The influence of sulfuric acid salts and radiation on the activity of the enzyme carbonic anhydrase in cotton ontogenesis
Автор: Alakbarova Sh.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.20, 2024 года.
Бесплатный доступ
Article deals γ-radiation doses of 5, 10, 50, 100, 200, 300 Gy, sulfate (Na2SO4, ZnSO4) concentrations of 5, 10, 50, 100, 200, 300 mM of cotton Gossypium hirsutum L . The dynamics of changes in the activity of carbonic anhydrase (CA, carbonate hydrolyase, EC 4.2.1.1) enzyme were studied in the ontogeny of the Ganja-182 cultivar of the species Gossypium hirsutum L. in type of true leaf emergence (LP), budding (BP), flowering (FP) and opening of seed boll phase (OBP). It was determined that chloride and sulfate salts have different effects on CA activity. Thus, CA activity increases at 200 Gy dose of γ-radiation, 100 mM of NaCl, and 200 mM of ZnSO4. It seems that the increase in CA activity in the medium containing ZnSO4 is related to the increase in the demand for CO2 under stress. The obtained results show that radiation and sulfate salts have a more regulatory effect on salt adaptation than other salts in cotton plant.
Gossypium hirsutum l, γ-radiation, salt stress, ca-activity, adaptation
Короткий адрес: https://sciup.org/143182787
IDR: 143182787
Текст научной статьи The influence of sulfuric acid salts and radiation on the activity of the enzyme carbonic anhydrase in cotton ontogenesis
It is shown in the literature that ~25% of the earth's surface in modern times (Hasan et al., 2011), and ~20% of arable land is saline. According to statistics, the world population is expected to reach 10 billion by 2025 (Cafi, 2003). One of the preventive measures taken to prevent the impending global threat on our planet can be the selection of durable and high-yielding cultivated plant varieties that can grow in extreme conditions. From this point of view, the study of physiological-biochemical mechanisms of adaptation to abiotic stress in plants has great scientific and practical importance. According to the results of Banzet et al., (1998), after γ-irradiation, 15 and 17 kDa polypeptides with a protective function are synthesized in the cytosol of the tomato plant, and 22 kDa molecular weight polypeptides are synthesized in the mitochondria (Banzet et al., 1998). Optimum dose of γ-irradiation of seeds accelerates the development of cultivated plants and shortens the ripening period, as a result, grain, potato, etc. increases the productivity of plants by approximately 5-20% (Calabrese, 2011; Jan et al., 2012; Kozmin et al., 2015). From this point of view, importance should be given to the application of radiation as an important biological method to increase the productivity of plants in agriculture (Sanzharova et al., 2016).
The growth, development, productivity and sustainability of plants also depend on the lability of physiological-biochemical processes and the amount of accumulated reserve substances (He, Hou, 2014). In this way, the membrane and cytosol of the plant cell, which has a role in creating photosynthetic productivity (Guliev et al., 2003), chloroplast and mitochondria (Yu et al., 2004), the CA enzyme localized in different subcellular fractions has different physiological functions (Wu et al., 2006). Various isoforms of CA are involved in photosynthesis, respiration, ion transport, carbon-fixing mechanism (CCM) in higher plants (Xiao et al., 2015; Fan et al., 2015; Price et al., 2008) role is to regulate the diffusion and exchange of CO 2 between the cell and the environment (Sun et al., 2014).
The main goal of this study is to compare the influence of different types of radiation, chloride and sulfate salts on the dynamics of CA enzyme activity in the ontogeny of the cotton plant.
MATERIALS AND METHODS
For the goal research purposes, were taken Ganja-182 species of cotton Gossypium hirsutum L. Cotton seeds were irradiated with doses of 5, 10, 50, 100, 200 and 300 Gr using Co60 as a source of radiation in the RUXUND 20,000-irradiation device at the "Isotope Sources of Radiation" scientific experiment department of the Institute of Radiation Problems of ANAS. Irradiated and non-irradiated seeds were disinfected in 3% H2O2 for 15 min before sowing, washed 2-3 times with distilled water after disinfection and transferred to a thermostat in Petri dishes. Seedlings obtained from nonirradiated seeds were planted in vegetation containers with 5, 10, 50, 100, 200, and 300 mM solutions of Na2SO4, and ZnSO4 separately, at a temperature regime of 25-28ºC, a photoperiod of 14 hours, humidity of 6070%, and light intensity. It is placed in an artificial climate chamber with 15-20 klux.
Gas exchange parameters - photosynthesis rate (P n , μmol CO 2 m-2s-1), concentration of CO 2 in intercellular areas (C i , μmol CO 2 mol-1), permeability of stomatal cells (g s , mol CO 2 m-2s-1) and transpiration rate (T r , mmol CO 2 m-2s-1) was measured in the leaf using an infrared photosynthetic analyzer (LI 6400 XT Postable Photosynthesis System; LI-COR 6400 Biosciences, USA).
To get the enzyme extract, the leaves were washed, dried with filter paper and homogenized at +4oC by adding 5 ml of homogenization solution to each gram of leaf. (5 mM DTT, 1 mM EDTA, 20 mM MgCl2, 0.5% PVP, and 0.5% Triton X-100 containing 100 mM , pH 7.8-8.0 by adding Tris-HCl). To obtain the enzyme extract, the leaves were washed, dried with filter paper, and 5 ml of homogenization solution (5 mM DTT, 1 mM EDTA, 20 mM MgCl2, 0.5% PVP, and 0.5% Triton X-100 containing 100 mM , pH 7.8-8.0 by adding Tris-HCl) and homogenized at +4oC temperature. Obtained homogenates were filtered through double capron, first 5 min at 1000g and then 15 min at 5000g to get rid of the nucleus and non-degradable plant remains. The sediment was discarded, and the supernatant liquid was used for further work.
CA activity was determined electrometrically according to Wilbur Anderson based on the separation of H+ ions formed by the reaction СО 2 + Н 2 О → Н+ + НСО 3 - (Wilbur, Anderson, 1948). Released H+-ions were recorded with pH-meter ЭВ-74 and XY-RECORDER endim 620.02 potentiometer. CA activity was calculated according to Risky (Rickli et al., 1964).
The total amount of proteins was determined spectrophotometrically (Ultrospec 3300 pro, Amersham) at a wavelength of 750 nm according to Lowry's method (Lowry et al., 195).
Statistical analyses. The values shown in the tables are mathematical averages and reflect the standard deviation. During the analysis of the results of the study, the average mathematical errors and deviations (M±m) were taken into account. Differences were considered significant when the accuracy probability was R≤0.05. The obtained results were processed using "Microsoft Office Excel 2010" computer programs.
RESULTS AND DISCUSSION
As we know, CA enzyme localized in leaves and roots of higher plants is strongly dependent on the level of gas exchange parameters. Therefore, we have studied the activity of CA enzyme in cotton leaves in relation to gas exchange parameters. The results obtained during the study of gas exchange parameters are given in table 1 (table 1). In our previous experiments, we determined the stimulating effect of 50 Gy dose of radiation and 50 mM concentration of salts on the development of biological processes in cotton plants. Therefore, when studying the parameters of gas exchange in cotton leaves, the optimal radiation dose was 50 g, and the optimal concentration of salts was 50 mM. Therefore, when studying the parameters of gas exchange in cotton leaves, who took 50 g as the optimal radiation dose and 50 mM as the optimal concentration of salts. Later, the effects of radiation and 2 types of salt on the parameters of gas exchange in the leaves of the cultivated cotton plant at this dose and concentration were studied comparatively. As can be seen from the table, the increase of Na2SO4 concentration up to 50 mM causes different changes as a result of the effect on Pn, gs, CO2 concentration (Ci) and Tr in the intercellular spaces. In green leaves, Tr and Pn are strongly regulated by gs. Accordingly, as Ci increases, Pn decreases. Compared to C in the GP, BP, and FP of Pn, it increased by 54.5, 37.4, and 15%, respectively.
As can be seen from the first table, the amount of P n and T r in 50 mM ZnSO 4 concentration decreases, while the amount of C i increases. Given the significant inverse correlation between the values of these three quantities, based on CO 2 exchange, we studied the activity of CA enzyme in the BP, FP and OBP phases of cotton plant ontogenesis, taking into account its important role in the formation of photosynthesis intensity and photosynthetic productivity. In the presence of ZnSO 4 , compared to C in the LP, BP and FP phases, Pn is 40.7, 51.9 and 17.1%, respectively, and C i is 23.9, 14.8 and 19.4 %, respectively increased (table 1).
In order to compare the changes in gas exchange parameters under the influence of 50 mM ZnSO 4 in the plant development phases, the values in the BP and FP phases were separately compared with the indicators in the LP phase. It was determined that the values of gs, T r and P n decreased over time depending on the phases. Among the gas parameters, only a small increase in the amount of C i was observed. C i increased by 5.6% in the BP phase compared to the GP phase, and by 13.6% in the FP phase compared to the BP phase. In the conditions where the amount of C i increased, the amount of P n in the boll phase decreased by 14.8% compared to the Leaf phase, and in the flowering phase by 39.9% compared to the green phase. The fact that gas parameters are also low in C in the last stages of vegetation can be explained by leaf senescence. Along with the parallel reduction of P n and T r due to the effect of salt, regeneration of CO 2 along with photochemical reactions is also inhibited (Fig. 1).
As we know, one of the most important functions of CA localized in plant cells is to carry out the transport and assimilation of inorganic carbon (CO2) generated in plant metabolism and fixed from the atmosphere to the carboxylation centers of photosynthesis. If we pay attention to the table, we can see that the CA activity is much higher in the variants with ZnSO4 than in the variants with other salts. In the second place are Na2SO4, and in the following places there are options with ZnSO4 salt. We attribute the fact that the activity of CA enzyme under the influence of ZnSO4 is higher than the activity of CA under the influence of other salts with the presence of the Zn atom, which plays the role of a coenzyme, in the active center of the CA enzyme. 50 mM ZnSO4 stimulates plant growth and development, productivity by increasing CA activity up to 50% compared to 50 mM Na2SO4. Such functions give the CA enzyme an adaptive property (Fig. 1).
CA transports CO 2 and HCO 3 - as an inorganic carbon carrier. CA participates in various biological processes by regulating the CO 2 /HCO 3 - ratio near the active center (Fan et al., 2015). Back in 2004, Moskvin et al. (2004) proved that PS II contains a 33 kDa molecular weight protein with CA activity in its oxygen-releasing complex.
As can be seen from table 1, CA loses its activity faster under the influence of chloride salts. This process is completely opposite to sulfate salts. Although the change of CA activity during ontogenesis in the presence of Na 2 SO 4 occurs similar to ZnSO 4 , the level of CA activity in the presence of ZnSO 4 is parallel higher than that of Na 2 SO 4 . Under the influence of this salt, the biological productivity of the plant increases significantly compared to others. The obtained results may be related to the physiological functions performed by the CA molecule in the spatial structure, organs and tissues.
Figure 1 shows the results obtained regarding the influence of chloride and sulfate salts on the dynamics of CA enzyme activity in cotton plant leaves. As can be seen from the figure, the CA activity in C in the roots of 10, 20 and 30-day-old plants gradually increases, while in the leaves of the plant, on the contrary, it decreases. On the 10th, 20th and 30th days of plant development, 50 and 100 mM NaCl causes a decrease in CA activity in roots and a parallel increase in leaves (Fig. 1).
During the effect of FeCl3 on the CA activity in the roots and leaves of cotton plants, it was determined that in contrast to NaCl, the CA activity in the roots and leaves decreases similarly under the influence of FeCl3 at a concentration of 50 - 100 mM in 10, 20 and 30-day-old plants. These show that the increase in salt concentration in roots and leaves at 50-100 mM concentrations of NaCl and FeCl3 further accelerates the inhibition of CA activity. Such an effect of chloride salts on CA activity in the roots and leaves of cotton plant can be explained by the decrease in the activity of H+-pumps as a result of the increase in the amount and concentration of salts in the rhizosphere, and in connection with this, the weakening of mineral nutrition, and the disruption of osmotic processes as a result of the accumulation of salts in cells. CA activity in roots and leaves of 10-day-old cotton plants decreases under the influence of ZnSO4 at concentrations of 50 and 100 mM from sulfate salts, while CA activity in 20-day-old plants increases rapidly and reaches the highest level in all variants. In contrast, although a weak reduction (1015%) occurred in 30-day-old cotton plants, CA activity in all variants was approximately 40-50% higher than in 10- and 20-day-old plants (Fig. 1).
As can be seen from the table, the effect of sulfate salts on CA activity in the roots and leaves of cotton plants is different from the effect of chloride salts. Thus, CA activity in C in 10-day-old plants gradually decreases under the influence of Na 2 SO 4 , CA activity takes the highest value under the influence of Na 2 SO 4 at a concentration of 50 mM, and this indicator decreases over time. Under the influence of Na 2 SO 4 at a concentration of 100 mM, the CA activity in the roots and leaves of 20-day-old plants increases, and on the contrary, it gradually decreases in the roots and leaves of 30-day-old plants.
The obtained results show that unlike chloride salts, sulfate salts, including ZnSO 4 , have a more regulatory effect on the physiological-biochemical processes carried out in the leaf and root cells of the cotton plant, depending on the stages of plant ontogenesis and salt concentration. We attribute this effect of ZnSO 4 to the presence of a Zn atom in the active center of the CA enzyme, which performs the coenzyme function. It is shown in the literature that the CA enzyme, which is localized in different subcellular fractions of cells and tissues of C 3 plants and performs various physiological and biochemical functions, has an oligomeric structure and each of its monomers contains 1 g-eqv zinc atom (Idayatov, 1990).
In order to confirm the role of Zn atom in the activation of CA enzyme localized in the leaf cells of cotton plant, we studied the inhibitory effect of orthophenanthroline (OPT), which forms a complex compound with heavy metal salts, on the enzyme activity. OPT precipitates by forming a complex combination with Zn atoms in the active center of CA and in the environment. As a result, the activity of the CA enzyme is completely inhibited, while P n is simultaneously reduced to a minimum. For this purpose, in order to study the inhibitory effect of OPT on the activity of the CA enzyme obtained from the leaves of the bamboo plant cultivated in the environment containing 50, 100 and 150 mM ZnSO 4 , the activity of the enzyme was determined at concentrations of 1-5
mM of OPT and the obtained results were comparatively analyzed (Fig. 2).
As can be seen from the picture, OPT at a concentration of 3•10-3M has completely inhibited CA by combining Zn atoms in the active center of CA enzyme. This process depends on the ambient temperature, pH and concentration of Zn2+ ions. Our experiments show that as the concentration of ZnSO 4 salt increases to 50100 mM, CA activity increases accordingly (Fig. 2).
The obtained results can be considered as one of the components of CCM, which arose as a way of adaptation to stress in evolution. CA activity, including flowering phase, increases as the age of the plant increases. After this phase, after remaining relatively unchanged for a certain period, it gradually weakens until the end of vegetation.
Table 1 : Effects of radiation, chlorine and sulfate salts on gas exchange parameters during the active development phases of cotton plant ontogeny
|
Parameters |
Control |
Sulfate salts, 50 mM |
Radiation, 50 Qy |
|
|
Na 2 SO 4 |
ZnSO 4 |
|||
|
True leaf formation phase (TL) |
||||
|
P n |
33,2±3,1 |
45,8±4,83 |
46,7±4,22 |
49,9±1,09 |
|
C i |
201±18,1 |
243±20,4 |
249±14,7 |
264±21,7 |
|
G s |
4,9 ±0,91 |
6,1±1,00 |
6,9±0,77 |
4,01±1,01 |
|
T r |
2,2 ±0,61 |
5,9±1,12 |
6,55±0,83 |
6,02±1,43 |
|
Budding phase (BP) |
||||
|
P n |
26,2±3,1 |
35,9±2,58 |
39,8±2,3 |
49,8±1,5 |
|
C i |
229±19,7 |
254±19,8 |
263±14,7 |
282±24,8 |
|
G s |
4,1± 0,98 |
5,5±1,23 |
4,6±1,12 |
4,91±0,93 |
|
T r |
1,8±0,42 |
4,18±0,94 |
4,21±0,99 |
6,14±0,95 |
|
Flowering phase (FP) |
||||
|
P n |
24,0±3,95 |
28,1±3,88 |
28,1±2,1 |
54,1±7,63 |
|
C i |
237±20,3 |
266±19,81 |
283±19,51 |
269±20,71 |
|
G s |
4,6±1,11 |
4,8±1,89 |
4,21±0,42 |
5,55±1,22 |
|
T r |
1,0±0,09 |
1,12±0,45 |
1,83±0,18 |
2,4±0,44 |
Note: P n -photosynthesis rate-μmol CO 2 •m-2•s-1; C i - the amount of CO2 in the intercellular spaces – μmol CO 2 •mol-1; g s -permeability of the stomata - mol H 2 O•m-2•s-1, T r - intensity of transpiration-mmol H 2 O•m-2•s-1
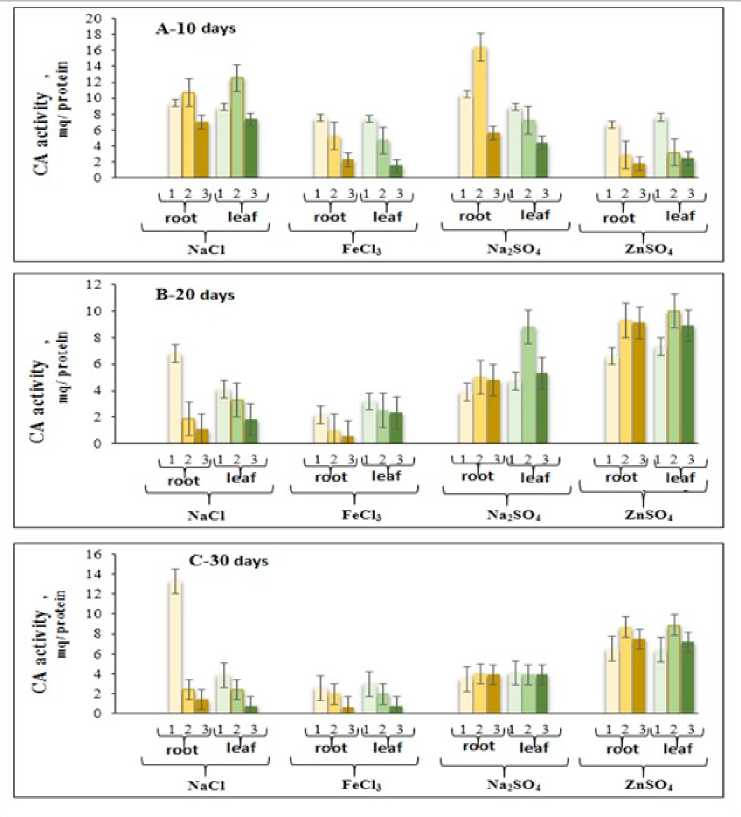
Figure 1. The effect of chloride and sulfate salts on the dynamics of CA enzyme activity in cotton plant leaves. 1-Control 2-50 mM salt; 3-100 mM salt.
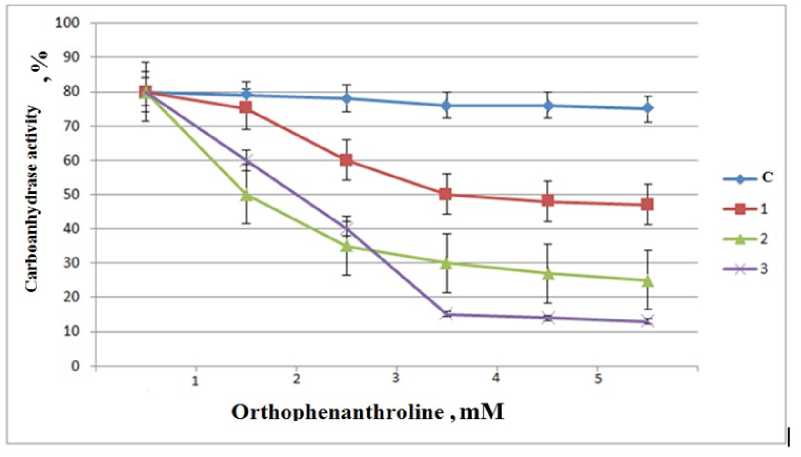
Figure 2. Inhibitory effect of OPT on CA activity in leaves of cotton plants grown in different concentrations of ZnSO 4 during the flowering phase (FP) of ontogeny. C-Control, 1-50 mM, 2-100 mM, 3-150 mM ZnSO 4
CONCLUSION
The processes occurring in the metabolism of the cotton plant under the influence of radiation and different types of salts: the interrelated changes in the indicators of gas exchange parameters, plant mineral nutrition, and the activity and functional diversity of the CA enzyme isoforms localized in roots and leaves are the physiological and physiological adaptation of plants to stress, can be considered as one of the components of biochemical mechanisms. Sulfate salts have a greater stimulating effect on CA enzyme activity than chloride salts. 50 Gy of radiation and 50 mM ZnSO 4 cause an increase in CA activity, further intensification of Pn and a high yield of FP in cotton ontogenesis. Under these conditions, the amount of C i decreases.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы The influence of sulfuric acid salts and radiation on the activity of the enzyme carbonic anhydrase in cotton ontogenesis
- Alekberova, Sh. E., Gaziev A. T. (2018) Dependence of photosynthetic activity and adaptability of corn crops on nutritional conditions. Noviye I ntradicionniye rasteniya I perspektivi eqo ispolzovaniya. No. 13, 225-228.
- Banzet N, Richard C, Deveaux Y. et al. (1998) Accumulation of small heat shock proteins, including mitochondrial HSP22, induced by oxidative stress and adaptive response in tomato cells. Plant Journal; 13, 519-527
- Cafi, M., Stewart, W.S. & Borland, A.M. (2003) Carbohydrate and Proline Contents in Leaves, Roots, and Apices of Salt-Tolerant and SaltSensitive Wheat Cultivars1. Russian Journal of Plant Physiology 50, 155-162.
- Sanzharova NI, Kozmin GV, Geraskin S.A. (2016) Collection of reports of the round table within the XX Mendeleev congress on general and applied chemistry. RIRAE ;109-112.
- Calabrese EJ, Blain RB. (2011) The hormesis database: the occurrence of hormetic dose responses in the toxicological literature. Reg. Toxicology and Pharmacology; 61: 73-81
- Fan J, Xu H, Luo Y, Wan M, Huang J, Wang W, Li Y. (2015) Impacts of CO2 concentration on growth, lipid accumulation, and carbon-concentrating-mechanism-related gene expression in oleaginous Chlorella. Appl Microbiol Biotechnol. 99(5):2451-62.
- Guliev NM, Babaev GG, Bairamov Sh.M. et al., (2003). Purification, properties, and localization of two Carbonic Anhydrases from Amaranthus cruentus leaves. Russian Journal of Plant Physiology; 50(2): 213-219
- Hasan D, Kovtun IS, Yefimova MV. (2011) Effect of chloride salinization on seed germination and seedling growth of Brassica napus L. Bulleten Tomskoqo Gosudarstvennoqo Universteta. 4: 108112
- Idayatov RB. (1990) Carbonic anhydrase and primary CO2 fixation in different wheat genotypes. Dissertation, Baku, Azerbaijan.
- Kozmin GV, Sanzharova NI, Kibina II, Pavlov AN, Tikhonov VN. (2015) Radiation technologies in agriculture and food industry. Achievements of science and technology; AIC (Agro-Industrial Complex), 5:87-92.
- Lowry OH, Roserbrough NJ, Farr AL, Candell RL. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chemistry; 193(1): 556-266
- Moskvin OV, Shutova TV, Khristin MS, Ignatova LK, Villarejo A, Samuelsson G, Klimov VV, Ivanov BN. (2004) Carbonic Anhydrase activities in pea tylakoids. A photosystem II core complex_associated Carbonic Anhydrase. Photosynthesis Research; 79: 93-100
- Price GD, Badger MR, Woodger FJ, Long BM. (2008) Advances in Understanding the Cyanobacterial CO2-Concentrating-Mechanism (CCM): Functional Components, Ci Transporters, Diversity, Genetic Regulation and Prospects for Engineering into Plants. Journal of Experimental Botany; 59: 14411461
- Rickley EE, Chazanfar SAS, Gibbons BH, Edsall JT. (1964) Carbonic Anhydrase from human erythrocytes. Preparation and properties of two enzymes. J. Biol. Chem.; 239: 1065-1078
- Sun Wei-Hong, Wu Yan-You, Sun Zhen-Zhen, Wu Qiu-Xia, Wen Xin-Yu. (2014) Enzymatic characteristics of higher plant Carbonic Anhydrase and its role in photosynthesis. J. of Plant Studies; 3(2): 39-44 Wilbur KM, Anderson NG. (1948) Electrometric and colorometric determination of Carbonic Anhydrase. J. Biological Chemistry; 176: 147-151.
- Wu YY, Li XT, Hao JC, Li PP, Wang BL. (2006) Study on the difference of the activities of Carbonic Anhydrase in different plants. Guihaia; 26: 366-369
- Xiao L, Lian B, Hao J, Liu C, Wang S. (2015) Effect of Carbonic Anhydrase on silicate weathering and carbonate formation at present day CO2 concentrations compared to primordial values. Sci Rep.; 5: 7733
- Yu S, Zhang XX, Guan QJ, TaCAno T, Liu SK. (2007) Expression of a carbonic anhydrase gene is induced by environmental stresses in Rice (Oryza sativa L.). Biotech. Letters; 29: 89-94


