The role of KRAS and NRAS mutation detection in determining the therapy strategy for colorectal cancer
Автор: Toropovskiy A.N., Nikitin A.G., Viktorov D.A., Solovev A.V., Khuzina R.M., Pavlova O.N.
Журнал: Вестник медицинского института "РЕАВИЗ": реабилитация, врач и здоровье @vestnik-reaviz
Рубрика: Клиническая медицина
Статья в выпуске: 5 (59), 2022 года.
Бесплатный доступ
Colorectal cancer is one of the most frequently diagnosed malignant tumors in men and women, which is a highly heterogeneous group of neoplasms consisting of subclasses with different molecular and clinical characteristics, and, as a consequence, patients with different types of tumors require different treatment protocols. Among the predictive factors of treatment response in patients with metastatic colorectal cancer, the most studied are the genes of the RAS family (KRAS, NRAS). Determination of RAS status is the first step in individual selection of drug therapy in patients with metastatic colorectal cancer. Patients with certain mutations in KRAS and NRAS genes are resistant to anti-EGFR therapy and have a lower median survival than WT (wild type) genotypes, indicating a negative prognosis in the presence of mutations.
Colorectal cancer, targeted therapy
Короткий адрес: https://sciup.org/143179585
IDR: 143179585 | УДК: 616.34/.35-006.6:616-08-059:575.224 | DOI: 10.20340/vmi-rvz.2022.5.CLIN.8
Текст научной статьи The role of KRAS and NRAS mutation detection in determining the therapy strategy for colorectal cancer
КЛИНИЧЕСКАЯ МЕДИЦИНА УДК 616.34/.35-006.6:616-08-059:575.224

Colorectal cancer (CRC) is one of the most frequently diagnosed malignant tumors in men and women and ranks third in the mortality rate from cancer in men and second in women [1]. Timely detection of colorectal cancer implies its diagnosis at early, preclin-ical stages, when there are no manifestations of the disease. Screening aimed at early detection of colorectal cancer is carried out by means of finger examination, endoscopic method and hemocult test. Currently, there is a program of primary screening of colorectal cancer, according to which all patients are tested for fecal occult blood at the dispensary examination annually by referral of the local therapist. If the analysis is negative, a finger examination of the rectum by a surgeon and, in women, by a gynecologist is performed annually. All persons over 50 years old undergo sigmoscopy and rectoscopy once every 3 years. The offered examination program allows to detect actively about 50 % of cases of primary cancer of colon and 57 % of cases of rectal cancer. Sigmoscopy and total colonoscopy are important methods of colorectal cancer screening, but the possibility of using these techniques for wide screening seems doubtful because of their great complexity and high cost. In general, the diagnosis of CRC is difficult, because the collection of biopsy material is difficult and the morphological integrity of the samples is destroyed during analysis; therefore, mostly CRC is detected only at late stages [2, 3].
CRC is a highly heterogeneous group of tumors consisting of subclasses with different molecular and clinical characteristics [3, 4]. Patients with different tumor types require different treatment protocols [5].
The basis of CRC pathogenesis is proliferation of atypical epithelial cells of intestinal mucosa resulting from aberrant methylation, dysregulation of transcription factors or mutation in oncogenes ( KRAS , NRAS , BRAF and PIK3CA ) and oncosupressors ( APC, TP53, SMAD4 and PTEN ) [6]. These disorders affect key signaling pathways including Wnt/ β -catenin, epidermal growth factor receptor ( EGFR ), mitogen-activated protein kinase, MAPK ), phosphoinositide-3-kinase ( PI3K ), the Ras superfamily of small guanosine triphosphatases, and transforming growth factor beta ( TGF - β ).
The listed abnormalities can be divided into two groups: CRC with chromosomal instability associated with loss of APC protein function and mutations in genes encoding Wnt- and Ras-signaling pathways and
CRC with microsatellite instability, which is often associated with mutations in genes of the mismatched nucleotide repair (MMR) system.
CRC with chromosomal instability is the most common group. Mutations of APC gene initiate initial stages of CRC: APC is a negative regulator of β -catenin, and in the presence of mutation the concentration of β -catenin in cytoplasm increases significantly and leads to activation of Wnt-signaling pathways which in turn stimulate tumor cell division and migration [7, 8].
Transformation of adenoma into carcinoma occurs when the structure of GTPases, which are involved in the transduction of extracellular MAPK signals, is disrupted [9–11]. An amino acid substitution in Ras proteins prevents their hydrolysis, resulting in activation of protein cascades: RAF/MEK/ERK and PI3KAKT signaling pathways responsible for cell growth and division [12]. As a result, the cell is in a permanently activated state, which allows it to avoid apoptosis and start uncontrolled division. In 80 % of cases of CRC, EGFR hyperexpression was found to be present, which leads to increased growth and division of tumor cells due to hyperactivation of RAS/MAPK signaling cascade [13].
Currently, much attention is being paid to the therapy of CRC. In the last decade, the survival rate of patients with metastatic CRC has significantly increased. This is due to the introduction of targeted therapies such as anti- EGFR monoclonal antibodies (MoAbs). Anti- EGFR MoAbs can be used both in monotherapy and in combination with conventional chemotherapy [14]. Currently, two targeted anti- EGFR drugs are approved for clinical practice – the chimeric immunoglobulin G (IgG1) cetuximab and a fully humanized antibody of the immunoglobulin G2 class – panitumumab, which have high dermal toxicity. These drugs are most effective in "wild-type" KRAS , NRAS , BRAF genes and the presence of mutated KRAS , NRAS or BRAF genes leads to the formation of the same name mutant protein that activates EGFR-RAS-RAF signaling pathway (fig. 1), which in turn results in uncontrolled cell division, impaired regulation of proliferation and resistance to apoptosis [15]. That is why the issue of identifying a target group of patients sensitive to EGFR inhibitors is urgent.
Binding of extracellular EGFR receptor leads to blocking of intracellular tyrosine kinase domain and, consequently, deactivates Ras signaling pathways and thus prevents tumor growth and development [16]. The efficacy of anti-EGFR MoAbs treatment depends on molecular genetic changes in the tumor: EGFR status, the presence of mutations in other members of the signaling cascade – KRAS and BRAF oncogenes and some other factors. In the absence of mutations in the KRAS gene, the efficacy of CRC treatment is very high, life expectancy increases and the number of recurrences decreases. At the same time, in the presence of activating mutations in the KRAS gene in tumor cells, the use of anti-EGFR antibodies does not lead to positive results [17].
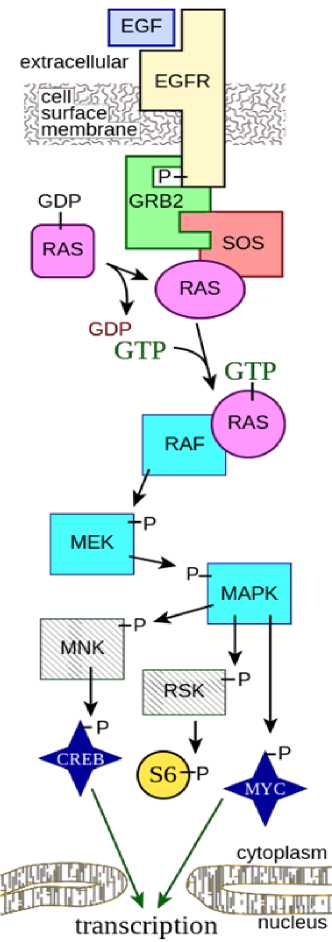
Figure 1. Schematic of the RAS-RAF-MEK-ERK signaling pathway. P stands for phosphorylation
[]
The following mutations in KRAS codons 12 and 13 (85–90 %), which correlate with tumor resistance to anti-EGFR therapy, are detected in 30–40 % of cases of CRC: G12C, G12S, G12R, G12A, G12V, G12D, G13D. Mutations in codons 61 (5 %) and 146 (5 %) are detected much less frequently [17]. Studies by Pisareva et al. (2016) of mutations in the KRAS gene in codons 12 and 13 also revealed that the most frequent mutations are G13D (15%) and G12D (13 %), which agrees with the studies of other authors [10, 17].
In general, mutations in the KRAS and NRAS genes, detected in approximately 50 % of patients with CRC, have been found to be associated with resistance to anti- EGFR therapy [17]. Moreover, recent studies on the development of resistance to anti-EGFR therapy show that patients with wild-type KRAS and NRAS genes may have small subpopulations of cells carrying mutations in RAS (Retrovirus Associated) genes [18]. In such cases, resistance to MoAbs therapy rapidly develops within a few months.
The most known oncogenic mutations are mutations in exons 2, 3, and 4 in the KRAS and NRAS genes, mostly all of which are pathogenic and play a role in determining targeted therapy for CRC (table 1). The presence of a mutant allele in one of these genes indicates an unfavorable prognosis for the patient and insensitivity to anti- EGFR therapy. Currently, there is no officially registered drug that inhibits Ras GTPase, but mutation analysis allows us to identify a group of patients who are responsive to anti- EGFR therapy.
KRAS mutations are most common, which may be due to the presence of a large number of rare codons in the KRAS gene, leading to reduced translation of the protein [19]. Patients with such mutations have a more aggressive nature of malignancy and are difficult to treat [20]. Therefore, targeted drugs inhibiting Ras family proteins are currently being tested. The main compounds capable of inhibiting Ras proteins are considered to be small molecules – chemical compounds with a molecular weight of no more than 900 Dalton [21]. However, inhibition of mutant Ras proteins is associated with high toxicity to normal tissues due to the fact that the Ras family has up to 300 substrates [22]. Another important reason for analyzing the spectrum of KRAS and NRAS mutations is that some patients with mutations in these genes are still sensitive to anti- EGFR therapy. For example, G13D mutation carriers turn out to be sensitive to cetuximab therapy [23]. A potential explanation for this phenomenon lies in the fact that
RAF- and PI3K-signaling pathways are mainly activated in cells with G12D mutations, while G12V, G12C or G13D mutations affect the activation of RAL-signaling pathways.
Initially, there was a hypothesis in oncology about the identity of metastases and the primary tumor, because it was assumed that if the cells of metastases originated from the cells of the primary tumor, they should carry the same specific genetic changes [24]. But thanks to the development of molecular genetic diagnostic methods, including the method of evaluation of individual nucleotide polymorphisms with high coverage density, it was possible to detect differences in the genome of metastasis and primary tumor cells. It is assumed that the changes in the metastasis tumor cell genome are related both to the metastatic process itself and to the adaptation of the cells to the new microenvironment [25]. Therefore, for this kind of patients, when prescribing targeted anti- EGFR drugs, not only the status of the primary focus, but also that of the metastases, should be taken into account.
The presence of a predictive marker of the effectiveness of anti-EGFR (epidermal growth factor receptor) monoclonal antibodies in patients with metastatic CRC – mutational status of RAS (KRAS, NRAS) and BRAF genes – determines the relevance of studying possible changes in the mutational status of genes in metastases. The results of early studies on concordance between the primary tumor and CRC metastases by the mutational status of the KRAS gene are contradictory. Some noted the absence of such concordance [26], while others, on the contrary, revealed a high percentage of concordance [27].
F. Loupakis et al. studied the mutational status of KRAS gene and PTEN and AKT expression in 106 patients with metastatic CRC who received targeted therapy (irinotecan with cetuximab). At the same time in 53 patients this analysis was performed in the primary tumor and in the metastasis. The researchers found correspondence of changes between the primary tumor and metastases by AKT expression in 68 %, by PTEN expression – in 60 % and by KRAS gene mutation status – in 95 % of patients. The authors of the work also found that along with mutation in KRAS gene, ineffectiveness of anti- EGFR effect was noted in loss of PTEN expression in metastases [28]. In contrast, researchers from Italy have also previously shown a significant difference in the expression of EGFR , pAKT , and components of MAPK -signaling pathway between primary tumors and metastases, which may indicate biological disorders accumulating in the course of disease progression [29].
Table 1. Mutation spectrum in KRAS and NRAS genes
|
KRAS |
NRAS |
||||
|
Exon |
Codon |
Title mutations |
Exon |
Codon |
Title mutations |
|
2 |
12 |
p.G12A; p.G12C; p.G12C; p.G12D; p.G12F; p.G12H; p.G12R; p.G12S; p.G12V; p.G12I; p.G12N; p.G12L; p.G12Y; p.G12F; p.G12R; p.G12L; p.G12C; p.G12W; p.G12D; p.G12A; p.G12V; p.G12fs*3 |
2 |
12 |
p.G12A; p.G12C; p.G12D; p.G12S; p.G12R; p.G12N; p.G12P; p.G12Y; p.G12V; p.G12E |
|
2 |
13 |
p.G13C; p.G13S; p.G13R; p.G13C; p.G13N; p.G13I; p.G13Y; p.G13F; p.G13D; p.G13R; p.G13A; p.G13V; p.G13E; p.G13E; p.G13D; p.G13V; p.G13V; p.G13_V14>DI |
2 |
13 |
p.G13R; p.G13V; p.G13S; p.G13C; p.G13N; p.G13Y; p.G13D; p.G13A; p.G13V |
|
3 |
59 |
p.A59T; p.A59S; p.A59P; p.A59E; p.A59G; p.A59V; p.A59del |
3 |
59 |
p.A59T; p.A59P; p.A59S; p.A59D; p.A59G; p.A59V |
|
3 |
61 |
p.Q61K; p.Q61E; p.Q61*(Ter); p.Q61H; p.Q61H; p.Q61L; p.Q61P; p.Q61K; p.Q61R; p.Q61Q; |
3 |
61 |
p.Q61H; p.Q61K; p.Q61L; p.Q61R; p.Q61E; p.Q61K; p.Q61P; p.Q61R; p.Q61L; p.Q61R; p.Q61H; p.Q61Q; p.Q61L; p.Q61_E62 > HK |
|
4 |
117 |
p.K117R; p.K117N; p.K117E; p.K117Q; p.K117T; p.K117I; p.K117N |
4 |
117 |
p.K117R; p.K117N; p.K117E; p.K117Q; p.K117T; p.K117M; p.K117N |
|
4 |
146 |
p.A146P; p.A146T; p.A146V; p.A146S; p.A146G; p.A146E |
4 |
146 |
p.A146P; p.A146T; p.A146V; p.A146S; p.A146G; p.A146D |
At the same time, other works have revealed high (78.0–94.7 %) concordance in EGFR expression in primary tumor and metastases [30]. Application of the most sensitive methods more often leads to detection of full concordance by mutational status of a primary tumor with CRC metastases [31]. However, there is a question about clinical relevance of low mutant alleles detected by highly sensitive methods to the efficacy of anti- EGFR monoclonal antibodies. Thus, in the work of D. Tougeron et al. the frequency of objective effect of combination of anti- EGFR antibodies with chemotherapy was 37.0 % in wild-type KRAS gene against 6.7 % in cases when even small percentage (< 10 %) of mutant alleles of this gene was detected [32]. Trivial falsepositive and false-negative test results cannot be excluded [33]. Note that if we compare mutational status of primary tumor cells and circulating tumor cells in blood the differences are more pronounced (up to 23 % for KRAS gene and 7 % for BRAF gene) [34]. Determination of circulating tumor DNA (cDNA) mutations in blood is considered to be perspective that can help to detect treatment-resistant tumor clones and select appropriate antitumor drugs. It should be noted that, as a rule, a particular mutation that determines resistance to current therapy does not appear de novo during treatment, but pre-exists in one of the tumor clones. Such findings lead researchers to believe that a combination of different targeted drugs should be used in order to overlap the entire spectrum of clinically significant molecular abnormalities in the tumor already in the 1st line of therapy [35].
In an additional study of cases of divergent mutational status of the KRAS gene in the primary tumor and metastases, genetic analysis in cells from additional sections of the primary tumor allowed the detection of cell clones with mutations in the KRAS gene [36]. As a result, if we accept that the tumor is initially heterogeneous by various mutational changes, then disease progression should be considered not as a sequential process but as a parallel development of the primary tumor and metastasis [36]. This is confirmed by the presence of differences in the mutational status of genes (driver mutations) between the primary tumor and metastases. Accordingly, metastases do not require the same set of mutational changes that are required for primary tumor growth [37]. Intratumor heterogeneity manifests itself not only in differences in mutational status, but also in the expression of unchanged genes. Hence, according to current research results, tumors, including CRC, are characterized by heterogeneity [38], and ongoing treatment through survival of resistant cell clone determines the phenomena of sub-clonal evolution at cellular level [39]. Conducting systemic therapy also leads to the selection of certain tumor clones, which may increase the incidence of discordance between the primary tumor and metastases. It is also possible that a biopsy of a single metastatic focus out of several, especially during specific treatment, will not reflect the entire molecular pattern of heterogeneous tumor clones. This may determine the ineffectiveness of an individually tailored targeted therapy based only on genetic changes obtained from 1 tumor sample [40]. Patients with CRC need to determine the mutation status of the KRAS gene, which plays an important role in terms of further chemotherapeutic treatment strategy. For example, in the presence of a mutation in codon 13, an effective combination of conventional chemotherapy and targeted drugs is possible.
Thus, analysis of the presence and spectrum of KRAS and NRAS mutations becomes a necessary requirement for the treatment of patients with CRC. Mutations in KRAS and NRAS genes are the most significant prognostic and therapeutic biomarkers in patients with CRC. The presence of a mutant allele in one of these genes indicates an unfavorable prognosis for the patient and insensitivity to anti- EGFR therapy. Currently, there is no officially registered drug that inhibits Ras GTPase, but mutation analysis allows us to identify a group of patients who are responsive to anti- EGFR therapy.
Список литературы The role of KRAS and NRAS mutation detection in determining the therapy strategy for colorectal cancer
- Davydov M.I., Aksel E.M. Statistics of malignant neoplasms in Russia and the CIS countries in 2012. Vestnik RONC im. N.N. Blokhina = Bulletin of N.N. Blokhin Russian Cancer Research Center. 2014 (In Russ). [Давыдов М.И., Аксель Е.М. Статистика злокачественных новообразований в России и странах СНГ в 2012 г. ВестникРОНЦ им. Н.Н. Блохина РАМН. 2014].
- Pancione M., Remo A., Colantuoni V. Genetic and epigenetic events generate multiple pathways in colorectal cancer progression. Pathol Res Int. 2012;2012:509348. https://doi.org/10.1155/2012/509348
- Bishehsari F., Mahdavinia M., Vacca M. et al. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014; 20(20): 6055-6072. https://doi.org/10.3748/wjg.v20.i20.6055
- Kondo Y., Issa J.P. Epigenetic changes in colorectal cancer. Cancer Metastasis Rev. 2004;23(1-2):29-39. https://doi .org/10.1023/a:1025806911782
- Cohen R., Pudlarz T., Delattre J.F. et al. Molecular targets for the treatment of metastatic colorectal cancer. Cancers (Basel). 2020;12(9):2350. https://doi.org/10.3390/cancers12092350
- Issa J.P. Colon Cancer: It's CIN or CIMP. Clin Cancer Res. 2008;14(19):5939-5940. https://doi.org/10.1158/1078-0432.CCR-08-1596
- Kwong L.N., Dove W.F. APC and its modifiers in colon cancer. Adv Exp Med Biol. 2009;656:85-106. https://doi.org/10.1007/978-1-4419-1145-2_8
- Brovkina O.I., Nikitin A.G. Mutations in KRAS and NRAS genes as biomarkers in colorectal cancer therapy and basic methods of their detection. Clinical Practice. 2021;12(1):66-71. [Бровкина О.И., Никитин А.Г. Мутации в генах KRAS и NRAS как биомаркеры в терапии колоректального рака и основные методы их детекции. Клиническая практика. 2021;12(1): 66-71 ]. https://doi.org/10.17816/clinpract63875
- Linardou H., Briasoulis E., Dahabreh I.J. et al. All about KRAS for clinical oncology practice: gene profile, clinical implications and laboratory recommendations for somatic mutational testing in colorectal cancer. Cancer Treat Rev. 2011;37(3):221-233. https://doi.org/10.1016/j.ctrv.2010.07.008
- Pisareva E.E., Ljubchenko L.N., Kovalenko S.P., Shamanin V.A. Analysis of mutations in kras and braf genes in colorectal cancer in Russian patients. Siberian Journal of Oncology. 2016;15(2):36-41. (In Russ). [Писарева Е.Е., Любченко Л.Н., Коваленко С.П., Ша-манин В.А. Анализ мутаций в генах KRAS и BRAF при раке толстой и прямой кишки в российской популяции. Сибирский онкологический журнал. 2016;15(2):36-41]. https://doi.org/10.21294/1814-4861 -2016-15-2-36-41
- Vaughn C.P., Zobell S.D., Furtado L.V. et al. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer. 2011;50(5):307-312. https://doi.org/10.1002/gcc.20854
- Molina J.R., Adjei A.A. The Ras/Raf/MAPK Pathway. J Thorac Oncol Elsevier. 2006;1(1):7-9.
- Porebska I., Harlozinska A., Bojarowski T. Expression of the tyrosine kinase activity growth factor receptors (EGFR., ERB B2., ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol. 2000;21(2):105-115.
- Grothey A., Lenz H.J. Explaining the unexplainable: EGFR antibodies in colorectal cancer. J Clin Oncol. 2012;30(15):1735-1737. https://doi.org/10.1200/JC0.2011.40.4194
- Shubin V.P., Pospekhova N.I., Tsukanov A.S., Rybakov E.G., Panina M.V., Sushkov O.I. et al. Frequency and spectrum KRAS mutations in different localization of colon cancer and anal cancer. Medicinskaâgenetika. 2014;13(5):3135. (in Russ). [Шубин В.П., Поспе-хова Н.И., Цуканов А.С., Рыбаков Е.Г., Панина М.В., Сушков О.И. и др. Частота и спектр мутаций в гене KRAS при раке толстой кишки разной локализации и раке анального канала. Медицинская генетика. 2014;13(5):3135].
- Jean G.W., Shah S.R. Epidermal growth factor receptor monoclonal antibodies for the treatment of metastatic colorectal cancer. Pharmacotherapy. 2008;28(6):742-754. https://doi.org/10.1592/phco.28.6.742
- Lièvre A., Bachet J.B., Corre D.L. et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992-3995. https://doi.org/10.1158/0008-5472.CAN-06-0191
- Diaz L.A., Williams R., Wu J. et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486(7404):537-540. https://doi.org/10.1038/nature11219
- Lampson B.L., Pershing N.L., Prinz J.A. et al. Rare codons regulate KRas oncogenesis. Curr Biol. 2013;23(1):70-75. https://doi.org/10.1016Zj.cub.2012.11.031
- Porru M., Pompili L., Caruso C. et al. Targeting KRAS in metastatic colorectal cancer: current strategies and emerging opportunities. J Exp Clin Cancer Res. 2018;37(1):57. https://doi.org/10.1186/s13046-018-0719-1
- Cox A.D., Fesik S.W., Kimmelman A.C. et al. Drugging the undruggable RAS: Mission Possible? Nat Rev Drug Discov. 2014;13(11):828-851. https://doi.org/10.1038/nrd4389
- Liu M., Sjogren A.K., Karlsson C. et al. Targeting the protein prenyltransferases efficiently reduces tumor development in mice with K-RAS-induced lung cancer. Proc Natl Acad Sci USA. 2010;107(14):6471-6476. https://doi.org/10.1073/pnas.0908396107
- Tejpar S., Celik I., Schlichting M. et al. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol. 2012;30(29):3570-3577. https://doi.org/10.1200/JC0.2012.42.2592
- Al-Mulla F., Keith W.N., Pickford I.R. et al. Comparative genomic hybridization analysis of primary colorectal carcinomas and their synchronous metastases. Genes Chromosomes Cancer. 1999;24(4):306-14.
- Munoz-Bellvis L., Fontanillo C., Gonzalez-Gonzalez M. et al. Unique genetic profile of sporadic colorectal cancer liver metastasis versus primary tumors as defined by high-density singlenucleotide polymorphism arrays. Mod Pathol . 2012;25(4):590-601.
- Albanese I., Scibetta A.G., Migliavacca M. et al. Heterogeneity within and between primary colorectal carcinomas and matched metastases as revealed by analysis of Ki-ras and p53 mutations. Biochem Biophys Res Commun. 2004;325(3):784-91.
- Zauber P., Sabbath-Solitare M,. Marotta S.P., Bishop DT. Molecular changes in the Ki-ras and APC genes in primary colorectal carcinoma and synchronous metastases compared with the findings in accompanying adenomas. Mol Pathol. 2003;56(3):137-40.
- Loupakis F., Pollina L., Stasi I. et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol . 2009;27(16):2622-9.
- Scartozzi M., Bearzi I., Berardi R. et al. Epidermal growth factor receptor (EGFR) status in primary colorectal tumors does not correlate with EGFR expression in related metastatic sites: Implications for treatment with EGFR-targeted monoclonal antibodies. J Clin Oncol. 2004;22:4772-8.
- Italiano A., Saint-Paul M.C., Caroli Bosc F.X. et al. Epidermal growth factor receptor (EGFR) status in primary colorectal tumors correlates with EGFR expression in related metastatic sites: biological and clinical implications. Ann Oncol. 2005;16(9):1503-7.
- Etienne-Grimaldi M.C., Formento J.L., Francoual M. et al. K-Ras mutations and treatment outcome in colorectal cancer patients receiving exclusive fluoropyrimidine therapy. Clin Cancer Res. 2008;14(15):4830-5.
- Tougeron D., Lecomte T., Pages J.C. et al. Effect of low-frequency KRAS mutations on the response to anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol. 2013;24(5):1267-73.
- Baas J.M., Krens L.L., Guchelaar H.J. et al. Concordance of predictive markers for EGFR inhibitors in primary tumors and metastases in colorectal cancer: a review. Oncologist. 2011;16(9):1239-49.
- Mostert B., Jiang Y., Sieuwerts A.M. et al. KRAS and BRAF mutation status in circulating colorectal tumor cells and their correlation with primary and metastatic tumor tissue. Int J Cancer. 2013;133(1):130-41.
- Leder K., Foo J., Skaggs B. et al. Fitness conferred by BCR-ABL kinase domain mutations determines the risk of preexisting resistance in chronic myeloid leukemia. PLoS One. 2011 ;6(11):e27682.
- Oltedal S., Aasprong O.G., M0ller J.H. et al. Heterogeneous distribution of K-ras mutations in primary colon carcinomas: implications for EGFR-directed therapy. Int J Colorectal Dis. 2011;26(10):1271-7.
- Ramaswamy S., Ross K.N., Lander E.S.,Golub T.R. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33(1):49-54.
- McGranahan N., Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell. 2015;27(1):15-26 .
- Misale S., Yaeger R., Hobor S. et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486(404):532-6.
- Fedyanin M.Yu., Elsnukaeva H.H.-M., Tjulandin S.A. Heterogeneity and clonal evolution of colorectal cancer. Advances in molecular oncology. 2017;4:24-34. https://doi.org/10.17650/2313-805X-2017-4-1-24-34


