The relationship among metabolic rate of tree shrews (Tupaia belangeri) under cold acclimation
Автор: Lin Zhang, Gao Wenrong, Jiang Wenxiu, Wang Zhengkun
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.8, 2012 года.
Бесплатный доступ
Many small mammals inhabiting cold environments display enhanced capacity for seasonal changes in nonshivering thermogenesis (NST) and thermoregulatory maximum metabolic rate (MMR). However, it is not known how this plasticity remains in a mammal that rarely experiences extreme cold fluctuations. In order to answer this question, we determined body mass (Mb), basal metabolic rate (BMR), NST, and MMR on a tree shrews (Tupaia belangeri), acclimated to cold (5 ºC) conditions. NST was measured as the maximum response of metabolic rate (NSTmax) after injection of norepinephrine (NE) in thermoneutrality minus BMR. Maximum metabolic rate was assessed in animals exposed to enhanced heat-loss atmosphere (He-O2) connected with an open-flow respirometer. Body mass and metabolic variables increased significantly after cold acclimation with respect to control group but to a high extent (BMR, 87.97%; NST, 69.77%; and MMR, 32.35%). However, aerobic scope (MMR/BMR), and calculated shivering thermogenesis (ST) did not significantly change with control group. Our data suggest: 1). The body mass and the capacity of heat production in the cold acclimated group were higher; 2). The increase of BMR and MMR during cold acclimation was the main pattern of heat production in the tree shrews.
Cold acclimation, metabolic rate, tupaia belangeri, thermogenesis plasticity
Короткий адрес: https://sciup.org/14323688
IDR: 14323688
Текст научной статьи The relationship among metabolic rate of tree shrews (Tupaia belangeri) under cold acclimation
The study of phenotypic plasticity has been a central topic in evolutionary ecology (Schlichting and Pigliucci, 1998). Phenotypic plasticity can be defined as reversible changes in the traits of organisms due to changes in internal or external environmental conditions (Naya et al. 2008). Eutherian nonhibernating small mammals shown several mechanisms to survive under cold environments. Thermogenic capacity include basal metabolic rate (BMR), non-shivering thermogenesis (NST), and shivering thermogenesis (ST; Bozinovic and Rosenmann, 1989); the maximum metabolic rate (MMR) included the BMR, NST and ST. NST was summarized by Jansky (1973) is the most plastic of metabolic variables. However, cold acclimation may increase BMR or decrease as a strategy to minimize energy expenditure in an environment of diluted energy offer (Veloso and Bozinovic 1993). ST is another way to produce heat; however, it is more energetically expensive than NST because energy is delivered into heat and muscular work. Indeed, it is generally believed that ST is used mainly during periods of low metabolic load (Jansky 1973; Lilly and Wunder 1979; Bockler and Heldmaier 1983).
Tree shrews, Tupaia belangeri (Mammalia: Scandentia: Tupaiidae), are the unique species small mammals, the remainder of the order’s extanr diversity is in mainland Southeast Asia, from China to Malaysia, and through the Malaysian and Indonesian islands of the Sunda region. T. belangeri live at the highest latitude, with the Yunnan-Kweichow Plateau being its northern limit (Wang et al. 1991). Previous studies demonstrate that tree shrews shown a seasonal increased in body mass and thermogenic capacity to adapt to the increase of energy requirements for thermoregulation (Wang et al. 1994, Zhu et al. 2012) , and seasonal variety in spermatogenesis of adult tree shrews (Cao, 1989). Tree shrews cold acclimated for 28 d decreased their serum leptin levels and increased thermogenesis (Zhang et al. 2011, 2012a), they acclimated to ward after cold acclimation for 28 d increased their serum leptin levels and decreased thermogenesis (Zhang et al. 2012b), short photoperiod can induce thermgenic capacity increased (Zhang et al. 2012c). However, we know nothing about the action of photoperiod with changes in serum leptin levels and its role in body mass regulation and thermogenesis in tree shrews.
Small mammals that regularly encounter seasonal variation conspicuous variables such as temperature, photoperiod and food availability in their habitats commonly alter their thermogenic capacity after cold acclimation (Hayes and Chappell 1986; Bozinovic et al. 1990; Kronfeld-Schor et al., 2000). We designed our study to examine how regulate body temperature in relation to changes in cold conditions. That is, we measured the effect of changes in Ta on body mass (Mb), BMR, NST, and MMR. Considering the low variability of cold environment, we predict low plasticity on these variables.
MATERIALS AND METHODS
Animal
All animal procedures were licensed under the Animal Care and Use Committee of Institute of Zoology, the Chinese Academy of Sciences. Tree shrews, T. belangeri were captured (25º25'~26º22' N, 102º13'~102º57' E, 1679 m in altitude) around boscage at Luquan County in November 2009. The average yearly temperature was 15.6 ºC, mean monthly temperature ranges from 7.8 ºC in winter to 19.6 ºC in summer. After being captured, then brought and bred at the School of Life Sciences, Yunnan Normal University, Kunming (1910 m in altitude). Each weight-matched tree shrew was housed individually in a wire cage (40 cm × 40 cm × 40 cm) with no bedding, all animals (23 males) were healthy adults. The photoperiod, ambient temperature and humidity were maintained at 12L: 12D (light on at 08.00 am), (25±1)ºC, and 85%-92% relative humidity, respectively. They were kept for at least 2 weeks, and then were randomly assigned into two groups. The two groups were control group (0 d) and cold acclimation groups ( 28 d), and there are 8 animals in every group. The control group was acclimation to light (12L: 12D, lights on 0800 am) and temperature (25±1ºC); the cold acclimation group was acclimation to light (12L: 12D, lights on 0800 am) and temperature (5±1ºC). All pregnant, lactating or young individuals were excluded. Body mass in T. belangeri showed no significant variations before the experiment (F(1, 14) =1.57, P>0.05, n=16). They were fed the mixed food; the food mixture contained 25.0% crude protein, 6.3% crude fat, 4.6% crude fibred, 7.4% ash, and 0.96 KJ/g gross energy (Zou et al. 1991) , with a ratio of every two-day interval feed appropriate apples, pears and other fruits, , and water were provided ad libitum. The tree shrews were fed once daily at 10:00 am, additionally, with a ratio of 3:1 every two-day interval they received apples, pears and other fruits. Measurement of Metabolic Rate (MR)
At the end of the acclimation period, metabolic rate was measured using open-system respirometry. A cylindrical, opaque respirometer chamber (760ml) was housed in an incubator (SPX-300, Shanghai Leaps Forward the Medical Instrument Co. Ltd, Shanghai, China) to maintain constant ambient temperature; ambient temperature was controlled with a temperature controlled cabinet at 30±0.5°C (The thermal neutral zone(TNZ) of T. belangeri is 30 - 35 ºC, Wang et al., 1994). The air was forced through the chmbers at a flow rate of 1000 ml/min using precision rotameters (7300 and 7400 series, Matheson, Montgomeryville, PA); flow rate was continuously monitored with a mass flowmeter (G265, Qubit Systems, Kingston, ON, Canada) and recorded to a computer; the subsample rate was approximately 200 ml/min. Each animal was generally in the metabolic chamber for at least 1.5 h, then we measured the BMR and all measurements were made daily between 1000 and 1400. The fractional concentrations of O2 and CO2 entering and exiting the chamber were measured using a gas analyzer (PowerLab ML206, ADinstruments Co. Sydney, Australia); air was dried (Drierite, W.A. Hammond Drierite Co., Xenia, OH, USA) prior to analysis. Ambient temperature (Ta) was measured via a T-type thermocouple situated within the respirometer chamber, just below the lid; unless otherwise noted, Ta was 30°C for all measurements. Gas flow rate, and O2 and CO2 concentrations were recorded to computer during each experiment (PowerLab ML870, ADinstruments Co. Sydney, Australia). The animal’s body mass was measured routinely before and after experiment 1. The method used for calculating the metabolic rate is detailed in Hill (1972):
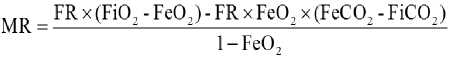
Note: FR = flow rate (ml/min), FiO 2 = O 2 input fractional concentration, FiCO 2 = CO 2 input fractional concentration, FeO 2 = O 2 excurrent fractional concentration, FeCO 2 = CO 2 excurrent fractional concentration.
Maximum NST defined as the maximum metabolic response to norepinephrine(NE), and was measured as the highest oxygen consumption following by scapular subcutaneous injection norepinephrine (NE) bitartrate (Shanghai Harvest Pharmaceutical Co. LTD, Shanghai, China). The dosage of NE was calculated based on preliminary experiments and the equation described by Jansky (1973) and Heldmaier (1971): NE dosage (mg/kg) =6.6Mb-0.458(g). Maximum NST was considered the highest 3-min average after NE injection (Heldmaier et al. 1982; Van Sant and Hammond 2008). Oxygen consumption reached peak values within 15-30 min after NE injection (Zhang et al. 2011).
Maximum metabolic rate (MMR) was measured as described by Zhang et al.(2012d). We measured MMR in a He-O 2 atmosphere according to the procedure of Rosenmann and Morrison (1974) in an open-circuit respirometer, as described by Chappel and Bachman (1995). In brief, a mixture of He (79%) and O 2 (21%) was passed through a volumetric flowmeter before entering the chamber (i.e., a positive pressure system), which was maintained at 1000 ml/min. The MMR measured as in the case of
BMR measurements. Chamber temperature (5.0±0.5 ºC) and Tb were measured. We defined instantaneous VO 2 as the highest O 2 averaged over 2 min of the last 5 min of 15 min Heliox exposure.
Shivering Thermogenesis (ST)
We calculated ST in each acclimation using the equation MMR = BMR+NST+ST for small eutherian mammals (Jansky 1973; Wunder and Gettinger 1996; Degen 1997).
Statistical analyses
Statistical analyses were performed using sigmaplot10.0, SPSS for Windows16.0 statistical package. Results are reported as mean ± SE and P <0.05 was considered to be statistically significant. RESULTS
Body mass
Prior to acclimation, there was no significant difference in body mass between the control group and the cold acclimation group ( t =0.087, P >0.05). During the 28 days, the body mass significantly increased 8.9% (Table 1). During the course of cold acclimation, there were significant differences in body mass within the treated group ( F =4.321, P <0.05), but not within the control group ( F =0.347, P >0.05).
Thermogenic capacity
BMR was influenced by ambient temperature.
Prior to acclimation, BMR in tree shrews showed no significant different between groups ( t =0.786, P >0.05). BMR in tree shrews gradually increased by 87.97% during the cold acclimation from 0 to 28 days (Table 1). NST was influenced by ambient temperature. Prior to acclimation, BMR in tree shrews showed no significant different between groups ( t =0.673, P >0.05). NST in tree shrews gradually increased by 69.77% during the cold acclimation (Table 1). During the course of cold acclimation, there were significant differences in body mass within the treated group ( F =14.412, P <0.05), but not within the control group ( F =0.532, P >0.05).
Basal metabolic rate, MMR and NST showed significant differences between the control group and the acclimation group (Table 1; Fig. 1). The mean values of NST + BMR (= NST max ) were significantly different between the control group and the acclimation group (Table 1; Fig. 1). Although Table 1 shows positive means of ST, most individuals had similar values of NST max and MMR, which gave low, and even negative, magnitudes of ST in some cases. Mean calculated ST was no significant different after both acclimations (Table 1). The MMR increased by 32.35% and was near twice as NST max in tree shrews during cold acclimation (Table 1; Fig. 1).
Table 1: Body mass ( M b ) and metabolic variable after cold acclimation
|
Control |
Cold acclimation (28 days ) |
P values |
|
|
M b (g) |
129.5±3.4 |
140.9±3.6 |
P <0.05 |
|
BMR(ml O 2 g-1 h-1) |
1.58±0.13 |
2.97±0.34 |
P <0.01 |
|
NST(ml O 2 g-1 h-1) |
3.54±0.11 |
6.01±0.23 |
P <0.01 |
|
MMR (ml O 2 g-1 h-1) |
7.79±0.37 |
10.31±0.37 |
P <0.01 |
|
ST (ml O 2 g-1 h-1) |
2.67±0.11 |
1.23±0.29 |
P >0.05 |
|
MMR/ BMR |
4.93 |
3.47 |
P <0.05 |
Note. Shivering thermogenesis (ST) was computed for ach individual as maximum metabolic rate (MMR) minus basal metabolic rate (BMR)
minus nonshivering thermogenesis (NST), Nonshivering thermogenesis (NST) was computed for ach individual as maximum nonshivering thermogenesis (NST max ) minus basal metabolic rate (BMR). Data are shown as mean ± SE; n = 8 in all cases.
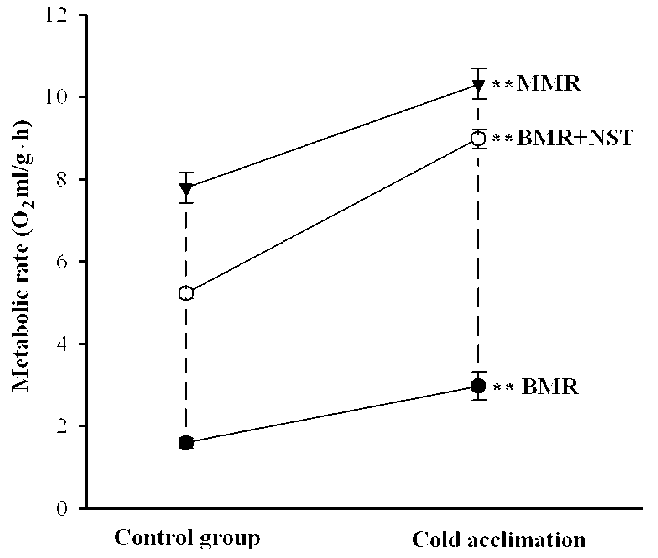
Figure 1. Maximum metabolic rate (MMR), nonshivering thermogenesis (NST), and basal metabolic rate (BMR) of tree shrews after cold acclimation. Asterisks denote significant differences between experimental group ( P <0.01).
DISCUSSION
Classic studies dealing with cold acclimation on the energetic features of small mammals of seasonal environments reported an increase in thermogenic capability, increasing survival probabilities. Classic studies dealing with cold acclimation on the energetic features of small mammals of seasonal environments reported an increase in thermogenic capability, increasing survival probabilities. For example, a reduced resting metabolic rate and a high NST capacity not only allows the maintenance of a low metabolic heat production during activity but also increases heat production quickly under cool to cold conditions (Degen, 1997).
Basal Metabolic Rate
Our values of BMR (1.58±0.13 and 2.97±0.34 ml O 2 g-1 h-1, in control group and cold acclimation group individuals, respectively), and it increased by
87.98% after cold acclimation, are similar current to those previously reported for other rodents, but higher than those of other rodents, such as Microtus agrestis (McDevitt and Speakman, 1994).
Regarding the influence of acclimation regime, BMR has two aspects. First, it can be considered as an adaptive strategy to minimize energy expenditure when metabolic loads are high (i.e., BMR is regulated strategically). Indeed, several studies reported that BMR decreased after acclimation to low energy availability and/or high energy demands (Veloso and Bozinovic, 1993; Corp et al., 1997). Second, the high BMR detected after cold acclimation can be the by-product of enhanced metabolic expenditure triggered by food processing and oxygen delivery systems and by thermogenic tissue (i.e., BMR is regulated homeostatically). When animals are faced with low-quality food, it is reasonable to expect compensations in BMR through reducing the size of organs other than those related to energy acquisition (Geluso and Hayes 1999). However, during cold acclimation, metabolically active organs cannot be reduced without limiting thermogenic capacity (Koteja 1996a, 1996b), and because of that, MMR is generally coupled with BMR (Bennet and Ruben 1979; Table 1). In those cases, BMR is predicted to increase after acclimation period to cold conditions (Wiesingeret al. 1990), as we found in cururos (Table 1). This result allows us to predict that cold-acclimated cururos increase organ masses to face with higher metabolic demands.
Maximum Metabolic Rate, Nonshivering Thermogenesis, and Shivering Thermogenesis
In extant eutherians, contribution of NST is higher in small eutherians, such as rodents, in comparison to larger eutherians (Cannon and Nedergaard, 2004). The body size of primitive eutherians is as summed to be small. Values of NST (3.54±0.11 ml O 2 g-1 h-1, in control group individuals) are close to other similarly sized rodents ( M b = 60250 g, NST = 0.67-3.30 ml O 2 g-1 h-1, Jansky, 1973; Haim, 1996; Nespolo et al., 1999; Kronfeld-Schor et al., 2000; Haim et al. 1984; Hislop and Buffenestein, 1994; Goldman et al., 1999), but the values of NST (6.01±0.23 ml O 2 g-1 h-1, in cold acclimation group individuals) was fairly high compared with them. Unfortunately, comparisons are complicated because these authors used anesthetized animals, a procedure that underestimates NST (Ellison and Skinner, 1990; Wunder and Gettinger, 1996), which is clearly shown in the NST measurements done in anesthetized and awake naked mole rats (Goldman et al., 1999).
However, MMR (7.79±0.37 and 10.31±0.37 ml O 2 g-1 h-1, in control group and cold acclimation group individuals, respectively), the values of MMR was higher than NSTmax after cold acclimation
(Table 1; Figs. 1) give large ST. Observed differences between MMR and NSTmax are considered within the range of error of the measurements, so we conclude that ST is near the NSTmax. In the previous studies, shivering is the main way of thermogenesis after cold acclimation in birds (Saarela and Heldmaier 1987; Dietz et al. 1997) and in humans under cold environment (Giesbrecht et al. 1997). In contrast, the role of ST during cold acclimation is lower understood, suggesting that this way of heat production is used only when NST is insufficient (Jansky 1973; Lilly and Wunder 1979; Bockler and Heldmaier 1983; Nespolo et al. 1999).
ACKNOWLEDGMENTS
We are grateful to anonymous reviewers and editors for their valuable suggestions and comments. This study was financially supported by the National Natural Science Foundation of China (No.31071925; No.31260097), the Natural Science Foundation of Yunnan Province (No. 2011FZ082) and the Science Research Foundation of Yunnan Province Education Department (No.ZD2009007; No.2011J065). We thank all the members of Animal physiological ecology group for their help on the experiment.
Список литературы The relationship among metabolic rate of tree shrews (Tupaia belangeri) under cold acclimation
- Bennett AF, Ruben JA. (1979). Endothermy and activity in vertebrates. Science 206:649-653.
- Bockler H, Heldmaier G. (1983). Interaction of shivering and non-shivering thermogenesis during cold exposure in seasonally-acclimatized Djungarian hamster (Phodopus sungorus). J Therm Biol 8:97-98.
- Bozinovic F, Novoa F, Veloso C. (1990). Seasonal changes in energy expenditure and digestive tract of Abrothrix andinus (Cricetidae) in the Andes Range. Physiol Zool 63: 1216-1231.
- Bozinovic F, Rosenmann M. (1989). Maximum metabolic rate of rodents: physiological and ecological consequenceson distributional limits. Funct Ecol 3: 173-181.
- Cannon B, Nedergaard J. (2004). Brown Adipose Tissue: Function and Physiological Significance. Physiol Rev 84: 277-359.
- Cao X. (1989). Seasonal changes in spermatogenesis of tree shrew (Tupaia belangeri Chinensis). Zool. Res. 10: 15-21.
- Chappell MA, Bachman GC. (1995). Aerobic performance in Belding's ground squirrels (Spermophilus beldingi): variance, ontogeny, and the aerobic capacity model of endothermy. Physiol Zool 68: 421-442.
- Corp N, Gorman ML, Speakman JR. (1997). Seasonal variation in the resting metabolic rate of male wood mice Apodemus sylvaticus from two contrasting habitats 15 km apart. J Comp Physiol 167B: 229-239.
- Degen A. (1997). Ecophysiology of Small Desert Mammals. Springer, Berlin.
- Dietz MW, van Mourik S, Toien O, Koolmees PA, Tersteeg-Zijderveld MHG. (199). Participation of breast and leg muscles in shivering thermogenesis in young turkeys and guinea fowl. J Comp Physiol 167B: 451-460.
- Ellison GTH, Skinner JD. (1990). Noradrenaline thermogenesis in conscious and anaesthetized pouched mice (Saccostomus campestris). Comp Biochem Physiol 97A: 23-26.
- Geluso K, Hayes JP. (1999). Effects of dietary quality on basal metabolic rate and internal morphology of European starlings (Sturnus vulgaris). Physiol Biochem Zool 72: 189-197.
- Giesbrecht GG, Goheen MSL, Johnston CE, Kenny GP, Bristow GK, Hayward JS. (1997). Inhibition of shivering increases core temperature afterdrop and attenuates rewarming in hypothermic humans. J Appl Physiol 83:1630-1634.
- Goldman BD, Goldman SL, Lanz T, Magaurin A, Maurice A. (1999). Factors influencing metabolic rate in naked mole-rats (Heterocephalus glaber). Physiol Behav 66:447-459.
- Haim A, Heth G, Avnon Z, Nevo E. (1984). Adaptive physiological v ariation in nonshivering thermogenesis and its significance in speciation. J Comp Physiol 154B: 145-147.
- Haim A. (1996). Food and energy intake non-shivering thermogenesis and daily rhythm of body temperature in the bushy-tailed gerbil Sekeetamus calurus: the role of photoperiod manipulations. Journal of Thermal Biology, 21: 37-42.
- Hayes JP, Chappell MA. (1986). Effects of cold acclimation on maximum oxygen consumption during cold exposure and treadmill exercise in deer mice, Peromyscus maniculatus. Physiol Zool 59:473-481.
- Heldmaier G. (1971). Nonshivering thermogenesis and body size in mammals. J. Comp. Physiol. 73: 222-248.
- Heldmaier G, Steinlechner S, Rafael J, Latteier B. (1982). Photoperiod and ambient temperature as environmental cues for seasonal thermogenic adaptation in the Djungarian hamster, Phodopus sungorus. Int. J. Biometeorol. 26: 339-345.
- Hislop MS, Buffenstein R. (1994). Noradrenaline induces nonshivering thermogenesis in both the naked mole-rat (Heterocephalus glaber) and the Damara mole rat (Cryptomys damarensis) despite very different modes of thermoregulation. J Therm Biol 19: 25-32.
- Jansky L. (1973). Non-shivering thermogenesis and its thermoregulatory significance. Biol Rev 48:85-132.
- Koteja P. (1996a). Limits to the energy budget in a rodent, Peromyscus maniculatus: does gut capacity set the limit? Physiol Zool 69: 994-1020.
- Koteja P. (1996b). Limits to the energy budget in a rodent, Peromyscus maniculatus: the central limitation hypothesis. Physiol Zool 69: 981-993.
- Kronfeld-Schor N, Haim A, Dayan T, Zisapel N, Klingespor M, Heldmaier G. (2000). Seasonal thermogenic acclimation of diurnally and nocturnally active desert spiny mice. Physiological and Biochemical Zoology, 73: 37-44.
- Lilly FB, Wunder BA. (1979). The interaction of shivering and nonshivering thermogenesis in deer mice (Peromyscus maniculatus). Comp Biochem Physiol 63C:31-34.
- McDevitt RM, Speakman JR. (1996). Summer acclimatization in the short-tailed field vole, Microtus agrestis. J Comp Physiol 166B:286-293.
- Naya DE, Ebensperger LA, Sabat P, Bozinovic F. (200). Digestive and metabolic flexibility allows female Degusto cope with lactationcosts. Physiol. Biochem. Zool. 81(2), 186-194.
- Nespolo RF, Opazo JC, Rosenmann M, Bozinovic F. (1999). Thermal acclimation, maximum metabolic rate and nonshivering thermogenesis in Phyllotis xanthopygus (Rodentia) inhabiting the andean range. Journal of Mammalogy, 80: 742-748.
- Rosenmann M, Morrison P. (1974). Maximum oxygen consumption andheat loss facilitation in small homeotherms by He-O2. Am. J. Physiol. 226: 490-495.
- Saarela S, Heldmaier G. (1987). Effect of photoperiod and melatonin on cold resistance, thermoregulation and shivering/nonshivering thermogenesis in Japanese quail. J Comp Physiol 157B: 625-633.
- Schlichting C, Pigliucci M. (1998). Phenotypic Evolution: A Reaction Norm Perspective. Sinauer, Sunderland, Mass.
- van Sant MJ, Hammond KA. (2008). Contribution of shivering and nonshivering thermogenesis to thermogenic capacity for the deer mouse (Peromyscus maniculatus). Physiol. Biochem. Zool. 81(5): 605-611.
- Veloso C, Bozinovic F. (1993). Dietary and digestive constraints on basal energy metabolism in a small herbivorous rodent. Ecology 74: 2003-2010.
- Wunder BA, Gettinger RD. (1996). Effects of body mass and temperature acclimation on the nonshivering thermogenic response of small mammals. Pp. 131-139 in F. Geiser, A. J. Hulbert, and S.C. Nicol, eds. Adaptations to the Cold: Tenth International Hibernation Symposium. University of New England Press, Armidale.
- Wiesinger H, Klaus S, Heldmaier G. (1990). Increased nonshivering thermogenesis, brown fat cytochrome c oxydase extivity, GDP binding, and uncoupling protein mRNA levels after short daily cold exposure of Phodopus sungorus. Can J Physiol Pharmacol 68: 195-200.
- Wunder BA, Gettinger RD. (1996). Effects of body mass and temperature acclimation on the nonshivering thermogenic response of small mammals. Pp. 131-139 in F. Geiser, A. J. Hulbert, and S.C. Nicol, eds. Adaptations to the Cold: Tenth International Hibernation Symposium. University of New England Press, Armidale.
- Wang YX, Li CY, Ma SL. (1991). The classification and ecology of tree shrews. In Peng, Y., Ye, Z., Zou, R., et al. (eds). Biology of Chinese Tree shrews (Tupaia belangeri Chinensis). Yunnan Scientic and Technological Press, Kunming. Pp: 24-69.
- Wang ZK, Sun RY, Li QF. (1994). Characteristics of the resting metabolic rate of the treeshrews. J Beijing Normal University (Natural Science) 30(3): 408-414.
- Zhang L, Wang R, Zhu W, Liu P, Cai J, Wang Z, Sivasakthivel S, Lian X. (2011). Adaptive thermogenesis of liver in tree shrew (Tupaia belangeri) during cold acclimation. Anim. Biol. 61: 385-401.
- Zhang L, Liu P, Zhu W, Cai J, Wang Z. (2012a). Variations in thermal physiology and energetics of the tree shrew (Tupaia belangeri) in response to cold acclimation. J. Comp. Physiol. B 182: 167-176.
- Zhang L, Zhang H, Zhu W, Li X, Wang Z. (2012b). Energy metabolism, thermogenesis and body mass regulation in tree shrew (Tupaia belangeri) during subsequent cold and warm acclimation. Comp. Biocheim. Physiol. A 162: 437-442.
- Zhang L, Zhu W, Wang Z. (2012c). Role of photoperiod on hormone concentrations and adaptive capacity in tree shrews, Tupaia belangeri. Comp. Biocheim. Physiol. A. 163: 253-259
- Zhang L, Cai J, Wang Z. (2012d). Metabolism and thermoregulation in the tree shrews, Tupaia bealngeri. Journal of Stress Physiology & Biochemistry. 8(2): 167-178.
- Zhu WL, Zhang H, Wang ZK. (2012). Seasonal changes in body mass and thermogenesis in tree shrews (Tupaia belangeri): The roles of photoperiod and cold. Journal of Thermal Biology. 37: 479-484.
- Zou R, Ji W, Yan H, Lu J. (1991). The captivities and reproductions of tree shrews. In Peng, Y., Ye, Z., Zou, R., et al. (eds). Biology of Chinese Tree shrews (Tupaia belangeri Chinensis). Yunnan Scientic and Technological Press, Kunming. Pp: 71-130.


