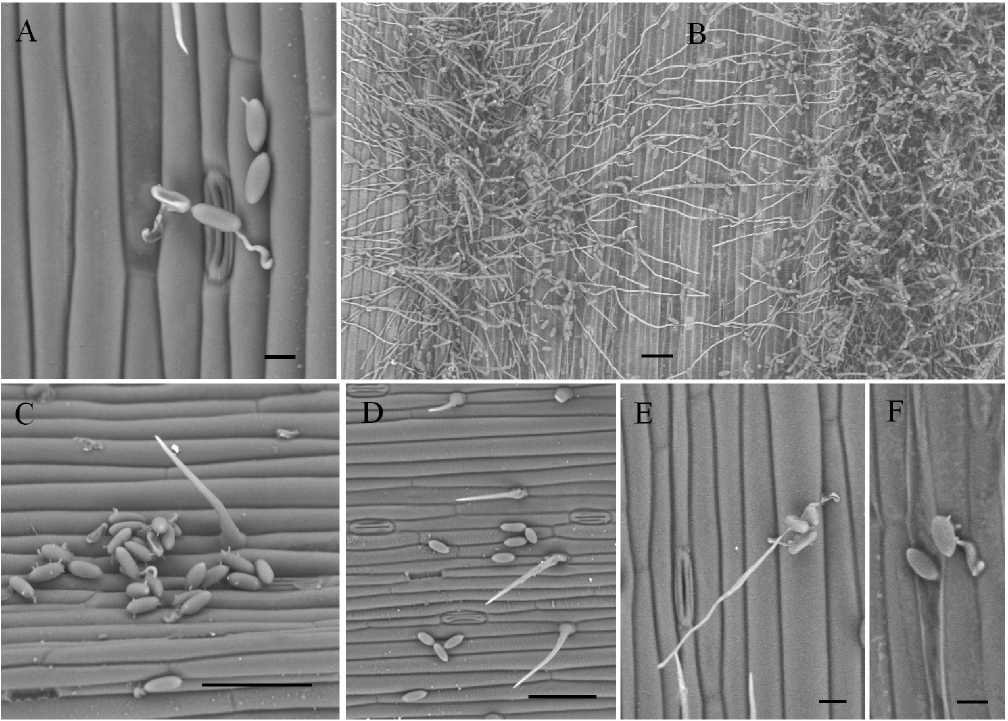
Figure. 2. Features of the development of conidial inoculum of Blumeria graminis on infected wheat leaves (scanning electron microscope, 3–7 days after inoculation): A, B – control; C, E – 5 mM H2O2; D, F – 4 mМ 3-АТА. Scale bars: A – 20 µm; B, C, D – 100 µm; E – 20 µm; F – 30 µm.
Table 1: Effect of Н 2 О 2 and 3-АТА treatment on the development of infection structures of powdery mildew pathogen in wheat leaves (15-20 preparations). Data are represented as arithmetic means and standard errors of the mean.
|
Variants of the experiment |
Non-germinated conidia |
Germinated conidia: |
|
|
with normal differentiation of infectious structures |
with abnormal differentiation of infectious structures |
||
|
Control (distilled water) |
1.8 ± 1.1 |
7.0 ± 1.2 |
0.1 ± 0.8 |
|
5 mM H 2 O 2 |
3.8 ± 1.4 |
3.1 ± 1.3 |
5.2 ± 1.1 |
|
4 mM 3-ATA |
5.7 ± 1.2 |
2.5 ± 1.2 |
3.0 ± 1.3 |
CONCLUSION
As a result of the study of the development of the disease caused by the phytopathogenic fungus Blumeria graminis on soft wheat plants, it was found out that induced oxidative stress is the cause of a change in the nature of the germination of the pathogen powdery mildew on wheat leaves. It has been shown that hydrogen peroxide and 3-АТА regulate the development of fungal inoculum, directing it to the abnormal side, thus preventing penetration into the plant cells. In conclusion, when the causative agent of powdery mildew interacts with wheat plants, the increased generation of ROS in plant tissues affects the result of the interaction, leading to the suppression or disruption of the pathological process.
ACKNOWLEDGEMENT
The study was carried out within the framework of the state programme № 122042700002-6.
CONFLICT OF INTERESTS
The author declare that she has no conflict of interest.
Список литературы The influence of induced oxidative stress on the development of powdery mildew pathogen in soft wheat leaves
- Aroca R, Amodeo G, Fernandez-Illescas S, Herman EM, Chaumont F et al (2005) The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiology 137(1):341-353. https://doi.org/10.1104/pp.104.05104
- D'yakov YT, Ozeretskovskaya OL, Dzhavakhiya VG, Bagirova SF (2001) General and Molecular Phytopathology. M.: Phytopathology society. 301 p.
- Baek KH, Rajashekar CB (2000) Hydrogen peroxide reduces hypoxia in germinating bean seeds. HortScience 35(3):427-428. https://doi.org/10.21273/HORTSCI.35.3.427F
- Casano LM, Martin M, Sabater B (2001) Hydrogen peroxide mediates the induction of chloroplastic ndh complex under photooxidative stress in barley. Plant Physiology 125(3): 1450-1458. https://doi.org/10.1104/pp.125.3.1450
- Chapin FS (1991) Integrated responses of plants to stress. BioScience 41(1):29-36. https://doi.org/10.2307/1311538
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IS (1998) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiology 116(4):1351-1357. https://doi.org/10.1104/pp.116A1351
- Deverall BJ (1995) Plant protection using natural defence systems of plants. In Advances in plant pathology. Academic Press 11:211-228. https://doi.org/10.1016/S0736-4539(06)80013-9
- Dumas B, Freyssinet G, Pallett KE (1995) Tissue-specific expression of germin-like oxalate oxidase during development and fungal infection of barley seedlings. Plant Physiology 107(4):1091-1096. https://doi.org/10.1104/pp.107A1091
- Feng H, Li X, Duan J, Li H, Liang H (2008) Chilling tolerance of wheat seedlings is related to an enhanced alternative respiratory pathway. Crop Sci 48(6):2381-2388. https://doi.org/10.2135/cropsci2007.04.0232
- Foyer CH, Descourvieres P, Kunert KJ (1994) Protection against oxygen radicals: An important defense mechanism studied in transgenic plants. Plant Cell Environ 17(5):507-523. https://doi.Org/10.1111/j.1365-3040.1994.tb00146.x
- Hückelhoven R, Dechert C, Kogel KH (2003) From the Cover: Overexpression of barley BAX inhibitor 1 induces breakdown of mlo-mediated penetration resistance to Blumeria graminis. PNAS 100(9):5555-5560. https://doi.org/10.1073/pnas.0931464100
- Kauss H, Jeblick W (1996) Influence of salicylic acid on the induction of competence for H2O2 elicitation (comparison of ergosterol with other elicitors). Plant Physiology 111(3):755-763. https://doi.org/10.1104/pp.11L3.755
- Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 53(372):1237-1247. https://doi.org/10.1093/jexbot/53.372.1237
- Okuda T, Matsuda Y, Sugawara M, Sagisaka S (1992) Metabolic response to treatment with cold, paraquat, or 3-amino-1,2,4-triazole in leaves of winter wheat. Bioscience, biotechnology and biochemistry 56(12):1911-1915.
- Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13(1):179-191. https://doi.org/10.1105/tpc.13.L179
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. The Plant Journal 11(6):1187-1194. https://doi.org/10.1046/j.1365-313X.1997.11061187.x
- Trujillo M, Altschmied L, Schweizer P, Kogel KH, Hückelhoven R (2006) Respiratory Burst Oxidase Homologue A of barley contributes to penetration by the powdery mildew fungus Blumeria graminis f. sp. hordei. J. Exp. Bot. 57(14):3781-3791. https://doi.org/10.1093/jxb/erl191
- Vanacker H, Carver TLW, Foyer CH (2000) Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hyper-sensitive response in the barley-powdery mildew interaction. Plant Physiology 123(4):1289-1300. https://doi.org/10.1104/pp.123A1289
- Vranova E, Inze D, Van Breusegem F (2002) Signal transduction during oxidative stress. J. Exp. Bot. 53(372):1227-1236. https://doi.org/10.1093/jexbot/53.372.1227
- Wan XY, Liu JY (2008) Comparative proteomics analysis reveals an intimate protein network provoked by hydrogen peroxide stress in rice seedling leaves. Mol. Cell. Proteomics 7(8):1469-1488. https://doi.org/10.1074/mcp.M700488-MCP200
- Wilson TJG, Thomsen KK, Petersen BO, Duus J0, Oliver RP (2003) Detection of 3-hydroxykynurenine in a plant pathogenic fungus. Biochemical Journal 371(3):783-788. https://doi.org/10.1042/bj20021797 Yarwood CE (1957) Powdery mildews. The Botanical Review 23:235-301.
- Zhou F, Zhang Z, Gregersen PL, Mikkelsen JD, De Neergaard E et al. (1998) Molecular characterization of the oxalate oxidase involved in the response of barley to the powdery mildew fungus. Plant Physiology 117(1):33-41. https://doi.org/10.1104/pp.117.L33


