The prospects of utilizing the modified sorption material to intensify purification of waste water from electroplating production
Автор: Kurilina Tatiana А., Dubrovskaya Olga G., Kulagin Vladimir A., Matyushenko Anatoly I., Bobrik Anastasiya G.
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Статья в выпуске: 2 т.12, 2019 года.
Бесплатный доступ
One of the sources of environmental pollution with hazardous substances, primarily heavy metals, is the electroplating industry. The prevention of pollution of water bodies with wastewater containing heavy metal ions is closely related to reducing the consumption of fresh water for the technological needs of production and to reducing the amount of effluent. One of the solutions to this problem is to create low-waste and waste-free environmentally safe technological processes of wastewater treatment using treated effluents in the recirculation system, which reduces the negative impact on the environment. The results of studying the sorption properties of the natural modified mineral Akdolit-Gran prove a high efficiency of this sorption filling when conditioning electroplating shop effluents contaminated with a complex of heavy metals. Taking into account a relatively low cost of this natural mineral, Akdolit-Gran has the advantages in terms of its economic feasibility, plus the high degree of extraction of metals using this sorbent together with its low consumption allows designing recirculation systems for industrial enterprises meeting the requirements for physical and chemical parameters of service water.
Sorption neutralization, heavy metal ions, modified sorbent, dolomite rocks
Короткий адрес: https://sciup.org/146281183
IDR: 146281183 | УДК: 628.33; | DOI: 10.17516/1999-494X-0127
Текст научной статьи The prospects of utilizing the modified sorption material to intensify purification of waste water from electroplating production
Research methods
This paper studies the sorption method for removal of a complex of heavy metals, for example, Cu (II); Ni (II) and Zn (II), from aqueous solutions with a modified sorbent which bases on dolomite raw materials. This sorbent, Akdolit Kesselburger Pelm Gran CM3 (Akdolit-Gran) is produced in Germany and is widely used in Europe and the European part of Russia.
The sorbent material is thermally activated and modified by calcining the natural mineral. Calcination contributes to loosening of the rock with the formation of structures with greater porosity and specific surface [10, 11]. The approximate chemical composition of Akdolit-Gran: calcium carbonate CaCO3 – 68.9%; calcium oxide CaO – 1.4%; magnesium oxide MgO – 25.4%; magnesium carbonate MgCO 3 – 0.6%; iron oxide Fe 2 O 3 – 0.6%; aluminum oxide Al 2 O 3 – 2.7%; silicon oxide SiO 2 – 0.3%; water H2O – 2.7%. The percentages of the substances obtained on the basis of a regular physical and chemical testing and are the average statistical values.
Research results
The objective was to study the physical and chemical and sorption properties of Akdolit-Gran.
To carry out the sorption in the laboratory, we used the method of variable sorbent weights and constant volumes of the simulated solution with the initial ion concentration: Cu (II) = 60 mg/dm3; Ni (II) = 15 mg/dm3; Zn (II) = 20 mg/dm3. These concentrations are most common in the electroplating industry wastewater. The residual concentration was determined on an ICAP-6500 Inductively Coupled Plasma Atomic Emission Spectrometer.
The sorbent mineralogical composition was determined based on the data from X-ray crystallography carried out on a DRON-3 diffractometer, in Cu-K α radiation.
The analysis of the diffractogram indicates that the main phase in the sorbent is calcite CaCO 3 (d=0.38; 0.30; 0.23; 0.19; 0.18 Å); besides, there is a rather significant amount of magnesium oxide MgO
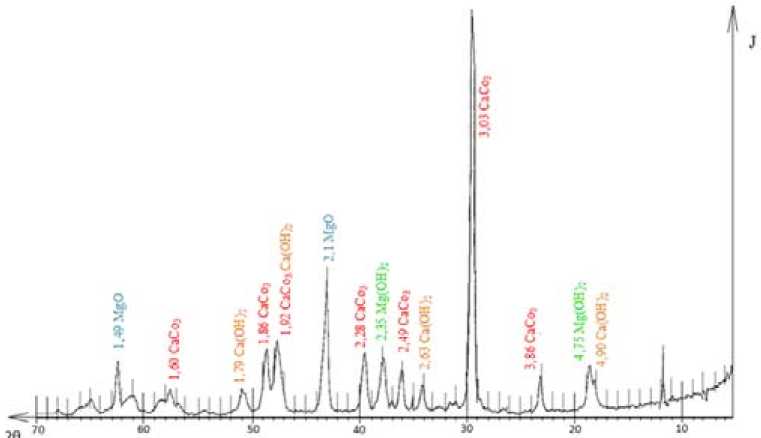
Fig. 1. The diffractogram of Akdolit-Gran
(d=0.21; 0.15 Å). Low-intensity diffraction maxima correspond to magnesium hydroxide Mg(OH)2 (d=0.21; 0.15 Å) and calcium hydroxide Ca(OH) 2 (d=0.49; 0.26 Å) resulting from hydrolysis oxides of magnesium and calcium contained in the sorbent. The remaining substances specified in the technical documentation for the sorbent (MgCO 3 , Fe 2 O 3 , Al2O 3 , and SiO 3 ) were not detected due to their low concentration. For a more detailed study of the sorbent sample, we carried out a thermal analysis on an STA 449 F1 (simultaneous thermal analyzer), by NETZSCH (Germany), in an argon medium. The thermogram of the Akdolit-Gran sample is shown in Fig. 2. The number, shape and position of various exothermic and endothermic peaks relative to the temperature scale were used to qualitatively identify the sample being studied.
The sample thermal analysis data show 4 endo-effects on the DSC. A minor endo-effect at 109 °С refers to removal of the adsorbed water, the endo-effect at 430 °С is caused by dehydration of Mg(OН) 2 :
Mg(OН) 2 → MgO+Н 2 О;
meanwhile, the sample mass, as shown by the TC, decreases ~ 5.59% in this temperature range, the compound of Mg(OН) 2 in the sample is ~ 18%, it is followed by the endo-effect at 4760 which is caused by dehydration of Са(ОН)2:
Са(ОН) 2 →СаО+Н 2 О;
the mass decreases by 0.59%; a large endo-effect is observed at t=844 °С, which is related to calcite decarbonization, i.e. calcite CaCO3 decomposes with formation of СО2:
СаСО 3 →СаО+СО 2 ↑;
the sample mass is reduced by approximately 27%, the content of СаСО 3 in the sample is 61.4% according to the thermogram.
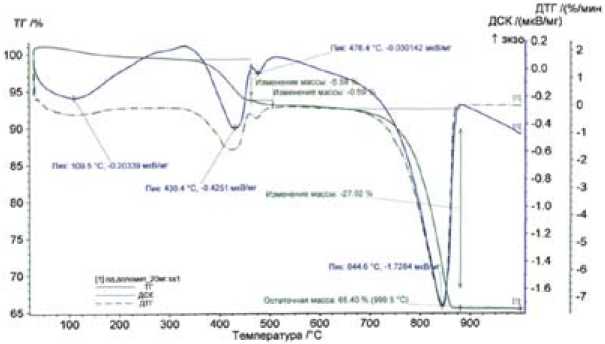
Fig. 2. Thermogram of Akdolit-Gran: DTC is a differential thermogravimetric curve (shows the rate of change of mass, this is the first derivative of TC), TC is a thermogravimetric curve which shows the change of mass during heating (the mass increases or decreases), DSC is a differential scanning curve (DSC and DTA show endo- and exo-effects occurring during heating, DTA is an analysis from one point, DSC is an analysis from the whole surface)
According to the data of differential-thermal and X-ray phase analyses of the sorbent, we can conclude that during heat treatment, chemical transformations also take place, which results in the formation of calcium carbonate and magnesium oxide.
We determined the technical characteristics of the sorbent abiding by the standard techniques (see Table 1).
The dose values of the proposed sorption material are found experimentally and given in Table 2.
The experiment performed (see Table 2) showed that the cleaning effect with the use of Akdolit-Gran sharply reduces in an acidic medium. The reason for this can be a change in the colloid-chemical properties of the sorbent, the isoelectric point of which corresponds to pH = 5.4 approximately, therefore, when the pH is below this value, the reaction centres on the sorbent granules are deactivated,
Table 1. Technical characteristics of the sorbent
|
Total pore volume, V ∑ (cm/g) |
0.103 |
|
Bulk density, ρ b (g/cm 3) |
1.15 |
|
Real density ρ (g/cm 3) |
2.37 |
|
Average density ρ 0 (g/cm 3) |
2.26 |
|
Porosity P (%) |
4.64 |
|
Water absorption W (%) |
10.3 |
Table 2. The experimental results
When analysing the sorption parameters of the studied Akdolit-Gran, we determined the following: A sb – absorbing capacity of the sorbent, k d – a coefficient of metal distribution between the simulated solution and the sorbent, ksb – a preset degree of the sorbent capacity exhaustion, R – a degree of metal extraction from the solution, D sb – dose of the sorbent [12–14]. The calculus results are given in Tables 3–5.
The sorption capacity of Akdolit – Gran varies in respect of the studied materials. Evaluation of the sorbent efficiency for extraction of metals from aqueous solutions using a specific sorption capacity
Table 3. The absorbing capacity of Akdolit-Gran (Asb, mg/g)
|
Item |
Dsb – dose of Akdolit – Gran, mg/dm3 |
Cu2+ |
Ni2+ |
Zn2+ |
|
1 |
1 |
57.74 |
13.01 |
19.87 |
|
2 |
1.2 |
48.16 |
11.71 |
16.37 |
|
3 |
1.4 |
41.28 |
10.27 |
14.14 |
|
4 |
1.6 |
36.76 |
8.63 |
12.35 |
|
5 |
1.8 |
32.26 |
7.26 |
10.54 |
|
6 |
2.0 |
28.15 |
6.37 |
9.19 |
Table 4. The coefficient of metal distribution between the simulated solution and the sorbent (kd, g/dm3)
|
Item |
Dsb – dose of Akdolit – Gran, g/dm3 |
Cu2+ |
Ni2+ |
Zn2+ |
|
1 |
1 |
25.59 |
6.55 |
155.23 |
|
2 |
1.2 |
21.81 |
12.48 |
45.98 |
|
3 |
1.4 |
18.68 |
16.48 |
67.98 |
|
4 |
1.6 |
31.20 |
7.29 |
52.55 |
|
5 |
1.8 |
16.69 |
3.77 |
10.28 |
|
6 |
2.0 |
7.61 |
2.83 |
5.64 |
Table 5. The degree of metal extraction from the solutions (R, %)
Ionic potential, i.e. a surface charge of the ion can be used to assess a surface dissociation degree.
The ionization potential is determined by the formula
IP = —,
r where n is a number of electrons; e is an electron charge.
Cu2+, Zn2+ and Ni2+ have got 2 electrons, and the electron charge is 1.602. There is a dependence [12] stating that the larger the radius of the ion is, the smaller the ionization potential will be. The radius of the atom for Cu2+ is 1.278 A , the radius of the Zn2+ atom is 1.333 A , and the radius of the for the Ni2+ atom is 1.246 A ; and according to this feature, the studied metals arrange in a row Zn2+ > Cu2+ > Ni2+, which corresponds to the experimental findings.
Table 6 shows the results of the study and calculation of the kinetics of the sorption process of copper (II), nickel (II) and zinc (II) ions.
Which also gives the following sequence of distribution of metals in the extraction rate:
Zn 2+ > Cu 2+ > Ni 2+
As the sorption process is exothermic, when temperature increases, the capacity of the sorbent with respect to metals decreases [15, 16], which is confirmed by the results (Table 7).
The phenomena of physical and chemical sorption clearly differ in some rare cases. There usually occur intermediate variants when the bulk adsorbed substance is relatively weakly bound, and only a small part of it binds firmly [17–19]. When the temperature is rising, an increase in chemical adsorption begins blocking a fall in physical sorption, starting from a certain temperature; therefore, the temperature dependence of sorption has a clear minimum in this case (Table 8).
These experimental data were used to develop a project to restore wastewater treatment facilities using the proposed sorption material.
Table 6. The results of the calculation of the sorption rate constant, depending on the dose of the sorbent
|
Dsb – dose of the sorbent mg/dm3 |
К, sec-1 |
||
|
Cu2+ |
Ni2+ |
Zn2+ |
|
|
1 |
3.28 |
2.02 |
5.05 |
|
1.2 |
3.3 |
2.77 |
4.02 |
|
1.4 |
3.3 |
3.18 |
4.56 |
|
1.6 |
3.9 |
2.54 |
4.44 |
|
1.8 |
3.4 |
2.05 |
2.97 |
|
2.0 |
2.78 |
1.89 |
2.51 |
Table 7. The dependence of the sorption capacity (Asb, mg/g) and the solution temperature, mg/g
|
Item |
Temperature ºС |
A sb , mg/g |
||
|
Cu(II) |
Ni(II) |
Zn(II) |
||
|
1 |
11.5 |
41.78 |
10.22 |
14.06 |
|
2 |
17.0 |
42.75 |
10.59 |
14.28 |
|
3 |
25.0 |
42.76 |
10.63 |
14.26 |
|
4 |
33.0 |
42.76 |
10.67 |
14.28 |
|
5 |
38.5 |
42.77 |
10.70 |
14.27 |
|
6 |
60.0 |
42.76 |
10.62 |
14.22 |
|
7 |
70.5 |
42.71 |
10.61 |
14.20 |
|
8 |
80.0 |
42.71 |
10.57 |
14.14 |
Table 8. The calculation results for the dependence of the residual concentration and the temperature of the aquatic medium
|
Temperature ºС |
res. |
||
|
Cu(II) |
Ni(II) |
Zn(II) |
|
|
11.5 |
2.201 |
0.689 |
0.308 |
|
17.0 |
0.151 |
0.161 |
0.005 |
|
25.0 |
0.128 |
0.112 |
0.031 |
|
33.0 |
0.131 |
0.056 |
0.0053 |
|
38.5 |
0.121 |
0.013 |
0.0101 |
|
60.0 |
0.136 |
0.128 |
0.0805 |
|
70.5 |
0.207 |
0.146 |
0.108 |
|
80.0 |
0.210 |
0.202 |
0.196 |
Conclusion
The results of studying the sorption properties of the natural modified mineral Akdolit-Gran prove a high efficiency of this sorption filling when conditioning electroplating shop effluents contaminated with a complex of heavy metals. Taking into account a relatively low cost of this natural mineral, Akdolit-Gran has the advantages in terms of its economic feasibility, plus the high degree of extraction of metals using this sorbent together with its low consumption allows designing recirculation systems for industrial enterprises meeting the requirements for physical and chemical parameters of service water.
Based on these results, we can conclude the following:
-
1. It is reasonable to use this sorbent as a potential ion exchange sorbent to treat the electroplating effluents since calcium and magnesium ions function as the exchangeable ions.
-
2. The cation sorption occurs both via the mechanism of ion exchange (exchange with cations in inter-packet spaces and via formation of complex compounds.
-
3. The optimal amount of Akdolit-Gran is about 1.4–1.6 gr/dm3 for solutions with an initial concentration: Cu (II)=60 gr/dm3; Ni (II)=15 gr/dm3; Zn (II)=20 gr/dm3, the temperature range is set within 33,0 – 38,0 °С.
-
4. The effect of cleaning the effluent from heavy metals using Akdolit-Gran is 93–98% in an alkaline environment, but it sharply decreases in an acidic environment.
Acknowledgment
The reported study was funded by RFBR and the government of Krasnoyarsk region according to the research projects №№ 18-48-242001, 18-41-242004, 18-41-242008
Список литературы The prospects of utilizing the modified sorption material to intensify purification of waste water from electroplating production
- Bek R.Yu. The impact of electroplating industry on the environment and ways to reduce the damage. Analytical review of the Academy of Sciences of the USSR. Siberian Branch. Institute of Solid State Chemistry and Mineral Processing; State Public Scientific and Engineering Library. Novosibirsk, 1991. 96 p.
- Tarasevich Yu.I. Natural sorbents in water purification processes. Kiev. Naukova Dumka. 1981. 206 p.
- Alykov N.M., Pavlova A.V., Nguen K.Kh., Abuova G.B., Utyubaeva N.V. A new sorbent for purification of water from ions of toxic metals. Natural Sciences. Journal of Fundamental and Applied Research. 2009. No. 4 (29). Pp. 150-158.
- Dzhigola L.A., Simakova Yu.M., Rubleva A.V., Nikitina O.V., Urazalieva A.K. The study of sorption on moulding boxes and diffusion of heavy metal ions in clays. Natural Sciences. Journal of Fundamental and Applied Research. 2009. No. 4(29). Pp. 175-180.
- Rogaleva E.V., Vorontsova N.V., Pilipenko A.I., Ganyaev V.P. Purification of natural water from heavy metal ions by sorption and ozonation. Proceedings of Higher Educational Institutions. Oil and Gas. 2008. No. 2. Pp. 102-104.
- Khuramshina I.Z., Nikiforov A.F., Migalatiy E.V., Baranov O.Yu. The interaction of copper (II) with a natural mineral sorbent in the processes of aqueous solutions purification. Water purification. Water treatment. Water supply. 2008. 2(74). Pp. 22-26
- Nikiforov A.Yu. The use of natural dolomite and its thermally modified forms to purify wastewater from heavy metal cations. Proceedings of Higher Educational Institutions. Chemistry and Chemical Engineering. 1999. No. 4.
- Nadeem R., Hanif M., Shaheen F. et al. Physical and chemical modification of distillery sludge for Pb(II) biosorption. J. Hazard. Matter. 2008. V. 150. Р. 335-342.
- Padmavathy V. Biosorption of nickel (II) ions by baker's yeast: Kinetic, thermodynamic and desorption studies. Bioresource. Technol. 2008. V. 99. Р. 3100-3109.
- Godymchuk A.Yu., Reshetova A.A. Studying the extraction of heavy metals on natural minerals (electronic source). Bulletin of the Department of Earth Sciences RAS (Electronic Scientific Information Journal) No. 1(21) 2003 URL: http://www.scgis.ru/russian/cp1251/h_dgggms/1-2003/informbul-1/hydroterm-17.pdf
- Niyazi F.F., Maltseva V.S., Sazonova A.V. The kinetic patterns of sorption of iron (II, III) ions by modified carbonate rocks. Vestnik South-West State University. Series «Physics and Chemistry». 2012. No. 1. Pp. 40-47.
- Kulagin V.A., Kurilina T.A., Dubrovskaya O.G., Matyushenko A.I. Using the akdolit-gran modification for sorption treatment of wastewater of electroplatin]. Water treatmen. 2018. No. 10. Pp. 12-27.
- Domracheva V.A. Extraction of metals from wastewater and anthropogenic formations. Publishing house of Irkutsk State Technical University. Irkutsk. 2006. 151 p.
- Levchenko S.I. Physical and colloidal chemistry. Rostov-on-Don. 2004. 77 p. http://www.physchem.chimfak.rsu.ru/
- Bikulova V.Zh., Latypova F.M., Mukhametdinova L.Kh. Adsorption purification of industrial wastewater from zinc ions. Water, Chemistry and Ecology. 2013. No. 3.
- Orazova S.S., Belov V.M., Evstigneev V.V. Efficiency of using natural sorbents of Eastern Kazakhstan in water purification from heavy metal ions Сu2+ Vestnik of Tomsk Polytechnic University. 2007. No. 2, vol. 311.
- Andryshev A.K., Kolpakov V.P., Lopukhov Yu.I., Daumova G.K. The effective purification technology for chromium-containing wastewater using modified sorbents. Water purification. Water treatment. Water supply. 2014. No. 9.
- Shcherbakov A.V. Sewage treatment to purify from heavy metal salts. Energy Efficiency and Water Treatment. 2013. No. 3.
- Dubrovskaya O.G., Eldarzade E.A., Andrunyak I.V. Research and production of sorption materials based on the technology of recycling waste from the heat and power industry. Safety and Monitoring of Anthropogenic and Natural Systems: Materials of International Scientific Conference, Krasnoyarsk, September 18-21, 2018; ed.-in-chief V.V. Moskvichev. Krasnoyarsk: Publishing House of Siberian Federal University. 2018.


