The seasonal changing of the chlorophyll content and stomata parameters in leaves of wild species of pear
Автор: Rajametov Sherzod
Журнал: Биология в сельском хозяйстве @biology-in-agriculture
Рубрика: Современные проблемы отраслей растениеводства и садоводства
Статья в выпуске: 3 т.8, 2015 года.
Бесплатный доступ
In this experiment, we analyzed seasonal changing of chlorophyll concentration from July to late September with interval 20 days, and also stomata parameters leaves of wild species of pear. Leaves for investigation was collected from mid part of annual shoots with length 0.8-1.0 m. Preliminary work showed that chlorophyll content and stomata parameters in leaves are different during vegetation. So, the lowest chlorophyll showed P. korshinskyi. The highest measured in P. aromatica, P. nivalis, and P. pyrifolia. Almost the same pattern of the chlorophyll content was detected under calculating per fresh weight of leaf per cm 2 unit area. As in chlorophyll concentration between wild species stomata parameters were also characterized with ranging. The biggest area of stomata showed P. bretshneideri, P. ussuriensis, P. pyrifolia, P. aromatic, and the smallest P. betulifolia. However, it should be noted that morning time when the plant well saturated with water the wild species P. korshinskyi, P. aromatica, P. calleryana and P. bretshneideri showed the biggest stomata area. Subsequently, at 03 PM they start to reduction of stomata, whereas P. betulifolia and P. ussuriensis showed not significant ranging regardless of environment condition. The highest of density stomata per certain leaf unit (mm 2) and total area showed drought tolerance species- P. korshinskyi.
Chlorophyll, stomata, leaf, wild species, density, area
Короткий адрес: https://sciup.org/14770301
IDR: 14770301 | УДК: 58.056
Текст научной статьи The seasonal changing of the chlorophyll content and stomata parameters in leaves of wild species of pear
In the process of evolution plants had been formed specific structures that make the process of photosynthesis. The main organ of photosynthesis in higher plants is the leaf. Features of the structure of this organ allow for the process of absorption of solar energy, convert it into energy and organic compounds to provide autotrophic type of source that is typical for the plant organism. And, in this case chlorophyll and stomata is the most visible manifestation of life dur- and stomata characteristics of leaves wild species of
В данной работе были проанализированы сезонные изменения концентрации хлорофилла в течение июля по сентябрь 2013 года с интервалом 20 дней, а также параметры устьиц листьев дикорастущих видов груши. Листья для опыта собирали из средней части однолетних побегов длиной 0,8-1,0 м. Предварительная работа показала, что содержание хлорофилла и параметры устьиц в листьях отличаются в период вегетации. Так, самый низкий хлорофилл отмечен у вида Р. korshinskyi . Самая высокая степень в P. aromatica , P. nivalis и Р. pyrifolia . Почти такая же картина содержания хлорофилла была обнаружена при расчете на сырой вес единицы площади листа (на см2).
Как и по концентрации хлорофилла, параметры устьиц у диких видов груши характеризовались изменчивостью. Самая большая площадь устьиц была выявлена у Р. bretshneideri , Р. ussuriensis , Р. pyrifolia , P. aromatica и наименьшая у P. betulifolia .
Тем не менее, следует отметить, что в утреннее время, когда растения хорошо насыщены водой, дикорастущие виды P. korshinskii , Р. аromatica , Р. calleryana и Р. bretschneideri имели наибольшую площадь устьиц.
Далее к 3 часам вечера устьица начинали уменьшения в размерах, в то время как Р. betulifolia и Р. ussuriensis значительно не реагировали на окружающую среду. Самая высокая плотность устьиц на определенную единицу листьев (мм2) и общей площади были отмечены у засухоустойчивого вида P. korshinskyi .
ing photosynthesis, gas exchange, respiration and so on, which clearly plays a very prominent role in the recycling of nutrients in plants physiology (Kräutler et al. 1997; Rodoni et al. 1998; Rotondi and Predieri, 2002; Rüdiger et al. 2006; Kräutler, 2008; Cruz et al. 2012; Gashi et al. 2013). Additionally, Martin et al. (1997) reported, that lower vigor in red-fruited trees was strongly correlated with lower rubisco activity and lower photosynthetic rates, and was (more weakly) correlated with lower chlorophyll content, the same pattern reported Rotondi and Predieri (2002), where detected low a leaf content of chlorophyll which contributed to decrease photosynthetic rate in pear.
However, histological studies of leaves and chlorophyll concentration may show a difference in plants and period. Chlorophyll and stomata can be changed by water content in plant and soil, light-induce, ages, habit and temperature (Trejo and Davies 1991; Baba-ni and Lichtenthaler 1996; Rotondi and Predieri 2002; Xu et al . 2008; Ghasemi et al . 2011; Gashi et al . 2013), soil fertilizing condition (Blackmer and Shep-ers 1995; Kräutler, 2008; Ghasemi et al . 2011) and genes induced by specific plant hormones (Rodriquez and Davies 1982; Gomes et al . 1988; Zhang et al . 1990a; Hartung and Slovik 1991; Trejo et al . 1993) etc.
In literature presented limit information about chlorophyll concentration and leaf stomata characteristics in pear wild species. So, according to Ma et al . (2005), the leaf chlorophyll content of wild species of pear rootstock seedlings decreased markedly with the increasing soil pH 8.2, where severe leaf chlorosis occurred at the high pH, whereas it was not observed in low soil pH 5.5. However, chlorophyll content values differed in the three pear rootstock. The same pattern was observed in the experiment of grafting with “Hosui” pear cultivar, which showed different concentration of chlorophyll in different rootstock. It means that different rootstocks can response differently on grafted plants. Therefore, many authors reported that the success of commercial varieties grafted on these tolerant rootstocks is mainly determined by the rootstock genotype (Byrne et al . 1989; Alcantara et al . 2003; Ma et al . 2005).
Ghasemi et al . (2011), estimated of leaf chlorophyll and nitrogen content in Asian pear ( Pyrus serot-ina Rehd.) by using CCM-200, and identified positive and linear correlation between CCM-200 data and chlorophyll a , chlorophyll b , total chlorophyll, and total nitrogen content in leaves. They reported that, CCM-200 can be used in order to predict both chlorophyll and nitrogen content in Asian pear leaves. However, the conclusion of results raises doubts, since that experiment was carried out only in June, whereas CCM-200 data was used and presented in certain limit study period of vegetation.
Coskun et al. (2011), hypothesized that differences among chlorophyll content of varieties may be due to different response of varieties to high temperature stress. Additionally, chlorophyll concentration, biosynthesis and photosynthesis ratios in plants leaf decreases at temperature 35-38°C (Berry et al . 1980; O’Mahony et al . 2000).
So, far, limited studies and application of data on chlorophyll concentration and leaf stomata parameters for breeding purposes have not been performed enough on Pyrus wild species during vegetation. And many researchers are concentrated they investigation only in certain commercial crops, period and under special treatments. Therefore, in this paper we presented research work about the characteristics of the seasonal changes of the chlorophyll concentration and stomata parameters in comparison to area and density of leaves of wild species of pear.
MATERIALS AND METHODS
Plant materials
The experiment was carried out in Pear Research Station, NIHHS, RDA (Republic of Korea) during the summer 2013. Study of changing chlorophyll composition and stomata parameters in pear leaves was done from July to late September with interval 20 days in wild species P.korshinskyi Litv., P.boissieriana Buhse, P.betulifolia Bung, P.pyrifolia Nakai, P.ussurienseis Maxim, P.nivalis Jacq, P.calleryana Decne, P.bretschneideri Rehd and P.aromatica . Sampling of leaves to measure of total chlorophyll content was done afternoon at 03 PM, and stomata parameters at 08 AM morning and at 03 PM afternoon, from middle part of annual shoots with length 80-100 cm.
Evaluation of Chlorophyll content and stomata parameters
Chlorophyll content extract was assayed in leaves by Eon Microplate Spectrophotometer USA- at 651 and 664 nm (mg/g-1 fresh weight).
The stomata area of leaves was determined from middle part of shoots by electron microscope AXIO (Carl Zeiss, Germany, and magnification- x50-400).
The leaf area (cm2) was measured from middle part of annual shoots by LI-3100 Area meter (USA).
RESULTS
Chlorophyll concentration in leaves during vegetation
Seasonal variation of chlorophyll content in leaves is presented Fig. 1. Chlorophyll content of wild species showed stable rates during vegetation period. However, in certain species P.aromatica, P.nivalis Jacq and P.pyrifolia Nakai identified a higher and greatly fluctuation of data, where P.aromatica showed a maximal values- 2.455 mg/g1(f.w) in midAugust, whereas in P.korshinskyi Litv it was significantly lower than other species- 0.225 mg/g1 (f.w) in late July.
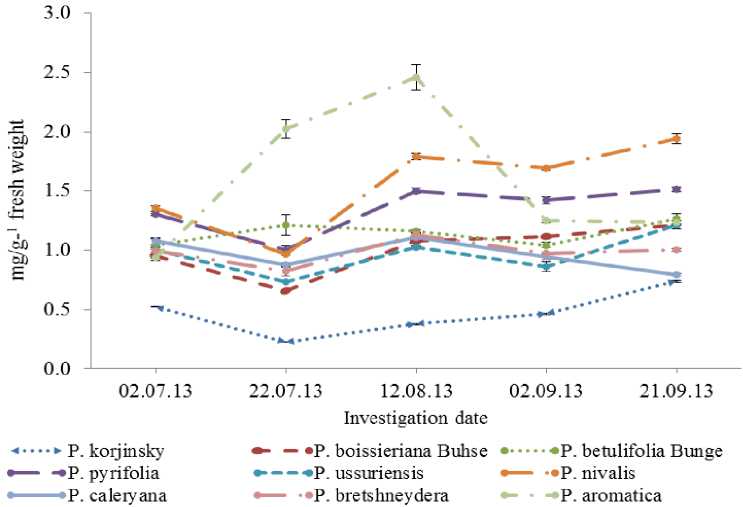
Fig. 1. The seasonal changing of the total chlorophyll content in leaves of wild species of pear during vegetation. Data represented by Mean ± SD (n= 10).
Almost the same pattern detected by calculating fresh weight chlorophyll content per unit cm2 area of leaf (Fig. 2), where in P.korshinskyi Litv determined the lowest value- 0.005 mg (f.w) in late July, whereas P.aromatica showed the highest rate- 0.066 mg (f.w) in mid-August of vegetation.
Appendix
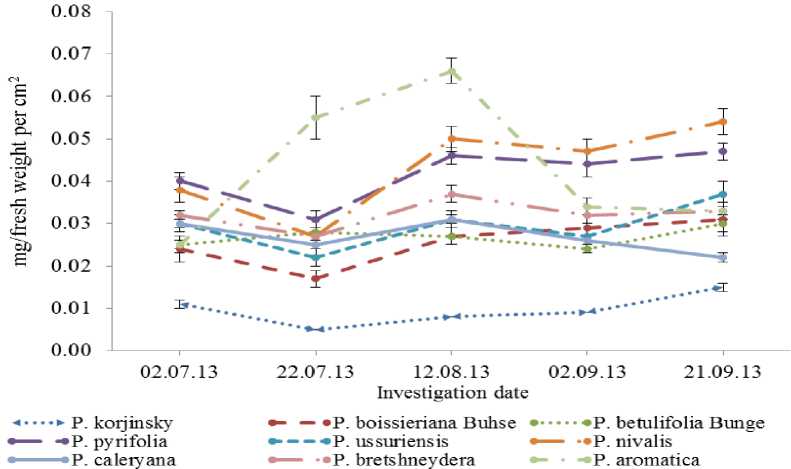
Fig. 2. Concentration of chlorophyll in cm2 leaf area during vegetation. Data represented by Mean ± SD (n= 10).
As well known the wild species of pear have a different size of leaves and in comparison to the total leaf area the total chlorophyll content per fresh weight of leaf were also varied (Fig. 3). So, the wild species P.aromatica, P.pyrifolia, P.ussuriensis, P.calleryana, and P.bretshneideri which had a great- est leaf area, also respectively, showed the highest concentration of chlorophyll, whereas species P.nivalis Jacq, P.betulifolia Bung, P.boissieriana Buhse which were identified with high composition of chlorophyll in certain per area in this case showed the lowest rate.
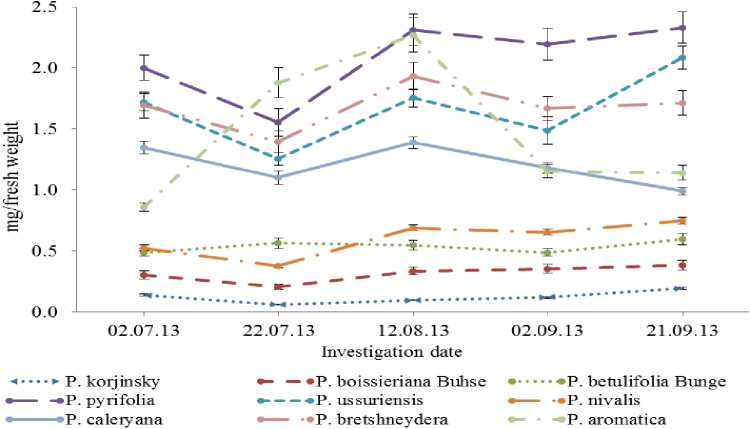
Fig. 3. Changes of the chlorophyll content per total leaf during vegetation. Data represented by Mean ± SD (n= 10).
A leaf size and stomata property of the pear wild species during vegetation
The biggest leaf area and fresh weight detected in wild species P.bretshneideri, P.pyrifolia, P.ussuriensis and P.calleryana where data was higher than 40 cm2 and 1.2 gram, respectively (Tab. 1). The smallest one detected in P.korshinskyi Litv, P.boissieriana Buhse and P.nivalis Jacq- below 13 cm2 and 0.4 gram, respectively. However, leaf weight per cm2 area unit showed difference values. In this case P.pyrifolia, P.bretshneideri, P.ussuriensis and P.nivalis Jacq distinguished with high leaf weight per unit- over 0.030 grams.
Table. 1. Leaf parameters of the different pear wild species.
|
Name |
Leaf weight, (g) |
Leaf area, (cm2) |
Leaf weight (g), per cm2 |
Leaf length, cm |
width, cm |
|
P.ussurienseis Maxim |
1.712±0.060 az |
55.2±1.5 a |
0.031±0.002 a |
12.1±0.2 b |
7.0±0.1 a |
|
P.bretschneideri Rehd |
1.636±0.066 a |
54.6±1.8 a |
0.030±0.001 a |
13.7±0.3 a |
6.3±0.1 b |
|
P. pyrifolia Nakai |
1.489±0.052 b |
49.5±1.3 b |
0.030±0.001 a |
10.5±0.2 d |
7.2±0.1 a |
|
P. calleryana Decne |
1.243±0.031 c |
44.1±0.9 c |
0.028±0.001 ab |
11.2±0.2 c |
6.4±0.1 b |
|
P. aromatica |
0.962±0.034 d |
34.5±1.0 d |
0.028±0.001 ab |
9.5±0.1 e |
6.1± 0.1 b |
|
P. betulifolia Bung |
0.459±0.017 e |
18.9±0.6 e |
0.025±0.001 b |
7.1±0.2 f |
4.4±0.1 c |
|
P. nivalis Jacq |
0.395±0.009 ef |
13.0±0.4 f |
0.031±0.001 a |
6.0±0.1 g |
3.2±0.1 e |
|
P.boissieriana Buhse |
0.300±0.021 f |
12.5±0.7 f |
0.026±0.003 b |
4.8±0.2 h |
3.6± 0.1 d |
|
P. korshinskyi Litv. |
0.294±0.015 f |
12.1±0.5 f |
0.025±0.002 b |
4.1±0.1 i |
3.7± 0.1 d |
Data represented by Mean ± SD (n=20).
zmean separation within columns by LSD test, P<0.05
As in chlorophyll concentration the stomata parameters were also characterized with ranging between species during vegetation period.
Among the species stomata area ranged depends on period of day and species. And in certain species it was not significantly differed during a day. So, the biggest stomata area regardless of investigation period identified in species P.bretshneideri, P.ussuriensis, P.pyrifolia, P.aromatica where values showed over-700 ^m2 (Tab. 2), and smallest one detected in P.betulifolia Bung over- 500 ^2. However, many species were sensitive to changing of daily temperature regardless of the water balance in soil and plant, and some species such as P.ussuriensis and P.betulifolia Bung was not significantly ranged. Whereas, morning at 08-00 AM species P.korshinskyi Litv, P.calleryana, P.bretshneideri and P.aromatica showed the biggest stomata area (Fig. 4), and conversely at 03-00 PM of day was detected reduction of stomata area, and in some case it was significantly in P.bretshneideri and P.aromatica.
Table 2. Characteristics of changing of pear leaf stomata during a day and its density Stomata parametersy
Name
08-00 AM
P.calleryana Decne
P.korshinskyi Litv.
Time of investigation
P.bretschneideri Rehd
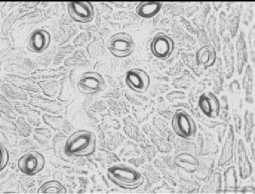
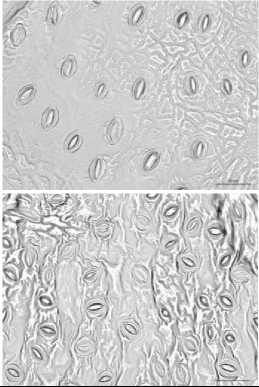
03-00 PM
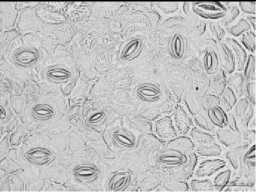
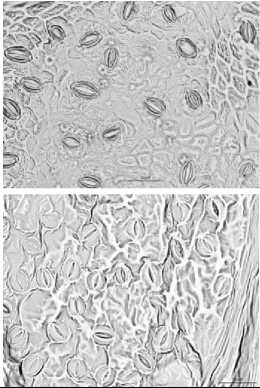
Fig. 4. Changing of the stomata in leaves of some wild species of pear during a day
In contrast, there was not found in leaves relation between reduction of stomata size and somatal porelength and width. In contrast, at morning time stomata in leaves of P.boissieriana Buhse, P.pyrifolia, P.nivalis Jacq which compacted and not opened well start to increase in size. Additionally, the density (number) of stomata per mm2 of unit and per leaf area was varied. In calculating of stomata per mm2 of leaf P.korshinskyi Litv showed a maximal- 370, whereas P.bretshneideri and P.ussuriensis had a minimal the density below- 170 pieces, respectively. However, in whole of the leaf P.calleryana, P.bretshneideri, P.pyrifolia, P.ussuriensis showed higher concentration of stomata in comparison to density per mm2 of unit leaf, where it was over 9000.0 thousand. Also, covered area of stomata in whole of the leaf was fluctuated and maximal data detected in P.korshinskyi Litv, which distinguished with high density per mm2 unit of leaf area, and respectively, stomata covered about 23.6% of the whole leaf and the lowest- 12.7% identified in P.aromatica.
DISCUSSION
Monitoring of the chlorophyll content showed that it is not steady and might be changed in different pear wild species during summer. However, there were revealed some species in which that rate was not significantly fluctuated. And, it means that plant has different response to environment on concentration of chlorophyll. So, according to our work the maximal high level of chlorophyll detected in wild species of P.aromatica in mid-August, but then rapidly decreased to late September, whereas P.korshinskyi Litv showed respectively low steady content during vegetation period. It should be noted that wild species P.korshinskyi Litv was originated under semi-arid condition in Central Asia, and, considered as drought tolerant species of pear (Likhonos et al . 1983; Ra-jametov et al . 2010), whereas P.aromatica originated under well humid East –Asian area (Rubtsov, 1944; Kajiura et al . 1983).
Comparison of total chlorophyll content between certain fresh weight (mg/g-1) and total leaf fresh weight according to leaf area showed significantly difference. So, P.nivalis Jacq which concentrated high chlorophyll in certain fresh weight of leaf showed low rates in comparison to total leaf fresh weight. Also, the same pattern showed P.betulifolia and P.boissieriana Buhse. In contrast, P.pyrifolia, P . bretshneideri and P . ussuriensis which have the greatest leaf area identified with high level of chlorophyll in total leaf fresh weight.
During vegetation as in chlorophyll concentra- tion between wild species stomata parameters were also characterized with ranging. Presumptive calculation showed that the biggest area of stomata had P.bretshneideri, P.ussuriensis, P.pyrifolia, P.aromatic, and the smallest P.betulifolia. However, it should be noted that morning time 08 AM when the plant well saturated with water the wild species P.korshinskyi, P. aromatica, P.calleryana and P.bretshneideri showed the biggest stomata area. Subsequently, afternoon at 03 PM they start to response to environment condition, where temperature and air humidity significantly increased and decreased, respectively, and contributed to reduction of stomata to reduce transpiration rate of leaves. But, there were identified species in which stomata area was not significantly ranged regardless of environment condition such as P.betulifolia and P.ussuriensis. According to Davies (1978) and Trejo et al. (1991, 1993), rise the temperature and increases of specific hormones in plants demonstrated a great role of ABA in the physiological process leading to stomatal closure, where usually activated dehydration-responsive genes. However, dehydration-responsive genes can be induced by the plant hormone ABA, but others are not (Shinozaki and Yamaguchi-Shinozaki, 1997).
Determination of density of stomata per leaf area showed different data. The highest of density stomata per certain leaf unit (mm2) and total area showed P . korshinskyi . As said above, P . korshinskyi originated under dry area, it is might be reason of the high density of stomata in leaf. In conclusion, concentration of chlorophyll and stomata in leaves of wild species is different and ranged during vegetation. Additionally, in wild species of pear should be continued more detail physiological and histological studies of leaves to give complex value of traits for breeding program.
ACKNOWLEDGMENT
This work was supported by fellowship funds of NIHHS, RDA, Republic of Korea.
Список литературы The seasonal changing of the chlorophyll content and stomata parameters in leaves of wild species of pear
- Alcantara E, Cordeiro AM, Barranco D. 2003. Selection of olive varieties for tolerance to iron chlorosis. J. Plant Physiol. 160: 1467-1472.
- Babani F, Lichtenthaler HK. 1996. Light-induced and age-dependent development of chloroplasts in etiolated barley leaves as visualized by determination of photosynthetic pigments, CO2 assimilation rates and different kinds of chlorophyll fluorescence ratios. J. Plant Physiol. 148:555-566
- Berry JA, Bjorkman O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Ann Rev Plant Physio 31:491-543.
- Blackmer TM, Shepers JS. 1995. Use of a chlorophyll meter to monitor nitrogen status and schedule fertigation for corn. J. Prod. Agr. 8:56-60.
- Byrne DH, Bacon T, Egilla JN. 1989. Developing peach rootstocks for calcareous soils. Compact Fruit Tree 22: 87-89.
- Coscun Y, Coskun A, Demirel U, Ozden M. 2011. Physiological response of maize (Zea mays L.) to high temperature stress. Australian J. Crop Sci. 5(8):966-972.
- Cruz ZN, Rodríguez P, Galindo A, Torrecillas E, Ondoño S, Mellisho CD, Torrecillas A. 2012. Leaf mechanisms for drought resistance in Zizyphus jujuba trees. Plant Sci. 197:77-83
- Davies WJ. 1978. Some effects of abscisic acid and water stress on stomata of Vicia faba L. J. Exp. Bot. 29 175-182
- Gashi B, Babani F, Kongjika E. 2013. Chlorophyll fluorescence imaging of photosynthetic activity and pigment contents of the resurrection plants Ramonda serbica and Ramonda nathaliae during dehydration and rehydration. Physiol. Mol. Biol. Plants. 19(3):333-341
- Ghasemi M, Kazem A, Yadollahi A. et al. 2011. Estimate of Leaf Chlorophyll and Nitrogen Content in Asian Pear (Pyrus serotina Rehd.) by CCM-200. Notulae Scientia Biologicae; Vol. 3 Issue 1, p91.
- Gomez J, Sanchez-Martinez D, Stiefel V, Rigau J, Puigdomenech P, Page`s M. 1988. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycinerich protein. Nature 334:262-264.
- Hartung W, Slovik S. 1991. Physicochemical properties of plant growth regulators and plant tissues determine their distribution and redistribution: stomatal regulation by abscisic acid in leaves. New. Phytol. 9: 361-382
- Kajiura I, Nakajima M, Sakai Y, and OOgaki C. 1983. A species-specific flavonoid from Pyrus ussuriensis Max. and Pyrus aromatic Nakai et Kikuchi, and its geographical distribution in Japan. Jpn. J. Breeding 33:1-14.
- Kräutler B, Mühlecker W, Anderl M, Gerlach B. 1997. Breakdown of chlorophyll: Partial synthesis of a putative intermediary catabolite -Preliminary communication. Helv. Chim. Acta. 80:1355-1362.
- Likhonos PD, Tuz AS, Lobachev AY. 1983. Cultural flora of USSR. XIV-Pome crops. Moscow-“Kolos” 193-223..
- Ma Ch, Tanabe K, Itai A, Tamura F, Chun JP, Teng Y. 2005. Tolerance to Lime-induced Iron Chlorosis of Asian Pear Rootstocks (Pyrus spp.). J. Japan. Soc. Hort. Sci. 74(6):419-423.
- Martin M,M, Larsen F,E, Higgins S,S, Ku M,S,B, and Andrews P,K. 1997. Comparative growth and physiology of selected one-year-old red-and green-fruited European pear cultivars. Scientia Hortic. 71:213-226.
- O’Mahony P, Burke JJ, Oliver MJ. 2000. Identification of acquired thermo tolerance deficiency within ditelosomic series of „Chineese Spring‟ wheat. Plant Physiol Bioch 38:243-252.
- Rajametov ShN, Baimetov KI, Khasanov KhM. 2010. Survey of pear species (Pyrus L.) and distribution in Uzbekistan. 2010. Biodiversity, conservation and use underutilized spices. Regional conference junior scientist -Uzbekistan-Samarkand, 10-12 October 2005. Bioversity International (Italy) 2010. PP 27-30
- Rodoni S, Schellenberg M, Matile P. 1998. Chlorophyll breakdown in senescing barley leaves as correlated with phaeophorbide a oxygenase activity. J. Plant Physiol. 152:139-144.
- Rodriguez JL, Davies WJ. 1982. The effects of temperature and ABA on stomata of Zea mays L. J. Exp Bot. 33: 977-987.
- Rotondi A, Predieri S. 2002. Leaf anatomy and photosynthesis of pear trees with different growth habit. Acta Hort. 596:745-748.
- Rubtsov GA. 1944. Geographical distribution of the genus Pyrus and trends and factors in its evolution. Amer. Naturalist 78:358-366.
- Rüdiger W, Grimm B. 2006. Chlorophylls and Bacteriochlorophylls, Advances in Photosynthesis and Respiration. In: Grimm B, Porra RJ, Rüdiger W, Scheer H, editors. Vol. 25. Springer; Dordrecht. pp. 133-146.
- Shinozaki K, Yamaguchi-Shinozaki K. 1997. Gene expression and signal transduction in water-stress response. Plant Physiol. 115: 327-334.
- Trejo CL, Davies J, Mar L, Ruiz P. 1993. Sensitivity of Stomata to Abscisic Acid An Effect of the Mesophyll. J. Plant Physiol. 102: 497-502
- Trejo CL, Davies WJ. 1991. Drought-induced closure of Phaseolus vulgaris L. stomata precedes leaf water deficit and any increase in xylem ABA concentration. J. Exp Bot. 42:1507-1555.
- Xu DH, Li JH, Fang XW, Wang G, Su PX. 2008. Photosynthetic activity of poikilochlorophyllous desiccation tolerant plant Reaumuria soongorica during dehydration and re-hydration. Photosynth. 46(4):547-551.
- Zhang J, Davies W. 1990a. Changes in the concentration of ABA in xylem sap as a function of changing soil water status can account for changes in leaf conductance and growth. Plant Cell Environ. 13: 277-285.


