The presence of various populations of circulating tumor cells in the blood of breast cancer patients before treatment: association with five-year metastasis-free survival
Автор: Kaigorodova Evgeniya V., Tarabanovskaya Nataliya A., Surkova Polina V., Zelchan Roman V., Garbukov Evgenii Y.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 6 т.19, 2020 года.
Бесплатный доступ
Localized and metastatic tumors are known to lead to the formation of circulating tumor cell (CTC) clusters in the blood. Currently, there is a heightened interest in the study of molecular and biological characteristics of CTCs. Recent studies have shown the presence of different populations of CTCs in the blood of cancer patients. Some cells are cancer stem cells, some tumor cells undergo epithelial-mesenchymal transition (EMT), and most CTCs do not have features of either stem cells or EMT. The aim of the study was to evaluate the five-year metastasis-free survival rate in patients with invasive breast carcinoma, depending on the presence of various populations of circulating tumor cells in the blood before treatment. Material and Methods. A prospective study included 47 patients with newly diagnosed invasive breast cancer (T1-4N0-3M0), who were treated at Cancer Research institute, Tomsk National Research Medical Center. The patients aged 31 to 69 years. The presence of different populations of CTCs in the blood of patients before treatment was determined by multicolor flow cytometry on the BD FACS Canto system, using different fluorochrome-labeled monoclonal antibodies to EpCam, CD45, CD44, CD24, and N-cadherin. Five-year metastasis-free survival was evaluated by the Kaplan-Meier method. The differences were considered significant at p function show_abstract() { $('#abstract1').hide(); $('#abstract2').show(); $('#abstract_expand').hide(); }
Breast cancer, ctc heterogeneity, five-year metastasis-free survival, circulating cancer stem cells, epithelial-mesenchymal transition, liquid biopsy
Короткий адрес: https://sciup.org/140254399
IDR: 140254399 | УДК: 618.19-006.6-092.18-033.2-076-036:616.15 | DOI: 10.21294/1814-4861-2020-19-6-57-65
Текст научной статьи The presence of various populations of circulating tumor cells in the blood of breast cancer patients before treatment: association with five-year metastasis-free survival
Breast cancer (BC) is the most common cancer in women (20.9 %) and is the leading cause of cancer-related death (17.0 %) in women. Approximately 60,000 new cases of breast cancer are diagnosed annually, with approximately 23,000 deaths from it [1].
Hematogenous metastasis is the major cause of cancer mortality. Currently, it is believed that circulating tumor cells (CTCs) are a population of tumor cells that enter the bloodstream. It has been shown that even localized tumors without clinical evidence of metastases are sources of CTCs [2]. However, CTCs are a rare population of cells. The number of
CTCs in 1L of blood (erythrocytes – 1012; granulocytes – 109–1010; lymphocytes – 109, etc.) is 1–107 cells. Besides, CTCs are quite heterogeneous in size and density, which creates great difficulties for their complete isolation [3]. Not more than 0.01 % of all CTCs initiate metastases [4].
Today, there is a heightened interest in studying the molecular and biological characteristics of circulating tumor cells. CTCs are a heterogeneous population: some cells are cancer stem cells, some of them undergo epithelial-mesenchymal transition (EMT), and most CTCs do not have features of either stem cells or EMT [5–7].
Circulating cancer stem cells may have specific features that allow them to survive in the bloodstream and cause metastatic lesions. Cancer stem cell populations have a number of molecular markers, which include antigens CD44, CD24, CD133, CD166, the ALDH1 enzyme (aldehyde dehydrogenase 1), ABC transport proteins (ABCG2, ABCB5), and the epithelial cell adhesion molecule (EpCam) [8–11].
In 2003, M. Al-Hajj et al., using breast cancer as an example, for the first time proved the existence of cancer stem cells in solid tumors. They showed that only cells with a specific phenotype – CD44 + CD24-/ low Epcam + – were able to induce human breast cancer in immunodeficient mice models. It should be noted that the tumor developed with the introduction of only 200 such cells, while the introduction of even several tens of thousands of breast cancer cells without this phenotype did not induce tumor development [12].
The aim of this study was to evaluate five-year metastasis-free survival in patients with invasive breast carcinoma, depending on the presence of various populations of circulating tumor cells in the blood before treatment.
Material and Methods
A prospective study included 47 patients with newly diagnosed invasive breast cancer (T1–4N0–3M0), who were treated at the Cancer Research Institute, Tomsk National Research Medical Center between 2013 and 2016. The patients aged 31 to 69 years. Venous blood in a volume of 5 ml taken before treatment was the material of the study. The study was approved by the local Ethics Committee of Cancer Research Institute, Tomsk National Research Medical Center (protocol No 2, 14.02.2014).
Different populations of CTCs were evaluated by flow cytometry on the BD FACS Canto system (Becton, Dickinson and Company (BD), USA) using BD FACSDiva and NovoExpress software:
(CTC1 – Epcam+CD45-CD44-CD24-Ncadherin-;
CTC2 – Epcam+CD45-CD44-CD24-Ncadherin+;
CTC3 – Epcam+CD45-CD44+CD24-Ncadherin+;
CTC4 – Epcam+CD45-CD44+CD24-Ncadherin-;
CTC5 – Epcam (m)-CD45-CD44+CD24-Ncadherin-;
CTC6 – Epcam (m)-CD45-CD44+CD24-Ncadherin+).
For this procedure, venous blood was incubated with different fluorochrome-labeled monoclonal antibodies to CD45 (clone HI30, APC/Cy7) (Biolegend, USA), EpCAM (clone 9C4, PE) (Biolegend, USA), CD44 (clone BJ18, FITC) (Biolegend, USA), CD24 (clone ML5, PE/Cy7) (Biolegend, USA), and N-cadherin (clone 8C11, PerCP/Cy5.5) (Biolegend,
USA). Then erythrocytes were lysed in a lysis solution (BD FACS lysing solution) and washed twice with the CellWash buffer. The cell pellet was resuspended in 1 ml BD Flow buffer. All samples were stored in the dark at 4 °С and analyzed using flow cytometry within 1 hour. To exclude autofluorescence, unstained samples were used.
The data obtained were processed using the Sta-tistica 10.0 software package (StatSoft Inc., USA). The significance of differences was assessed using Pearson’s χ2 test and Fisher’s exact test. The five-year metastasis-free survival rate was assessed by the Kaplan-Meier method and Log-rank (Mantel-Cox) test. The differences were considered significant at р<0.05.
Results
The presence of CTCs (CTC1 and/or CTC2-CTC6) in the blood of breast cancer patients before treatment was observed in 77 % of cases (36/47), while 23 % (11/47) of breast cancer patients were CTC-negative. In the group of patients with CTCs, hematogenous metastases were detected in 17 % (8/47) of cases; no evidence of hematogenous metastases were found in 60 % of cases. In the group of CTC-negative patients, no hematogenous metastases were observed.
Division of CTCs into 6 phenotypes (CTC1 – Epcam+CD45-CD44-CD24-Ncadherin-; CTC2 – Epcam+CD45-CD44-CD24-Ncadherin+; CTC3 – Epcam+CD45-CD44+CD24-Ncadherin+; CTC4 – Epcam+CD45-CD44+CD24-Ncadherin-; CTC5 – Epcam (m)-CD45-CD44+CD24-Ncadherin-; CTC6 – Epcam (m)-CD45-CD44+CD24-Ncadherin+) showed that CTCs without stemness and EMT features (CTC1) were observed in 51 % of cases. In the group of breast cancer patients with hematogenous metastases, the frequency of presence and absence of CTC1 was statistically insignificant (Fig. 1).
Non-stem CTCs showing signs of EMT (CTC2) were observed in 38% of cases. We observed CTC2 significantly more often in the group of patients with hematogenous metastases (p<0.05) (Fig. 2). Stem-like CTCs showing signs of EMT (CTC3) were observed in 32 % of patients with breast cancer. In the group of patients with hematogenous metastases of breast cancer, CTC3 population was found in 5 of 8 patients. In 3 of 8 patients, no CTC3 cells were detected (Fig. 3). Stem-like CTCs showing no signs of EMT (CTC4) were detected in 43 % of patients. In the group of patients with hematogenous metastases, the frequency of presence and absence of CTC4 was statistically insignificant (Fig. 4).
Stem-like CTCs without expression of Epcam on the membrane (Еpcam (m)-CD45-CD44+CD24-Ncadherin-) were observed in 57.45 % of cases. In retrospective studies we showed that CTCs with this phenotype were citokeratin-7-positive and they demonstrated expression of Epcam in the cytoplasm [13]. Their frequency in the blood of breast cancer patients
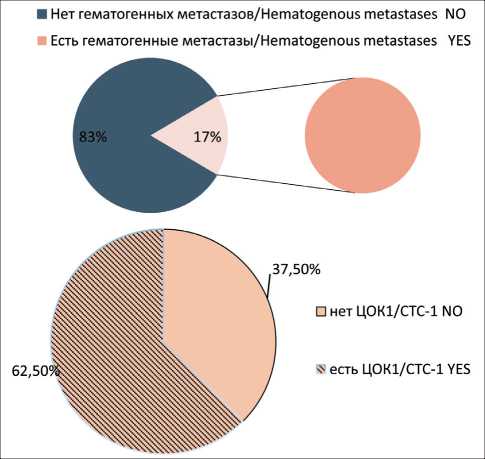
Рис. 1. Частота встречаемости популяции ЦОК1 у больных раком молочной железы с выявленными гематогенными метастазами. Точный критерий Фишера (двусторонний)=0,700, p>0,05.
Примечание: ЦОК1 Epcam+CD45-CD44-CD24-Ncadherin-Fig. 1. Frequency of CTC1 population in group of breast cancer patients with hematogenous metastases. Fisher’s exact test=0.700, p>0.05.
Note: CTC1 – Epcam+CD45-CD44-CD24-Ncadherin-
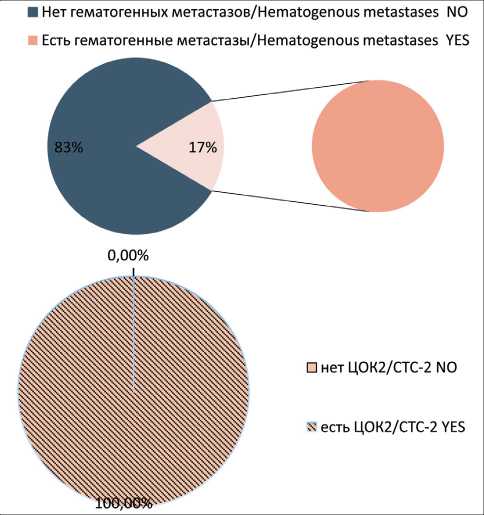
Рис. 2. Частота встречаемости популяции ЦОК2 у больных раком молочной железы с гематогенными метастазами. Точный критерий Фишера (двусторонний)=0,00014, p<0,05. Примечание: ЦОК2 – Epcam+CD45-CD44-CD24-Ncadherin+ Fig. 2. Frequency of CTC2 population in group of breast cancer patients with hematogenous metastases. Fisher’s exact test=0.00014, p<0.05.
Note: CTC2 – Epcam+CD45-CD44-CD24-Ncadherin+
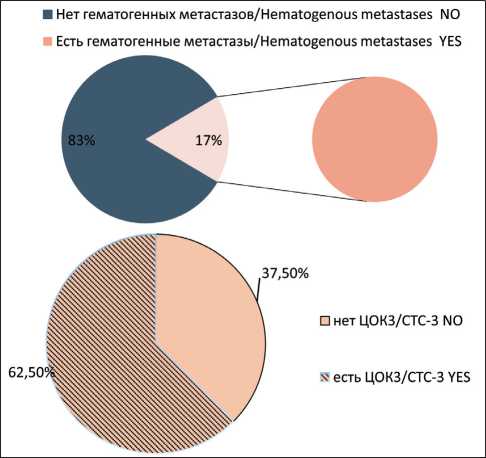
Рис. 3. Частота встречаемости популяции ЦОК3 у больных раком молочной железы с гематогенными метастазами. Точный критерий Фишера (двусторонний)=0,089, p>0,05.
Примечание: ЦОК3 – Epcam+CD45-CD44+CD24-Ncadherin+ Fig. 3. Frequency of CTC3 population in group of breast cancer patients with hematogenous metastases. Fisher’s exact test=0.089, p>0.05.
Note: CTC3 – Epcam+CD45-CD44+CD24-Ncadherin+
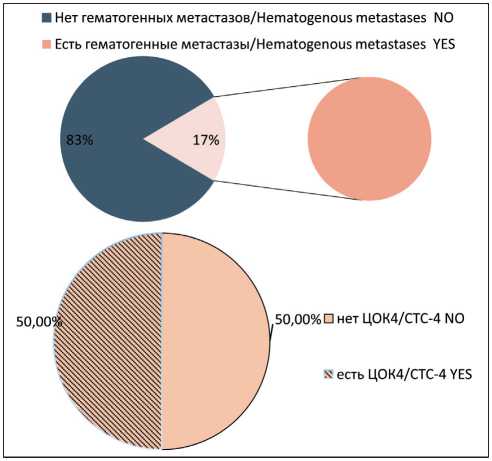
Рис. 4. Частота встречаемости популяции ЦОК4 у больных раком молочной железы с гематогенными метастазами. Точный критерий Фишера (двусторонний)=0,707, p>0,05.
Примечание: ЦОК4 – Epcam+CD45-CD44+CD24-Ncadherin-.
Fig. 4. Frequency of CTC4 population in group of breast cancer patients with hematogenous metastases. Fisher’s exact test=0.707, p>0.05.
Note: CTC4 – Epcam+CD45-CD44+CD24-Ncadherin-
with and without hematogenous metastases before treatment showed no statistically differences (Fig. 5). On the other hand, CTCs with the Еpcam(m)-CD45-CD44+CD24-Ncadherin+ phenotype were detected in the blood of 7 out of 8 patients breast cancer with hematogenous metastases; these CTCs were absent in other patient (p=0.002) (Fig. 6).
Evaluation of the five-year metastasis-free survival rate in breast cancer patients with different CTC populations in the blood before treatment showed that a statistically significant decrease in the metastasis-free survival was observed in the presence of non-stem CTCs showing signs of EMT (CTC2) (p=0.0016), in stem-like CTCs showing signs of EMT (CTC3) (p=0.0172), and in CTCs with stemness and EMT features and without expression of Epcam on the membrane (CTC6) (p=0.0112) (Fig. 7).
Discussion
The number of tumor cells in the peripheral blood is the result of three processes: recruitment and in-travasation in the tumor, destruction in the blood, and extravasation in distant organs. To predict the hematogenous spread of cancer, it is important to find out which of the mechanisms is characteristic of each of the studied subpopulations.
Intravasation is a key stage in the metastasis of malignant neoplasms, during which tumor cells enter the circulation, passing through the vascular wall, and become circulating tumor cells and potential metastatic seeds. There are several types of intravasation: asso- ciated with invasion; associated with macrophages; TMEM (Tumor Micro Environment of Metastasis) – mediated tumor cell intravasation; intravasation of tumor cells not related to invasion (metastasis by clusters of tumor cells); and intravasation in conditions of vasculogenic mimicry of tumor cells [14].
The existence of various types of intravasation (associated and not associated with invasion) partly explains the presence of various forms of CTCs in the bloodstream (single CTCs, clusters of CTCs, CTCs showing signs of EMT, CTCs showing no signs of EMT, atypical/hybrid forms of CTCs). A large percentage of CTC-positive breast cancer patients in the group of patients without hematogenous spread of the disease (60 %), as opposed to the group of patients with detected hematogenous metastases (17 %), confirms the hypothesis that not all CTCs are capable of seeding. The presence of CTCs is not a determining factor in the development of hematogenous metastases. It can be assumed that hematogenous metastases develop both in the presence of CTCs capable of seeding and in conditions favorable for the formation of premetastatic niches.
The findings of the study showed that non-stem CTCs showing signs of EMT (CTC2) and stem-like CTCs showing signs of EMT (CTC3 and CTC6) significantly reduced the metastasis-free survival. A common feature of these cells is the presence of EMT signs. The obtained results show that the ability of CTCs to undergo EMT is a predetermining characteristic for their acquisition of the seed properties. The results
■ Нет гематогенных метастазов/Hematogenous metastases NO Есть гематогенные метастазы/Hematogenous metastases YES
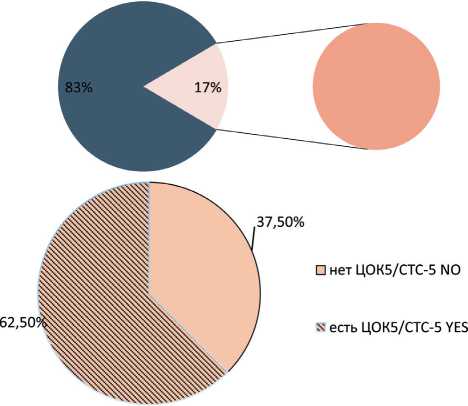
Рис. 5. Частота встречаемости популяции ЦОК5 у больных раком молочной железы с гематогенными метастазами. Точный критерий Фишера (двусторонний)=1,000, p>0,05. Примечание: ЦОК5 – Epcam (m)-CD45-CD44+CD24-Ncadherin-Fig. 5. Frequency of CTC5 in group of breast cancer patients with hematogenous metastases. Fisher’s exact test=1.000, p>0.05.
Note: CTC5 – Epcam (m)-CD45-CD44+CD24-Ncadherin-
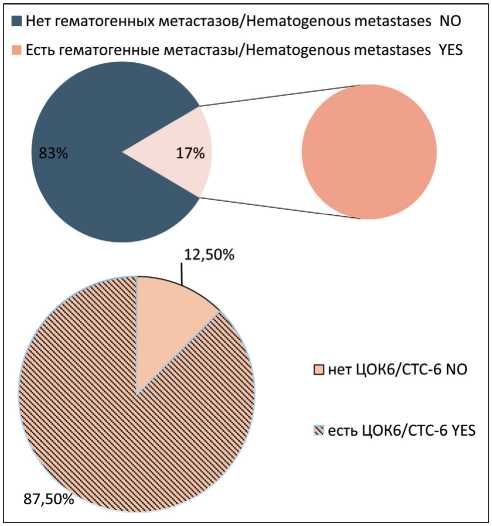
Рис. 6. Частота встречаемости популяции ЦОК6 у больных раком молочной железы с гематогенными метастазами. Точный критерий Фишера (двусторонний)=0,00473, p<0,05. Примечание: ЦОК6 – Epcam (m)-CD45-CD44+CD24-Ncadherin+ Fig. 6. Frequency of CTC6 in group of breast cancer patients with hematogenous metastases. Fisher’s exact test=0.00473, p<0.05.
Note: CTC6 – Epcam (m)-CD45-CD44+CD24-Ncadherin+
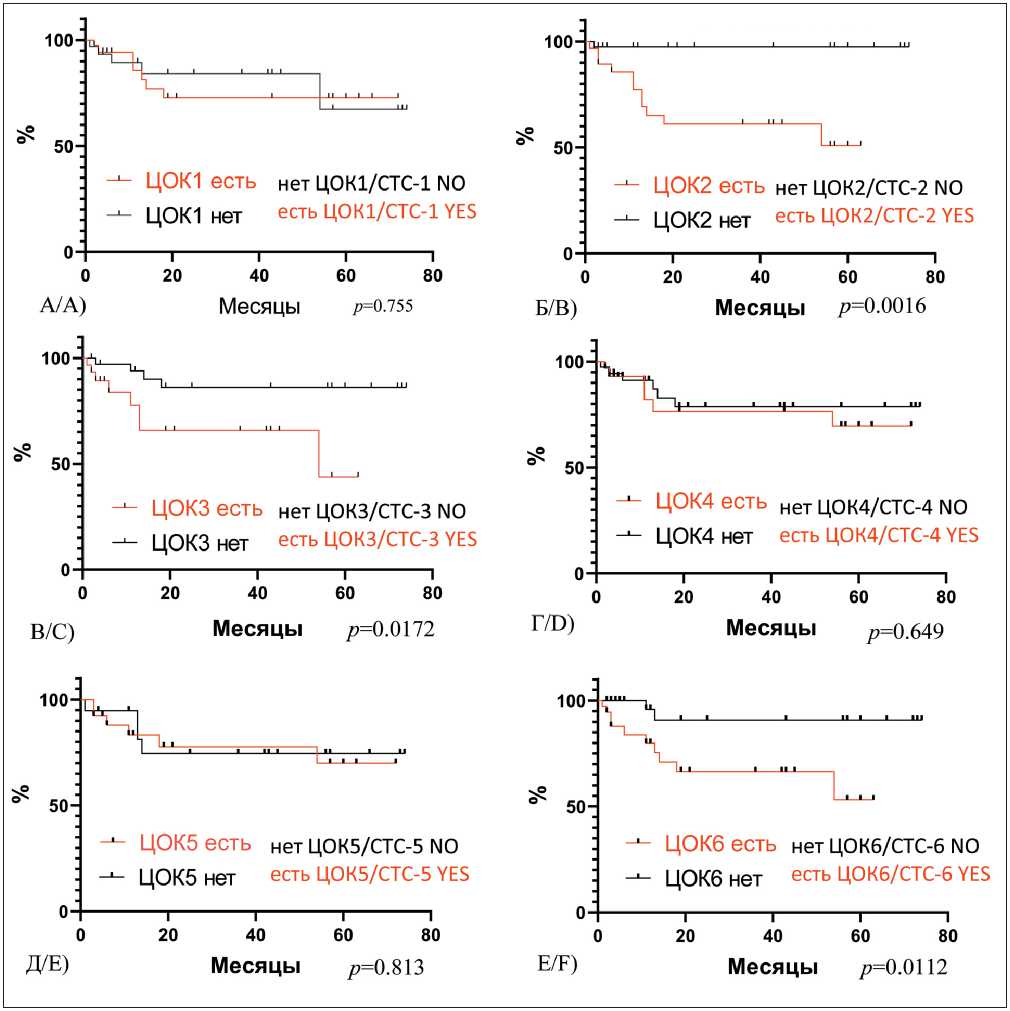
Рис. 7. Безметастатическая выживаемость у больных инвазивной карциномой молочной железы в зависимости от наличия различных популяций ЦОК в крови до лечения:
-
A) безметастатическая выживаемость у больных РМЖ в зависимости от наличия/отсутствия ЦОК1 – Epcam+CD45-CD44-CD24-Ncadherin-, Log-rank test р=0,755; Б) безметастатическая выживаемость у больных РМЖ в зависимости от наличия/отсутствия ЦОК2 – Epcam+CD45-CD44-CD24-Ncadherin+, Log-rank р=0,0016; В) Безметастатическая выживаемость у больных РМЖ в зависимости от наличия/отсутствия ЦОК3 – Epcam+CD45-CD44+CD24-Ncadherin+, Log-rank р= 0,0172; Г) безметастатическая выживаемость у больных РМЖ в зависимости от наличия/отсутствия ЦОК4 – Epcam+CD45-CD44+CD24-Ncadherin-, Log-rank р=0,649; Д) безметастатическая выживаемость у больных РМЖ в зависимости от наличия/отсутствия ЦОК5 – Epcam (м)-CD45-CD44+CD24-Ncadherin-, Log-rank р=0,813; Е) безметастатическая выживаемость у больных РМЖ в зависимости от наличия/отсутствия ЦОК6 – Epcam (м)-CD45-CD44+CD24-Ncadherin+, Log-rank р=0,0112
-
Fig. 7. Five-year metastasis-free survival rate in patients with invasive breast carcinoma, depending on the presence of different CTC populations in the blood before treatment.
-
A) Metastasis-free survival rate in patients with invasive breast carcinoma, depending on the presence of CTC1 – Epcam+CD45-CD44-CD24-Ncadherin-, log-rank test р =0.755; B) Metastasis-free survival rate in patients with invasive breast carcinoma, depending on the presence of CTC2 – Epcam+CD45-CD44-CD24-Ncadherin+, log-rank test р =0.0016; C) Metastasis-free survival rate in patients with invasive breast carcinoma, depending on the presence of CTC3 – Epcam+CD45-CD44+CD24-Ncadherin+, log-rank test р =0.0172; D) Metastasis-free survival rate in patients with invasive breast carcinoma, depending on the presence of CTC4 – Epcam+CD45-CD44+CD24-Ncadherin-, log-rank test р =0.649; E) Metastasis-free survival rate in patients with invasive breast carcinoma, depending on the presence of CTC5 – Epcam (m)-CD45-CD44+CD24-Ncadherin-, log-rank test р =0.813; F) Metastasis-free survival rate in patients with invasive breast carcinoma, depending on the presence of CTC6 – Epcam (m)-CD45-CD44+CD24-Ncadherin+, log-rank test р =0.0112
are consistent with the literature data. Thus, in breast cancer, a direct relationship was established between the EMT markers in primary and disseminated bone marrow tumor cells and aggressive clinical behavior of the tumor [15].
It is known that cancer stem cells are chemoresistant [8]. During EMT, cells change their epithelial phenotype (partially or completely) to the mesenchymal one [16]. This process, which is involved in organogenesis and wound healing, is intensively studied in relation to CTCs. It has been suggested that the epithelial-mesenchymal transition is associated with cancer aggressiveness and may increase cell migration [9]. It was shown that in tumor cells that hyperexpress RAS or HER2, a stem-like CD44+/ CD24-subpopulation had an increased potential for EMT [10]. It was also found that in CD44+/CD24- / low breast cancer cells, a subtype with the claudin-low phenotype was determined, which was characterized by the expression of many EMT-associated genes, such as FoxC2, Zeb, and N-cadherin [17].
It is worth noting that EMT inducers can cause the emergence of stemness features in cells. S.A. Mani et al. showed for the first time that EMT can lead to the formation of a population of cells with oncogenic and metastatic properties [18]. It should be stressed that EMT does not occur spontaneously; it is regulated by microenvironment signals. The control of EMT plasticity most likely depends on normal cells of the tumor microenvironment, including tumor-associated stromal cells, such as immune cells, fibroblasts, and endothelial cells. It has also been shown that tumor cells that undergo EMT are often able to survive genotoxic and other influences, and, as a rule, are resistant to chemotherapy and radiation therapy. M. Mego et al. (2012) demonstrated that CTCs showing signs of EMT can be detected in 26 % of patients with metastatic breast cancer. High expression of EMT markers predicted short metastasis-free survival in these patients [19]. Cells undergoing EMT were detected in the blood of 7 % of CTC-negative patients [20].
Список литературы The presence of various populations of circulating tumor cells in the blood of breast cancer patients before treatment: association with five-year metastasis-free survival
- Каприн А.Д., Старинский В.В., Петрова Г.В. Злокачественные опухоли в России в 2018 г. (Заболеваемость и смертность). М., 2019. 250 с. [KaprinA.D., Starinskiy V.V., Petrova G.V. Malignant neoplasms in Russia in 2018 (morbidity and mortality). Moscow, 2019. 250 р. (in Russian)].
- YangM.H., Imrali A., Heeschen C. Circulating cancer stem cells: the importance to select. Chin J Cancer Res. 2015 Oct; 27(5): 437-49. doi: 10.3978/j.issn.1000-9604.2015.04.08.
- Stoecklein N.H., Fischer J.C., Niederacher D., Terstappen L.W. Challenges for CTC-based liquid biopsies: low CTC frequency and diagnostic leukapheresis as a potential solution. Expert Rev Mol Diagn. 2016; 16(2): 147-164. doi:10.1586/14737159.2016.1123095.
- ZheX., CherM.L., BonfilR.D. Circulating tumor cells: finding the needle in the haystack. Am J Cancer Res. 2011; 1(6): 740-751.
- Giordano A., Gao H., Anfossi S., Cohen E., Mego M., Lee B.N., Tin S., DeLaurentiisM., Parker C.A., AlvarezR.H., Valero V., UenoN.T., De Placido S., Mani S.A., Esteva F.J., Cristofanilli M., Reuben J.M. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther. 2012 Nov; 11(11): 2526-34. doi: 10.1158/1535-7163.MCT-12-0460.
- КайгородоваЕ.В. Циркулирующие опухолевые клетки: клиническое значение при раке молочной железы (обзор литературы). Вестник Российской академии медицинских наук. 2017; 72(6): 450-457. [Kaigorodova E.V. Circulating tumor cells: clinical significance in breast cancer (Review). Herald of the Russian Academy of Medical Sciences. 2017; 72(6): 450-457. (in Russian.)]. doi: 10.15690/vramn833.
- Kaigorodova E.V., Tarabanovskaya N.A., Staheeva M.N., Saveli-eva O.E., Tashireva L.A., Denisov E.V., Perelmuter V.M. Effect of minor and major surgical injury on the level of different populations of circulating tumor cells in the blood of breast cancer patients. Neoplasma. 2017; 64(3): 437-443. doi: 10.4149/neo_2017_315.
- Theodoropoulos P.A., Polioudaki H., Agelaki S., Kallergi G., Saridaki Z., Mavroudis D., Georgoulias V. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010 Feb 1; 288(1): 99-106. doi: 10.1016/j.can-let.2009.06.027.
- Kasimir-Bauer S., Hoffmann O., WallwienerD., KimmigR., Fehm T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 2012 Jan 20; 14(1): R15. doi: 10.1186/bcr3099.
- Reuben J.M., Lee B.N., Gao H., Cohen E.N., Mego M., Giordano A., Wang X., Lodhi A., Krishnamurthy S., Hortobagyi G.N., Cristofanilli M., Lucci A., Woodward W.A. Primary breast cancer patients with high risk clinicopathologic features have high percentages of bone marrow epithelial cells with ALDH activity and CD44-CD24lo cancer stem cell phenotype. Eur J Cancer. 2011 Jul; 47(10): 1527-36. doi: 10.1016/j. ejca.2011.01.011.
- Armstrong A.J., Marengo M.S., Oltean S., Kemeny G., Bitting R.L., Turnbull J.D., Herold C.I., Marcom P.K., GeorgeD.J., Garcia-BlancoM.A. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011; 9(8): 997-1007. doi: 10.1158/1541-7786.MCR-10-0490.
- Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003 Apr 1; 100(7): 3983-8. doi: 10.1073/ pnas.0530291100.
- Kaigorodova E.V., Savelieva O.E., Tashireva L.A., Tarabanov-skaya N.A., Simolina E.I., Denisov E.V., Slonimskaya E.M., Choynzo-nov E.L., Perelmuter V.M. Heterogeneity of Circulating Tumor Cells in Neoadjuvant Chemotherapy of Breast Cancer. Molecules. 2018 Mar 22; 23(4): 727. doi: 10.3390/molecules23040727.
- Zavyalova M.V., Denisov E.V., Tashireva L.A., Savelieva O.E., Kaigorodova E.V., Krakhmal N.V., Perelmuter V.M. Intravasation as a Key Step in Cancer Metastasis. Biochemistry (Mosc). 2019; 84(7): 762-72. doi: 10.1134/S0006297919070071.
- KimM.Y., Oskarsson T., Acharyya S., NguyenD.X., ZhangX.H., Norton L., Massague J. Tumor self-seeding by circulating cancer cells. Cell. 2009; 139: 1315-26. doi: 10.1016/j.cell.2009.11.025
- Scatena R., Bottoni P., Giardina B. Circulating tumour cells and cancer stem cells: a role for proteomics in defining the interrelationships
- between function, phenotype and differentiation with potential clinical applications. Biochim Biophys Acta. 2013; 1835(2): 129-143. doi: 10.1016/j. bbcan.2012.12.002.
- Asiedu M.K., Ingle J.N., Behrens M.D., Radisky D.C., Knut-son K.L. TGFbeta/TNF(alpha)-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 2011 Jul 1; 71(13): 4707-19. doi: 10.1158/0008-5472.CAN-10-4554.
- Mani S.A., Guo W, LiaoM.J., EatonE.N., AyyananA., ZhouA.Y., BrooksM., Reinhard F., Zhang C.C., ShipitsinM., CampbellL.L., PolyakK., Brisken C., Yang J., WeinbergR.A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008; 133(4): 704-15. doi: 10.1016/j.cell.2008.03.027.
- Mego M, Gao H, Lee B. N, Cohen E. N, Tin S., Giordano A., Wu Q., Liu P., Nieto Y., ChamplinR.E., Hortobagyi G.N., CristofanilliM., Ueno N.T., Reuben J.M. Prognostic value of EMT-circulating tumor cells in metastatic breast cancer patients undergoing high-dose chemotherapy with autologous hematopoietic stem cell transplantation. J Cancer. 2012; 3: 369-80. doi: 10.7150/jca.5111.
- Aktas B., Tewes M., Fehm T., Hauch S., Kimmig R., KasimirBauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009. 11(4): R46. doi: 10.1186/ bcr2333


