The influence of the constant illumination on the ultrastructure of rat's hepatocytes
Автор: Areshidze David A., Kozlova Mariya A., Chernikov Valery P., Kondashevskaya Marina V.
Журнал: Морфологические ведомости @morpholetter
Рубрика: Оригинальные исследования
Статья в выпуске: 1 т.31, 2023 года.
Бесплатный доступ
The disorganization of natural biorhythms in the modern world is mostly attributed to the violation of circadian rhythms due to light pollution. Urbanization is directly interconnected with an excess of artificial lighting. As a one-time phenomenon, light pollution leads to a reversible shift in circadian rhythms, but in the case of constant influence, it leads to the development of desynchronosis. It is known that light pollution contributes to the development of nonalcoholic fatty liver disease, primary biliary cirrhosis, and metabolic disorders. The study aimed to investigate the changes in micro-morphometric parameters and ultrastructure of hepatocytes of Wistar rats under the influence of normal lighting and constant lighting exposure. This study was conducted on 120 outbred stock male Wistar rats at an age of 6 months, with a body weight of 350 g. The rats were divided into 2 equal groups. The control group included 60 rats, kept in standard laboratory conditions under a normal cyclical «light-dark» lighting regime (10:14, 10 hours of light - from 8:00 to 18:00, 14 hours of darkness - from 18:00 to 8:00) within 3 weeks. The experimental group included 60 rats kept in standard laboratory conditions under constant lighting within 3 weeks. To obtain the results, histological, micro morphometric methods and transmission electronic microscopy were used. The revealed changes of the hepatocytes under the influence of constant lighting indicate that a violation of the illumination regime is a potent factor causing damage and structural changes in the liver. Understanding the mechanisms underlying the liver's response to circadian rhythm disruption and associated damage is important to form patient-specific recommendations on lifestyle and behavioral regimens.
Hepatocyte, liver, micromorphometry, constant lightning, desynchronosis
Короткий адрес: https://sciup.org/143180239
IDR: 143180239 | DOI: 10.20340/mv-mn.2023.31(1).758
Текст научной статьи The influence of the constant illumination on the ultrastructure of rat's hepatocytes
Areshidze DA, Kozlova MA, Chernikov VP, Kondashevskaya MV. The influence of constant illumination on the ultrastructure of rat’s hepatocytes. Morfologicheskie Vedomosti – Morphological newsletter. 2022;30(4):758. (4).758
Арешидзе Д.А., Козлова М.А., Черников В.П., Кондашевская М.В. Влияние постоянного освещения на ультраструктуру гепатоцитов крыс. Морфологические ведомости. 2022;30(4):758. (4).758
Introduction. The normal functioning of living systems depends on several rhythmic environmental and internal cycles, which are referred to as biological rhythms. One of the most significant groups of rhythms is the group of diurnal, or circadian rhythms (onwards - CRs) [1]. Biological rhythms are known to be modulated under the influence of periodic environmental factors, the leading role among which is played by light exposure [2]. Prolonged contravention of natural biological rhythms leads to an adaptive irregularity, to desynchronosis, which can entail the development of a variety of pathological conditions in an organism [3]. The liver plays a key role in maintaining metabolic homeostasis and serves as a unique functional system That is involved in many mechanisms of regulation, reaction, and adaptations [4-5]. The autonomous biological clock of hepatocytes at the molecular genetic level includes the Bmal1 gene, paired with the Clock gene, Per genes (Per1, Per2, Per3), and Cry genes coding cryptochrome proteins (Cry1, Cry2), which are involved in the formation of specific genetic profiles with other numerous genes [6-7]. BMAL1/CLOCK also binds to the
E-Box DBSs present in the genes of the nuclear receptors Rev-Erba (NR1D1) and Re-vErbb (NR1D2) to activate their transcription, while the presence of ROR-response element (RORE) DBSs in the Rev-Erba/b genes mediate their autorepression. REV-ERBs also inhibit (through RORE DBSs) the transcription of their activators Bmal1 and Clock, thus constituting the second loop of the CC-oscillator [8]. The main central pacemakers of circadian rhythms in mammals are the suprachiasmatic nuclei (SCN) of the hypothalamus. The rhythm-organizing function of the SCN is modulated by environmental time-giver stimuli (timers), the main of which is light. The SCN transmits a «time signal» to other organs, synchronizing peripheral pacemakers [9]. These peripheral structures dependent on SCN regulation are found in the olfactory bulb, arcuate nucleus, pineal gland, and adrenal cortex and perform a synchronizing function by synthesis of regulating hormones [10-12]. Feeding habits and ambient temperature are also shown to act as circadian rhythm timers [13-14].
The disorganization of natural biorhythms in the modern world is mostly attributed to the violation of circadian rhythms due to light pollution. Urbanization is directly interconnected with an excess of artificial lighting. As a one-time phenomenon, light pollution leads to a reversible shift in circadian rhythms, but in the case of constant influence, it leads to the development of desynchronosis [15]. The level of light pollution correlates with such metabolic changes as a decrease in high-density lipoprotein levels, an increase in triglyceride levels, and carbohydrate metabolism disorders [16-17]. Violation of the light regime is one of the possible premises of the occurrence of metabolic syndrome and can increase the risk of developing type 2 diabetes mellitus and atherosclerosis [18-19]. In addition, there is evidence that changes caused by chronic desynchronosis can lead to the development of malignant liver tumors [20-21]. It is known that light pollution contributes to the development of nonalcoholic fatty liver disease, primary biliary cirrhosis, and metabolic disorders [22-26]. However, the effect of constant illumination on the ultrastructure of hepatocytes underlying the above pathologies remains practically unexplored. Functional changes in hepatocytes are reflected in a variety of morphological structure modifications and cell death [27-28].
The study aimed to investigate the changes in micro-morphometric parameters and ultrastructure of hepatocytes of Wistar rats under the influence of normal lighting and constant lighting exposure.
Materials and research methods. This study was conducted on 120 outbred stock male Wistar rats at an age of 6 months, with a body weight of 350 g. Animals were taken from the «Stolbovaya» Nursery affiliated with the Scientific Center of Biomedical Technologies of the Federal Medical and Biological Agency. All the animals were housed in plastic cages with free access to water and food. The rats were divided into 2 equal groups. The control group included 60 rats, kept in standard laboratory conditions under a normal cyclical «light-dark» lighting regime (10:14, 10 hours of light – from 8:00 to 18:00, 14 hours of darkness – from 18:00 to 8:00) within 3 weeks. The experimental group included 60 rats kept in standard laboratory conditions under constant lighting for 3 weeks. Illumination intensity was 300 luces for animals of both groups, the illuminance was equal for all the cages. Since the feeding regime can significantly affect the circadian rhythms of the liver, the animals were provided with constant, round-the-clock access to food and drink to eliminate the effect of this factor.
Withdrawal of animals from the experiment was carried out three weeks after the start of the experiment in a carbon dioxide chamber equipped with a device for the upper gas supply (100% CO 2 ) at 9:00, 15:00, 21:00 and 3:00. The chamber volume was filled with gas at a rate of 20% per minute to avoid dyspnea and pain in animals. After sacrifice, evisceration was performed. All animal experiments were performed according to compliance with EC Directive 86/609/EEC and with the Russian law regulating experiments on animals. Keeping of animals and experiments were performed following the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 18 March 1986). The study was approved by the Local Bioethics Committee of the Academician Avtsyn Scientific Research Institute of Human Morphology, Minutes № 27/3 (11/10/2021).
The liver was fixed in 10% neutral buffered formalin with further processing in alcohols of increasing concentration (50°, 60°, 70°, 80°, and 96°) and xylol, followed by placement in a Histomix histological medium. Liver samples were embedded in paraffin, and serial sections with a thickness of 5-6 μm were prepared. Histological sections were made on the sliding microtome Leica SM2010 R. Hematoxylin-eosin staining was carried out. Stained sections were put in a BioMount mounting medium. The microscopy of histological preparations was performed using a Leica DM 2500 microscope with the use of a Leica DFC 290 digital camcorder. 10 digital images of randomly selected visual fields were taken at a magnification of ×400 and ×1000 from each preparation. With the use of the digital «ImageJ» Program the crosssectional nuclear area (Sn), small (d) and long (D) diameters of a nucleus, perimeter of the nucleus (Pn), a cross-sectional area of the cell
(Sc), small (a) and long (b) diameters of the cell were studied. The measurements were carried out in micrometers after preliminary geometric calibration on an objectmicrometer scale digitized with the same magnification. Several parameters were calculated using the appropriate formulas: the nucleocytoplasmic ratio as NCR=Sn/(Sc–Sn); mean diameter of nucleus M=(D+d)/2, in which D – long diameter, d – small diameter; a volume of nuclei Vn=0,523·M 3 ; cell volume Vc=0,523·M 3 , in which M – mean diameter of cell; elongation index of nucleus EI=D/d, in which D – long diameter, d – small diameter. The ratio of the volume of the nucleus to its area was also determined [29]. For the calculation of the coefficient of nuclear form, the following formula was used: CF=4×π×Sn/Pn2 in which Sn – the area of the nucleus, Pn – the perimeter of a nucleus. The contoured index of a nucleus, which represents the relief of its surface, was calculated according to the formula: CI=Pn/√ Sn (Sn – the area of the nucleus, Pn – the perimeter of a nucleus) [29]. To calculate the proportion of binuclear hepatocytes, we examined 10 fields from each preparation with a magnification of ×400. The percentage of binuclear cells was expressed as a percentage of the total number of hepatocytes in the field of view.
For the electronic microscopy liver samples of 2 mm3 size were fixed with a 2,5% solution of glutaraldehyde in 0,1 M phosphate buffer (pH 7,4), additionally fixed in a 1% solution of osmium tetroxide (OsO4), dehydrated in ethanol according to the generally accepted scheme, contrasted with 1% uranyl acetate in 70% ethanol during dehydration and poured into the eponaraldite mixture according to the standard procedure. Ultrathin sections were obtained on an LKB-III ultramicrotome, the sections were additionally counterstained with lead citrate according to the Reynolds method and viewed with a JEM-100CX transmission electron microscope. Photo fixation of preparations was carried out using a Gatan ES500W Erlangshen camera at a magnification of ×5000 and ×6700. The shapes of the hepatocyte nuclei and the condition of their organelles (mitochondria, ribosomes) were evaluated, and the presence of lipid vacuoles was revealed during transmission electronic microscopy. The obtained data were analyzed by calculating average values, standard deviation, and arithmetic mean error. The data are presented as mean±SD. To assess the significance of differences, the Student's t-test was used. Changes were considered reliably significant at p≤0,05.
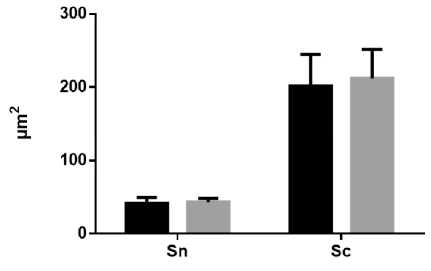
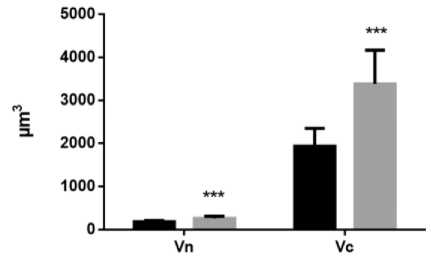
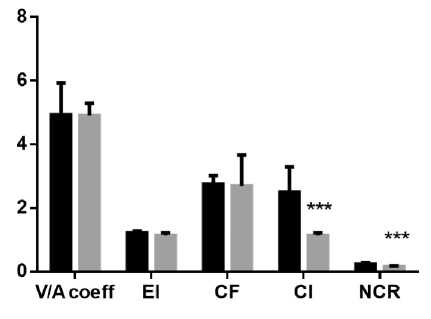
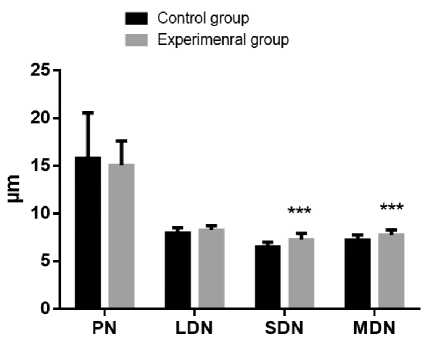
Results and Discussion. Exposure to constant light conditions for three weeks resulted in significant differences in micro morphometric parameters from the norm. We noted an increase in the area and volume of hepatocytes, causing a decrease in the nucleocytoplasmic ratio, as well as a decrease in the proportion of binuclear cells relative to the control. At the same time, the small and average diameters of the nuclei increased and the index of its contour decreased (Fig. 1, A, B, C, and D).
Electronic microscopy studies made it possible to establish several distinct changes in the ultrastructure of hepatocytes. The nuclei of a significant part of the cells, in comparison
A
B
■ Control group
■ Experimenral group
C
Fig. 1. Diagrams of indicators of changes in hepatocytes under the influence of constant lighting. A: Sn – cross-sectional area of nuclei; Sc - cross-sectional area of cells. B: Vn – volume of nuclei; Vc – cell volume. С: CI – contour index of nuclei; EI – elongation index of nuclei; СF – coefficient of a form of nuclei; NCR – the nucleocytoplasmic ratio. D: PN – the perimeter of the nucleus; SDN – small diameters of nuclei; LDN – long diameters of nuclei; MDN – mean diameters of nuclei. Common notes: * - p≤0,05, ** - p≤0,005, *** -p≤0,0005 – in comparison with the same parameters of animals of the control group
Рис. 1. Диаграммы показателей изменения гепатоцитов при воздействии постоянного освещения. A: Sn – площадь поперечного сечения ядра; Sc - площадь поперечного сечения клетки. B: Vn – объем ядра; Vc – объем клетки. С: CI – индекс контура ядра; EI – индекс удлиненности ядра; СF – коэффициент формы ядра; NCR – ядерно-цитоплазматическое отношение. D: PN – периметр ядра; SDN – малый диаметр ядра; LDN – большой диаметр ядра; MDN – средний диаметры ядер. Примечания: * - р≤0,05, ** - р≤0,005, *** - р≤0,0005 – в сравнении с аналогичными показателями животных контрольной группы
D
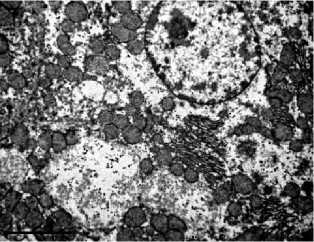
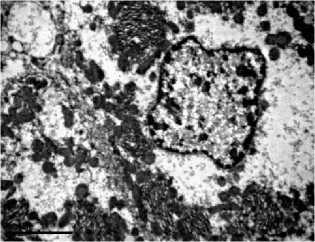
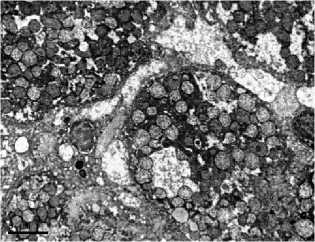
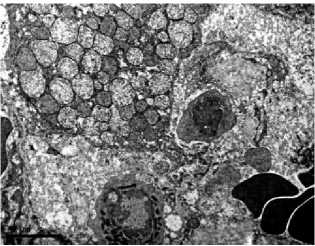
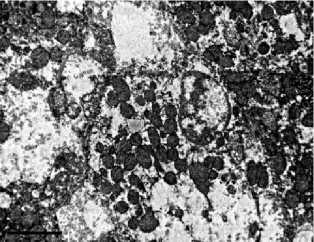
with the control (Fig. 2A), acquired sinuous contours and sometimes lost their rounded shape. The cytoplasm was poor with glyco- gen, the granular endoplasmic reticulum is noticeably reduced, so-called «ribosomal shedding», (which indicates a decrease in protein synthesis in hepatocytes) is observed (Fig. 2B). The significant number of cells contained a large amount of swollen mitochondria (Fig. 2C, 2D). Among hepatocytes, the single leukocytes (Fig. 2D) and a decrease in the proportion of binuclear cells were noted. Dying hepatocytes were also detected (Fig. 2E).
A
B
C
D
E
Fig 2. Ultramicrophotos of structural changes in hepatocytes of rats. A – hepatocyte of rat of a control group. The rounded nucleus, mitochondria with a dense matrix, and glycogen grains are observed. B-F – hepatocytes of rats of the experimental group. B – the nucleus with a sinuous contour and freely located ribosomes in the hepatocyte. C – hepatocyte containing numerous edematous mitochondria with an enlightened matrix. D – significantly pronounced degree of swelling of mitochondria (above) and a leukocyte (below). E – an area of necrosis development in the liver of rats of the experimental group. TEM. The magnification on A, D, E – ×6700, on B – ×8000, on C – ×5000
Рис. 2. Ультрамикрофотографии с труктурных изменений клеток печени крыс. А – гепатоцит крыс контрольной группы. Наблюдаются округлое ядро, митохондрии с плотным матриксом, зерна гликогена. B-F – клетки крыс опытной группы. B – ядро гепатоцита с извилистым контуром, свободно расположенные рибосомы. C – гепатоцит, содержащий многочисленные отечные митохондрии с просветленным матриксом. D – часть гепатоцита со хорошо заметно значительное набухание митохондрий гепатоцита выраженной степенью (вверху) и лейкоцита (внизу). E – зона развития некрозов в печени крыс экспериментальной группы. Электронная микроскопия. Ув.: А, D, E – ×6700, B – ×8000, C – ×5000
Our results show an increase in the nuclei diameters with a change in the elongation index of the nuclei. This phenomenon is a sign of commencing destruction of nuclei [30]. Micro-morphometry results allowed us to assert ongoing structural changes in the hepatocytes of rats of the experimental group. Notable hepatocyte hypertrophy can be seen in all individuals exposed to constant light within three weeks.
The noted decrease in the proportion of binuclear hepatocytes in the experimental group can be explained by the fact that al- most all cell divisions produced daughter mononuclear cells, regardless of the number of nuclei in mother hepatocytes [31]. At the same time, processes of compensatory cell hypertrophy are the signs of liver regeneration and compensatory changes seen in stressful conditions [32]. Considering literature data indicating that initial stages of hepatocyte adaptation to pathological influence are predominantly processes of intracellular regeneration, it can be assumed that in the experimental group, the process of adaptation to light pollution occurs mainly through intracellular regeneration which manifests itself by hepatocyte hypertrophy [33]. Hepatocyte hypertrophy, occurring after a partial hepatectomy (PHx), shows an average 150% size increase in hepatocytes, and the cells pass and move on to proliferation only after 1-2 days [34]. A similar effect can be seen in our results. Hepatocyte hypertrophy is the first reaction to liver damage, and proliferation occurs when hypertrophy is not enough to restore the initial mass of the organ. Hypertrophy of hepatocytes manifests through an increase in cellular organelles (mitochondria, lysosomes, endoplasmic reticulum, and ribosomes) and the accumulation of lipids and glycogen [35-36].
Such changes are also largely determined by the fact that the production of pineal melatonin, which demonstrates numerous hepatoprotective effects in several pathologies, almost stops under conditions of constant illumination [37-39]. Thus, melatonin can activate hepatocyte proliferation by inhibiting IKKα, JNK1, and cJUN (c-Jun N-terminal kinases), which inhibit mitotic and apoptotic activity, under standard light conditions, but in the absence of pineal melatonin, their acute decrease is observed [40-41]. It is known that one of the effects of melatonin is an increase in the ploidy and proportion of binuclear hepatocytes, and pinealectomy reduces the intensity of proliferation in the liver after its partial resection [40-42].
The changes seen in the hepatocytes of the experimental group in our study represent the activation of defensive and adaptive mechanisms and the onset of cellular damage in the liver. As such, these changes represent the negative influence of light pollution on hepatocyte health. As our study presents an extreme but short-term representation of light pollution, it does not directly represent
Список литературы The influence of the constant illumination on the ultrastructure of rat's hepatocytes
- VitaternaMH, Takahashi JS, TurekFW. Overview of circadian rhythms. Alcohol Res Health. 2001;25(2):85-93
- Boyce PR. Human factors in lighting. Third Edition. Boca Raton-London-New York: Crc Press, 2014.- 703pp
- Jasser SA, Blask DE, Brainard GC. Light during darkness and cancer: relationships in circadian photoreception and tumor biology. Canc Caus Con. 2006;17(4):515-523. DOI: 10.1007/s10552-005-9013-6
- Fazakas J, Mändli T, Ther G et al. Evaluation of liver function for hepatic resection. Transplant Proc. 2006;38(3):798-800. DOI: 10.1016/j.transproceed.2006.01.048
- Parkinson A, Ogilvie BW. Biotransformation of xenobiotics. Casarett and Doull's toxicology: the basic science of poisons. 2008;7:161-304
- Kim P, Oster H, Lehnert H et al. Coupling the Circadian Clock to Homeostasis: The Role of Period in Timing Physiology. Endocr Rev. 2019;40(1):66-95. DOI: 10.1210/er.2018-00049
- Shi D, Chen J, Wang J et al. Circadian Clock Genes in the Metabolism of Non-alcoholic Fatty Liver Disease. Front Physiol. 2019;10:423. DOI: 10.3389/fphys.2019.00423
- Mukherji A, Bailey SM, Staels B, Baumert TF. The circadian clock and liver function in health and disease. J Hepatol. 2019;71(1):200-211. DOI: 10.1016/j.jhep. 2019.03.020
- Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018;19(8):453-469. DOI: 10.1038/s41583-018-0026-z
- Balsalobre A, Brown SA, Marcacci L et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344-2347. DOI: 10.1126/science.289.5488.2344
- Guerrero-Vargas NN, Guzmân-Ruiz M, Fuentes R et al. Shift Work in Rats Results in Increased Inflammatory Response after Lipopolysaccha-ride Administration: A Role for Food Consumption. J Biol Rhythms. 2015;30(4):318-330. DOI: 10.1177/0748730415586482
- Guilding C, Hughes AT, Brown TM et al. A riot of rhythms: neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol Brain. 2009;2:28. DOI: 10.1186/1756-6606-2-28
- Brown SA, Zumbrunn G, Fleury-Olela F et al. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12(18):1574-1583. DOI: 10.1016/s0960-9822(02)01145-4
- Westerterp-Plantenga MS. Challenging energy balance - during sensitivity to food reward and modulatory factors implying a risk for overweight - during body weight management including dietary restraint and medium-high protein diets. Physiol Behav. 2020;221:112879. DOI: 10.1016/j .physbeh.2020.112879
- Leung JM, Martinez ME. Circadian Rhythms in Environmental Health Sciences. Curr Environ Health Rep. 2020;7(3):272-281. DOI: 10.1007/s40572-020-00285-2
- Aho V, Ollila HM, Kronholm E et al. Prolonged sleep restriction induces changes in pathways involved in cholesterol metabolism and inflammatory responses. Sci Rep. 2016;6:24828. DOI: 10.1038/srep24828
- Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018;84:11-27. DOI: 10.1016/j.metabol.2017.11.017
- Mota MC, Silva CM, Balieiro LCT et al. Social jetlag and metabolic control in non-communicable chronic diseases: a study addressing different obesity statuses. Sci Rep. 2017;7(1):6358. DOI: 10.1038/s41598-017-06723-w
- Anisimov VN. Light desynchronosis and health. Light & Engineering (2019);27.3:14-25
- Masri S, Sassone-Corsi P. The emerging link between cancer, metabolism, and circadian rhythms. Nat Med. 2018;24(12):1795-1803. DOI: 10.1038/s41591-018-0271-8
- Verlande A, Masri S. Circadian Clocks and Cancer: Timekeeping Governs Cellular Metabolism. Trends Endocrinol Metab. 2019;30(7):445-458. DOI: 10.1016/j.tem.2019.05.001
- Wei L, Yue F, Xing L et al. Constant light exposure alters gut microbiota and promotes the progression of Steatohepatitis in high fat diet rats. Front Microbiol. 2020;11:1975. DOI: 10.3389/fmicb.2020.01975
- Heathcote J. Treatment of primary biliary cirrhosis. J Gastroenterol Hepatol. 1996;11(7): 605-609. DOI: 10.1111/j.1440-1746.1996.tb00300.x
- van den Heiligenberg S, Deprés-Brummer P, Barbason H et al. The tumor promoting effect of constant light exposure on diethylnitrosamine-induced hepatocarcinogenesis in rats. Life Sci. 1999;64(26):2523-2534. DOI: 10.1016/s0024-3205(99)00210-6
- Walker WH, Bumgarner JR, Walton JC et al. Light Pollution and Cancer. Int J Mol Sci. 2020;21(24):9360. DOI: 10.3390/ijms21249360
- Nelson RJ, Chbeir S. Dark matters: effects oflight at night on metabolism. Proc Nutr Soc. 2018;77(3):223-229. DOI: 10.1017/S0029665118000198
- Li W, Li L, Hui L. Cell Plasticity in Liver Regeneration. Trends Cell Biol. 2020;30(4):329-338. DOI: 10.1016/j.tcb.2020.01.007
- Junatas KL, Tonar Z, Kubikovâ T, Liska V, Pâlek R, Mik P, Krâlickovâ M, Witter K. Stereological analysis of size and density of hepatocytes in the porcine liver. J Anat. 2017;230(4):575-588. DOI: 10.1111/joa.12585
- Smitha T, Sharada P, Girish H. Morphometry of the basal cell layer of oral leukoplakia and oral squamous cell carcinoma using computer-aided image analysis. J. Oral Maxillofac Pathol. 2011;15:26-33
- Häussinger D, Graf D, Weiergräber OH. Glutamine and cell signaling in liver. J Nutr. 2001;131(9 Suppl):2509S-14S; discussion 2523S-4S. DOI: 10.1093/jn/131.9.2509S
- Bardeck N, Paluschinski M, Castoldi M et al. Liver cell swelling leads to upregulation of miR-141-3p in perfused rat liver and primary rat hepatocytes. Zeitschrift für Gastroenterologie. 2021;59(01):1-16. DOI: 10.1055/s-0040-1721965
- Miyaoka Y, Ebato K, Kato H et al. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;22(13):1166-1175. DOI: 10.1016/j.cub.2012.05.016
- Lazzeri E, Angelotti ML, Conte C et al. Surviving Acute Organ Failure: Cell Polyploidization and Progenitor Proliferation. Trends Mol Med. 2019;25(5):366-381. DOI: 10.1016/j.molmed.2019.02.006
- Nagy P, Teramoto T, Factor VM et al. Reconstitution of liver mass via cellular hypertrophy in the rat. Hepatology. 2001;33(2):339-45. DOI: 10.1053/jhep.2001.21326
- Miyaoka Y, Miyajima A. To divide or not to divide: revisiting liver regeneration. Cell Div. 2013;8(1):8. DOI: 10.1186/1747-1028-8-8
- Anderson WR, Zieve L, Lindblad S. Ultrastructural study of hepatic regeneration following one-lobe, two-lobe, and subtotal hepatectomy in the rat. Exp Pathol. 1990;38(1):61-72. DOI: 10.1016/s0232-1513(11)80200-8
- Khraiwesh H, Löpez-Dominguez JA, Lôpez-Lluch G et al. Alterations of ultrastructural and fission/fusion markers in hepatocyte mitochondria from mice following calorie restriction with different dietary fats. J Gerontol A Biol Sci Med Sci. 2013;68(9):1023-1034. DOI: 10.1093/gerona/glt006
- Mathes AM. Hepatoprotective actions of melatonin: possible mediation by melatonin receptors. World J Gastroenterol. 2010;16(48): 6087-6097. DOI: 10.3748/wjg.v16.i48.6087
- Chojnacki C, Walecka-Kapica E, Romanowski M et al. Protective role of melatonin in liver damage. Curr Pharm Des. 2014;20(30):4828-4833. DOI: 10.2174/1381612819666131119102155
- Esteban-Zubero E, Alatorre-Jiménez MA, Löpez-Pingarrön L et al. Melatonin's role in preventing toxin-related and sepsis-mediated hepatic damage: A review. Pharmacol Res. 2016;105:108-120. DOI: 10.1016/j.phrs.2016.01.018
- Abbasoglu O, Berker M, Ayhan A rt al. I. The effect of the pineal gland on liver regeneration in rats. Journal of Hepatology. 1995;23(5):578-581. DOI:10.1016/0168-8278(95)80065-4
- Liang R, Nickkholgh A, Hoffmann K et al. Melatonin protects from hepatic reperfusion injury through inhibition of IKK and JNK pathways and modification of cell proliferation. J Pineal Res. 2009;46(1):8-14. DOI: 10.1111/j.1600-079X.2008.00596.x
- Kobayashi T, Saito Y, Ohtake Y et al. Effect of aging on norepinephrine-related proliferative response in primary cultured periportal and perivenous hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2012;303(7):G861-869. DOI: 10.1152/ajpgi.00081.2012
- Wilkinson PD, Duncan AW. Differential Roles for Diploid and Polyploid Hepatocytes in Acute and Chronic Liver Injury. Semin Liver Dis. 2021;41(1):42-49. DOI: 10.1055/s-0040-1719175
- Yanko R. The combined influence of the intermittent normobaric hypoxia and melatonin on morphofunctional activity of the rat's liver parenchyma. Bulletin of Taras Shevchenko National University of Kyiv -Problems of Physiological Functions Regulation. 2018;25(2):36-40


