The molecular detection of the Anaplasma ovis pathogens of the serological samples in small ruminants and ixodid ticks in Azerbaijan
Автор: Azizova A.
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Сельскохозяйственные науки
Статья в выпуске: 12 т.9, 2023 года.
Бесплатный доступ
A total of 704 blood samples (561 sheep, 143 goats) were examined for the Anaplasma ovis infection during a 1-year period. PCR and ELISA were used for the detection of the A. ovis antibodies. PCR assay identified A. ovis in 20 (8.1%) sheep and 15 (6.09%) goats. Using ELISA assay, 8.53% (31) were positive (21 sheep, 10 goats). A total of 246 blood smears were examined for the presence of intra-erythrocytic inclusions using Giemsa stain. Among the collected specimens, 60 were found positive with an overall prevalence of 24.3%. Among the 60 positive animals, 26 (43.3%) were sheep and 34 (56.7%) were goat. In the peripheral blood samples, the other piroplasmids - Babesia ovis, Theileria ovis, Th. recondita - were followed in an associative form. The ticks were collected, and the species composition was appointed in order to determine the ticks parasitized and have a pathogenic lifestyle in small ruminants. The intensive infection with the ticks of 2 genera - Rhipicephalus and Hyalomma was followed in small ruminants. It was determined that 45.8% of sheep and 35.1% of goats were infected intensively with the ticks of the Hyalomma genus. 110 samples prepared from the ticks of the Hyalomma genus were tested from the PCR test according to the Anaplasma ovis pathogen. 45 samples (40.9%) were assessed positively that 21 samples of them belonged to sheep and 24 of them to goats. 80 samples prepared from the internal organs of the ticks were examined according to the A. ovis parasite and the obtained results were analyzed. In 5 out of 35 samples which detected the parasites, A. ovis was followed, and in 30 samples, the associative parasites: Th. ovis, B. ovis piroplasmids. The Rickettsia and Coxiella pathogens were also detected in the samples.
Molecular identification, polymerase chain reaction, elisa, anaplasma, ixodidae, small ruminants
Короткий адрес: https://sciup.org/14129283
IDR: 14129283 | УДК: 619:576.89, | DOI: 10.33619/2414-2948/97/21
Текст научной статьи The molecular detection of the Anaplasma ovis pathogens of the serological samples in small ruminants and ixodid ticks in Azerbaijan
Бюллетень науки и практики / Bulletin of Science and Practice
Anaplasma ovis is gram-negative rickettsial bacterium transmitted by the tick belonging to the genus Anaplasma, family Anaplasmataceae, and order Rickettsiales. Anaplasma ovis is transmitted by the ticks and reproduces asexually by the infecting the erythorocytes of their hosts. In addition to the biotic factors, the age and gender composition of the animals are also influenced to the distribution of the Anaplasma ovis parasite in sheep and goats. The local sheep and goats genera were used in our experiments. They aren't very sensitive to Anaplasma ovis and the other primitive blood parasites, in contrast to the animals brought to the republic. But the intensive infection showes the complexities with the decrease in weight in the animals, the death of the young animals and the other invasion diseases (eimerioses and helminthiases) in the older animals and causes the serious economic damage to the animal husbandry [1, 2]. 6 species caused to the disease of the Anaplasma genus noted the parasitise in cattle and small ruminants: A. ovis, A. marginale, A. centrale, A. platys, A. bovis and A. phagocytophilum [3]. A.ovis causes to the anaplasmosis in sheep and goats, and A.bovis in cattle [4]. The A. ovis parasite was observed in subclinical or mild form in small ruminants. And in case of intensive infection, it results with the anemia, miscarriage in the animals [5].
Azerbaijan is an agricultural country and engage with the animal husbandry for 300 years. The animal husbandry sector plays an important role in its national economy. The invasion diseases influence negatively to the intensive development of the animal husbandry in the republic. In the recent years, our researches shows that the invasion diseases are observed more in an associative form (primitive parasites, helminths). In Azerbaijan, in cattle, 4 species of the piroplasmidoses (theileriosis, anaplasmosis, piroplasmosis, francaiellosis) are distributed, and in small ruminants, 5 species (babesiosis, theileriosis, anaplasmosis, piroplasmosis, francaiellosis). In Azerbaijan, in cattle and small ruminants, A.marginale in cattle and A.ovis in small ruminants from the piroplasmidoses are noted more intensively [6].
For the first time, the serological samples of sheep and goats and the transmitting ticks were researched by the molecular examinations according to the A.ovis parasite, compared by the microscopic examinations, the results were analyzed. The intensive infection with the A.ovis parasite is noted in goats in the researches conducted by us in the 1 year. In the animals with a high temperature, the examinations were conducted by the Romanovsky-Giemza dyeing,the causative parasites were detected in the erythrocytes. The A. ovis parasite was detected in the internal organs
(spleen) of the dead kids [7]. The researches were continued at the level of the molecular biology, and the obtained results were compared by the classical examination methods.
The various serological methods — PCR (polymerase chain reaction), enzyme-linked immunosorbent assay (ELISA) tests were used for the detection the specific antibodies to the anaplasma. The competitive ELISA (cELISA) is depending on the use of a monoclonal antibody (Mab) ANAF16C1 that recognizes the conserved (MSP-5) antigen of different Anaplasma ovis and has high sensitivity and specificity for detection of Anaplasma antibodies [8, 9].
The “Gold standard” method for the diagnosis of Anaplasma spp . relies on the combination of the microscopic examination and cELISA [10]. The indirect immunofluorescence antibody test is widely used for the diagnosis of blood protozoon and Rickettsia . The ELİSA test is commonly used in epidemiological studies because of its low costs.
Molecular identification methods such as Polymerase chain reaction (PCR) have several advantages compared to the traditional serologic and blood smear tests [11]. PCR is the most sensitive and reliable diagnostic tool that allows discriminating between Anaplasma subspecies. In addition, PCR can detect the coinfections with multiple Anaplasma subspecies [12]. The aim of the study was to assess the sensitivity and specificity of the different diagnostic tools used for detecting anaplasmosis in sheep and goats.
Material and methods
The researches were conducted in the livestock farms of the Shirvan-Salyan economic regions of Azerbaijan. The animals were researched for the anaplasmosis from March 2021 to April 2022. The microscopic examination of the blood smear was mainly used as the reference diagnosis of the anaplasmosis. ELISA are the most commonly used serological methods for the detection the antibodies against to the anaplasma. PCR is the most reliable diagnosis for the anaplasma invasion.
The collection of the blood samples for the molecular examination
A total of 704 blood samples were taken from 561 sheep and 143 goats of different age groups (from 6 months to 2 years and over 2 years). To separate sera, the additive-free blood was allowed to clot for about 15–30 min at room temperature. The tubes then centrifuged at 1000–2000 rpm for 10 min and serum was collected. The serum specimens were stored at - 20 °C for further use.
Competitive ELISA (cELISA) assay
Sera were screened for the Anaplasma immunoglobulin G (IgG) by a semi- quantitative indirect ELİSA commercial kit (Fuller, USA), according to the manufacturer instructions. Briefly, sera samples were diluted in phosphate-buffer saline (PBS) and 25 μl were transferred to the slide wells. The slides were incubated at 35°C for 30 min then washed with PBS followed by distilled water to remove the unreacted antibodies. Twenty five μl anti-ovine conjugate with DyLight 488 dye (Fuller, USA) were added and incubated then removed by washing as previously described. The slide was examined by the standard fluorescence microscopy (Olympus BX50, Japan) at 400X magnification, the positive reaction appears as green fluorescent small cocci with a red background.
DNA extraction and PCR
DNA extraction was carried out using the G-spinTM Total DNA Extraction Kit (iNtRON Biotechnology, Korea) according to the instructions of the manufacturer. PCR was performed to detect both Anaplasma phagocytophilum , Anaplasma ovis using Bioin Gentech Veterinary PCR Kits (Concepcion, Chile) according to the instructions of the manufacturer. The cycling conditions were initial denaturation at 94 °C for 2 min, 35 cycles (94°C 30 s, 57°C 30 s, 72°C 30 sec) and a final extension at 72°C for 5 min.
Microscopic examination
Thin blood smears were prepared for microscopic examination accordingly the standard protocol [13].
The slides were allowed to air-dry before being fixed with absolute methanol. Fixed smears were stained with 10% Giemsa (Cresent diagnostic, KSA) and examined by using compound microscope under oil immersion lens. About 25 fields were examined from each slide for the presence of Anaplasma and the number of infected erythrocytes. Anaplasma was identified on the basis of its morphology [14].
The collection of the ticks
Ticks were collected by the generally accepted method, namely, when examining the animal, the identified ticks were removed, placed in a clean, dry container or container with a tight-fitting lid. Ticks were also collected from walls and floors in places where animals were kept (on pastures, meadows, in the soil). The collected mites were fixed, placed in closed test tubes, labeled, and stored in a refrigerator at minus 20°C.
Analysis of the obtained results
The double samples of the examined animals: sheep 561 (79.7%) and 143 (20.3%) of goats were taken. The animals were classified into three age groups: the samples collected from the animals aged 6 months to 1 year (172; 24.4%) were belonged to the first group, > 1-2 years old (250; 35.5%) animals to the second group and the animals older than 2 years to the third group (282; 40.1%) (Table 1).
Table 1
THE BASIC DEMOGRAPHICS OF THE SAMPLED ANIMALS
|
Categories |
||
|
Gender |
Female |
324 (46,0%) |
|
Male |
380 (54,0%) |
|
|
Host |
Sheep |
561 (79,7%) |
|
Goat |
143 (20,3 %) |
|
|
Age |
Age group I (6 m-1 y) |
172(24,4%) |
|
Age group II (1 y -2 y) |
250 (35,5%) |
|
|
Age group III (>2 y) |
282(40,1%) |
|
Competitive inhibition ELISA (cELISA) assay
The overall prevalence of Anaplasma spp. using cELISA was 8.53% (n = 31), including 21 (67,4%) specimens from sheep and 10 (32,6%) from goats. In sheep, the infection rates were higher among males (66,7%), animals of the age group I (42,9%). While in goats, the prevalence was 60,0% among males, 60,0% among age group I animals (Table 2).
Table 2
ELISA TESTING RESULT
|
Regions |
Animal type |
Serumsample |
Testing result |
||
|
Positive |
Negative |
Suspected |
|||
|
Bilasuvar |
goat |
70 |
5 |
58 |
7 |
|
Salyan |
sheep |
53 |
5 |
43 |
5 |
|
Hajigabul |
sheep |
120 |
16 |
102 |
2 |
|
Shirvan |
goat |
120 |
5 |
115 |
- |
|
Total |
363 |
31 |
330 |
12 |
|
31 positive samples were analyzed by the regions and 5 infection cases were noted in the Shirvan region, 5 in the Bilasuvar region, 5 in the Salyan region, 16 in the Hajigabul region. The high infection by the A.ovis parasite was noted in sheep in the Hajigabul region.
Table 3
THE ELISA TEST RESULTS FOR THE HAJIGABUL REGION
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
|
A |
100 |
117,28 |
49,04 |
4,8 |
8,88 |
2,8 |
7,52 |
7,84 |
2,4 |
5,76 |
8,88 |
53,28 |
|
B |
0 |
33,04 |
9,76 |
4,8 |
86,08 |
5,76 |
7,12 |
66,64 |
19,2 |
0,4 |
2,4 |
5,76 |
|
C |
23,84 |
28,96 |
39,52 |
6,96 |
19,2 |
44,56 |
0,48 |
6,96 |
8,72 |
0,88 |
77,2 |
0,4 |
|
D |
58,16 |
107,76 |
53,44 |
7,36 |
8,72 |
0,88 |
0,56 |
7,36 |
8,72 |
0,24 |
8,72 |
2,8 |
|
E |
30,72 |
8,08 |
7,36 |
7,52 |
74,08 |
52,56 |
0,32 |
7,52 |
7,84 |
77,2 |
7,84 |
5,76 |
|
F |
13,92 |
76,4 |
7,52 |
7,12 |
9,44 |
0,48 |
6,96 |
70,08 |
6,96 |
0,56 |
6,96 |
0,4 |
|
G |
32,88 |
77,2 |
7,12 |
2,8 |
9,76 |
0,56 |
67,6 |
7,36 |
7,36 |
0,32 |
7,36 |
48,88 |
|
H |
27,04 |
20,4 |
-0,56 |
3,04 |
2,8 |
0,32 |
7,52 |
0,56 |
0,48 |
0,56 |
7,52 |
0,24 |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
PCR analysis
The overall prevalence of Anaplasma spp. using PCR was 14,2 % (35), of which 20 (57,1%) were sheep and 15 (42,9 %) were goats (Table 5). In sheep, the infection rate was higher among males (45,0%), animals of the age group I (55,0%). While in goats, the prevalence was 60,0% among males, 53,3% amongage group I animals (Table 5).
Table 4
MOLECULAR IDENTIFICATION
|
Region |
Animal type Sample type Quantity Testing result Positive Negative |
|
Bilasuvar,Salyan |
Sheep, goat blood 120 20 100 |
|
Shirvan |
sheep blood 46 5 41 |
|
Hajigabul |
Sheep, goat blood 80 10 70 |
|
Total |
246 35 211 |
110 samples prepared from the tick smears were tested from the PCR test according to the Anaplasma ovis parasite in order to detect the causative agent of A.ovis in the ticks. The results showed that the ticks are invasion with the parasites (Figure 1).
The microscopic examination of the peripheral blood samples of the animals involved to the PCR examination were conducted. A total of 246 blood smears were examined for the presence of intra-erythrocytic inclusions using Giemsa stain. Anaplasma spp. appeared as small spherical deep purple intraerythrocytic inclusions. Among the collected specimens, 60 were found positive with an overall prevalence of 24.3%. Among the 60 positive animals, 26 (43.3%) were sheep and 34 (56.7%) were goat (Table 2).
The difference in Anaplasma prevalence in sheep and goats was not significant (P > 0.05). While there were 35 positive evaluations in the PCR examinations, this number increased to 60 in the microscopic examinations. The other piroplasmids — Babesia ovis, Theileria ovis, Th. recondita were followed in an associative form in the peripheral blood samples (Figure 2).
The examinations were also conducted by the Giemsa method, 80 samples prepared from the internal organs (salivary gland, ovary, intestine) of the ticks were examined according to the Anaplasma ovis parasite. A. ovis was followed in 5 of 35 samples detected parasites, and the associated parasites — Babesia ovis, Theileria ovis, Th. recondita piroplasmids in 30 samples. The Rickettsia and Coxiella pathogens were also detected in the samples (Figure 3).
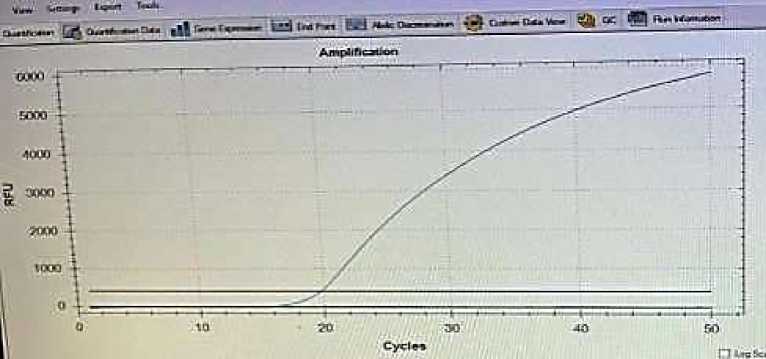
Figure 1. The infection level of the pathogenic ticks
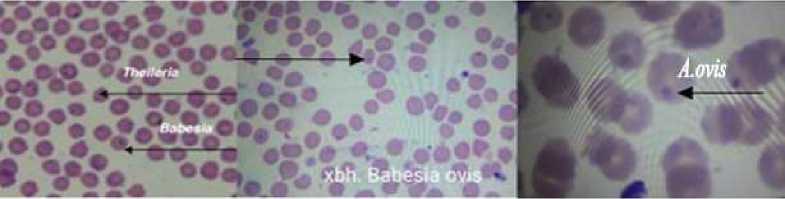
Figure 2. The piroplasmids in the erythrocytes
MICROSCOPIC EXAMINATION
Table 5
|
Host |
Demographic factor |
Positive animalspercentage |
|
|
Sheep ( n = 120) |
Gender |
Male (75) |
15 (12,5%) |
|
Female (35) |
11 (9,2%) |
||
|
Age |
Group I (33) |
10 (8,3%) |
|
|
Group II (37) |
9 (7,5%) |
||
|
Group III (40) |
7 (5,8%) |
||
|
Goat ( n = 126) |
Gender |
Male (76) |
19 (15,1%) |
|
Female (50) |
15 (11,9%) |
||
|
Age |
Group I (62) |
16 (12,7%) |
|
|
Group II (31) |
11 (8,7%) |
||
|
Group III (33) |
7 (5,6%) |
||
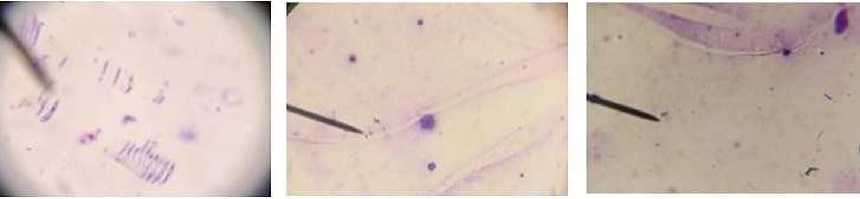
Figure 3. The Rickettsia and Coxiella pathogens (by the microscopy method)
The other piroplasmids ( Babesia ovis, Th. ovis, Th. recondita ), as well as the Rickettsia and Coxiella pathogens were detected during the classical examinations of the negative samples according to the A.ovis tests in the molecular examination.
Discussion
The anaplasmosis is noted in the agricultural animals, as well as in humans in the American, European and Asian countries [15-17]. Epidemiologic studies aimed to determine the prevalence of anaplasmosis uses different diagnostic tools, such as microscopic examination of stained blood smears, serological, and molecular tests. The reliability of the diagnostic tests is crucial for accurate diagnosis and estimation of the disease prevalence. Despite microscopic examination and serologic tests are practical and reliable diagnostics to detect Anaplasma spp. infection, they have limitations [18]. While the sensitivity against to one causative agent is checked in the molecular examinations, it is possible also to detect the other causative agents at the same time in the microscopic examinations. Our experiments confirmed that it is possible to detect the other piroplasmids and pathogens in addition to the A. ovis parasite by this method. As well as, the microscopic examinations are more efficient also financially for the big farmer farms. The accuracy of the stained blood smear examination can be hindered by the low number of the infected cells, lack of expertise of the examiner, and/or the occurrence of intracellular artifacts. In the early acute phase of the infection, serologic assays have limited value, due to the absence of the detectable antibodies [19].
In our researches, it was determined that the peripheral blood samples of the animals influenced negatively to the A.ovis tests were invasion with the other piroplasmids and pathogens. This shows that the microscopic examinations are practical for detecting more extensive invasions. Anaplasma is routinely diagnosed by the microscopic examination of the Giemsa stained blood smears and detection of intraerythrocytic Anaplasma inclusions. The microscopic examination is suitable for diagnosis of acute anaplasmosis, but it is not applicable for the detection of pre-symptomatic or carrier cases due to low numbers of Anaplasma infected cells in circulation, which falls below the detection limit [20].
In this study, based on the age factor, it was found that age group I (6 m — 1 yrs) had the highest rate of anaplasmosis. The showing a higher result of the anaplasmosis infection in the young animals in small ruminants was related not only with the immune system in the animals, but also with their associative infection with the other parasites. The infection with the piroplasmids at the same time with the dicroceliosis and strongyloides of the respiratory tract in the associative form were noted in sick sheep aged from 6 months to 1 year. The treatment was ineffective in the delayed form, the death was followed in the animals. In goats of that age group, the infection with the moniesiosis were detected in an associated form with the anaplasmosis that it also caused the death of the kids. The death was not followed in 1-2-year-old animals, the long-term treatment resulted with the recovery of the animals. And in animals older than 2 years, the clinical signs were showed weakly, the death was not followed, the decrease in weight was noted in the animals infected intensively with the ticks. The productivity decreased by 3.5-4 kg in sheep infected with the ticks and by 3 kg goats in 6 months. The animals’ skin was unusable for the leather production.
Several studies have reported that ELİSA may be used as an alternative to PCR [21] for the detection of anaplasmosis among sheep and goats [22]. A previous study reported a similar level of the specificity and sensitivity for ELİSA when compared with PCR.
Detection of carrier animals is very important, as they play a significant role in the disease epidemiology as reservoirs. Furthermore, it is essential for epidemiologic studies to discriminate between Anaplasma species [23]. PCR is reported to be more sensitive than conventional parasitological techniques in different hosts. It also enables the identification of different species. Therefore, we also evaluated PCR for the detection of the Anaplasma species in animals in comparison with ELISA.
35 out of 246 samples of our PCR tests were positive (14.2%). The sensitivity of the PCR results was 100% compared to the other diagnostic results. And Babesia ovis, Theileria ovis, Th.recondita parasites were detected in 25 more samples in the microscopic examinations. The results confirmed that 25 head animals were sick with the other piroplasmidoses. This shows that although the PCR tests are favorable in order to determine one parasite, it does not allow to determine the causative agents of the disease like the microscopic examinations. In recent years, our experiments confirmed that the A.ovis parasite, which is characteristic for the lowland landscapes, has distributed intensively among sheep and goats in the mountainous and foothill regions of the republic. And this indicates the increase of the infection risk of the anaplasma species with the zoonotic potential to humans.
Conclusions
Proper disease diagnosis requires reliable tests. Therefore, it is important to evaluate the existing diagnostic methods. The evaluation depends on several factors as; whether the test is suitable for the field and/or the laboratory settings; cost; and time required. The microscopic examination provides reliable results, but it is not suitable to diagnose carrier animals. cELISA is known for its ease of use, low cost, and for being quantitative and is an economical and easy method to perform. In the present study, ELİSA was highly specific and sensitive, but it requires special laboratory settings such as fluorescent microscope. PCR is the most sensitive and reliable diagnostic tool that achieves simultaneous differentiation between different Anaplasma subspecies.
Список литературы The molecular detection of the Anaplasma ovis pathogens of the serological samples in small ruminants and ixodid ticks in Azerbaijan
- Azizova, A. A. (2022). The associative invasions - the helminths, primary intestinal parasites, piroplasmids of the small ruminants in Azerbaijan. In 7th International New York Conference on evolvingtrends in interdisciplinary research and practices Proceedings book. New York, 97-103.
- Jonsson, N. N., Bock, R. E., & Jorgensen, W. K. (2008). Productivity and health effects of anaplasmosis and babesiosis on Bos indicus cattle and their crosses, and the effects of differing intensity of tick control in Australia. Veterinary parasitology, 155(1-2), 1-9. https://doi.org/10.1016/j.vetpar.2008.03.022
- Ybanez, A. P., Sashika, M., & Inokuma, H. (2014). The phylogenetic position of Anaplasma bovis and inferences on the phylogeny of the genus Anaplasma. Journal of Veterinary Medical Science, 76(2), 307-312. https://doi.org/10.1292/jvms.13-0411
- Hornok, S., Elek, V., de La Fuente, J., Naranjo, V., Farkas, R., Majoros, G., & Földvári, G. (2007). First serological and molecular evidence on the endemicity of Anaplasma ovis and A. marginale in Hungary. Veterinary microbiology, 122(3-4), 316-322. https://doi.org/10.1016/j.vetmic.2007.01.024
- Renneker, S., Abdo, J., Salih, D. E. A., Karagenç, T., Bilgiç, H., Torina, A., ... & Seitzer, U. (2013). Can Anaplasma ovis in small ruminants be neglected any longer?. Transboundary and emerging diseases, 60, 105-112. https://doi.org/10.1111/tbed.12149
- Azizova, A. A., & Rustamova, S. I. (2021). The distribution dynamics of the piroplasmid (Piroplasmida) and transmitting ticks in the agricultural animals. In Fundamental and applied scientific investigations in zoology: Actual problems, achievements and innovations. Baku, 23-27.
- Azizova A. A. (2022). The morphological parameters of the blood of sheep infected with the associative invasion. Endless Light in Science, (3-3), 350-357. https://doi.org/10.24412/2709-1201-2022-2022-350-357
- Bradway, D. S., de Echaide, S. T., Knowles, D. P., Hennager, S. G., & McElwain, T. F. (2001). Sensitivity and specificity of the complement fixation test for detection of cattle persistently infected with Anaplasma marginale. Journal of veterinary diagnostic investigation, 13(1), 79-81. https://doi.org/10.1177/10406387010130011
- Mason, K. L., Gonzalez, M. V., Chung, C., Mousel, M. R., White, S. N., Taylor, J. B., & Scoles, G. A. (2017). Validation of an improved Anaplasma antibody competitive ELISA for detection of Anaplasma ovis antibody in domestic sheep. Journal of Veterinary Diagnostic Investigation, 29(5), 763-766. https://doi.org/10.1177/104063871770949
- Ekici, O. D., & Sevinc, F. (2011). Comparison of cELISA and IFA tests in the serodiagnosis of anaplasmosis in cattle. African Journal of Microbiology Research, 5(10), 1188-1191.
- Dumler, J. S., & Brouqui, P. (2004). Molecular diagnosis of human granulocytic anaplasmosis. Expert Review of Molecular Diagnostics, 4(4), 559-569.https://doi.org/10.1586/14737159.4.4.559
- Torina, A., Agnone, A., Blanda, V., Alongi, A., D’Agostino, R., Caracappa, S., ... & de la Fuente, J. (2012). Development and validation of two PCR tests for the detection of and differentiation between Anaplasma ovis and Anaplasma marginale. Ticks and tick-borne diseases, 3(5-6), 283-287. https://doi.org/10.1016/j.ttbdis.2012.10.033
- Bowman, D. D. (2006). Successful and currently ongoing parasite eradication programs. Veterinary Parasitology, 139(4), 293-307. https://doi.org/10.1016/j.vetpar.2006.04.020
- Dumanli, N. (2016). Şanlıurfa yöresinde koyun ve keçilerde Anaplasma phagocytophilum’un moleküler yöntemlerle araştırılması.
- Noaman, V., & Sazmand, A. (2022). Anaplasma ovis infection in sheep from Iran: molecular prevalence, associated risk factors, and spatial clustering. Tropical Animal Health and Production, 54(1), 6. https://doi.org/10.1007/s11250-021-03007-4
- Johan S., Bakken J., Stephen Dumler.Human Granulocytic Anaplasmosis. Infect Dis Clin North Am. 2015 Jun; 29(2): 341–355.doi: 10.1016/j.idc.2015.02.007
- Bakken, J. S., & Dumler, J. S. (2015). Human granulocytic anaplasmosis. Infectious Disease Clinics, 29(2), 341-355. https://doi.org/10.1016/j.idc.2015.02.007
- Thomas, R. J., Dumler, J. S., & Carlyon, J. A. (2009). Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert review of anti-infective therapy, 7(6), 709-722. https://doi.org/10.1586/eri.09.44
- De Waal, T. (2012). Advances in diagnosis of protozoan diseases. Veterinary Parasitology, 189(1), 65-74. https://doi.org/10.1016/j.vetpar.2012.03.033
- Silva, V. M., Araújo, F. R., Madruga, C. R., Soares, C. O., Kessler, R. H., Almeida, M. A., ... & Torres Júnior, R. A. (2006). Comparison between indirect enzyme-linked immunosorbent assays for Anaplasma marginale antibodies with recombinant major surface protein 5 and initial body antigens. Memórias do Instituto Oswaldo Cruz, 101, 511-516. https://doi.org/10.1590/S0074-02762006000500005
- Zhang, X. C., Zhang, L. X., Li, W. H., Wang, S. W., Sun, Y. L., Wang, Y. Y., ... & Zhang, L. J. (2012). Ehrlichiosis and zoonotic anaplasmosis in suburban areas of Beijing, China. Vector-Borne and Zoonotic Diseases, 12(11), 932-937. https://doi.org/10.1089/vbz.2012.0961
- Niu, Q., Luo, J., Guan, G., Ma, M., Liu, Z., Liu, A., ... & Yin, H. (2009). Detection and differentiation of ovine Theileria and Babesia by reverse line blotting in China. Parasitology research, 104, 1417-1423. https://doi.org/10.1007/s00436-009-1344-x
- Dey, A., & Singh, S. (2009). Progress of science from microscopy to microarrays (Part 1): Diagnosis of parasitic diseases. Journal of Laboratory Physicians, 1(01), 002-006. https://doi.org/10.4103/0974-2727.54800


