The use of low-waste technologies of desalination of water in the reconstruction of the chemical plant
Автор: Vershinina T.A.
Журнал: Теория и практика современной науки @modern-j
Рубрика: Основной раздел
Статья в выпуске: 12 (30), 2017 года.
Бесплатный доступ
This article is the analysis of the application of water treatment by reverse osmosis. A comparison between chemical treatment and membrane method. The drawbacks of reagent and benefits of using chemical free.
Reverse osmosis, membrane module, water treatment, membrane desalination, wti
Короткий адрес: https://sciup.org/140270429
IDR: 140270429
Текст научной статьи The use of low-waste technologies of desalination of water in the reconstruction of the chemical plant
To receive softened water used in toxic and corrosive substances: technical brine (26% and 6-8%), hydrated lime (67%), iron sulfate (53%), caustic magnesite(83%), which creates the risk of poisoning of personnel and caustic burns.
The danger and toxicity of obtaining chemically purified water is caused by: - the possibility of allocation to production areas in emergency situations of harmful substances: technical brine (26% and 6-8%), hydrated lime (67%), iron sulfate (53%), caustic magnesia and toxic impact of their service personnel;
-
- the possibility of obtaining chemical and thermal burns when working with solutions of hydrated lime, iron sulphate, caustic magnesite, technical brine.
In the production of chemically treated water are dumped sludge waste. Wastewater of production of chemically treated water are divided into:
-
- regenerative, resulting in the regeneration of Na-cationite filters;
-
- wash formed by carrying out the flushing of mechanical filters;
-
- vent formed in the process of purging clarifiers.
Reverse osmosis and normal osmosis (dialysis) are directly related processes. In simple terms, if a selective membrane (i.e., a membrane freely permeable to water, but much less permeable to salt) separates a salt solution from pure water, water will pass through the membrane from the pure water side of the membrane into the side less concentrated in water (salt side) as shown in Figure 1.1. This process is called normal osmosis . If a hydrostatic pressure is applied to the salt side of the membrane, the flow of water can be retarded and, when the applied pressure is sufficient, the flow ceases.
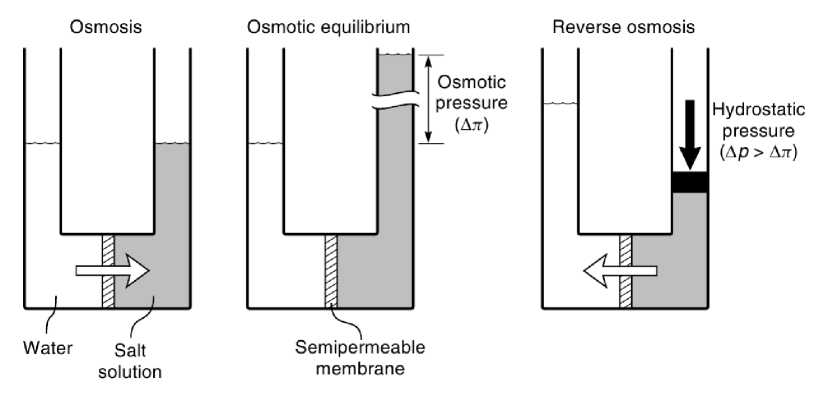
Figure 1.1 – A schematic illustration of the relationship between osmosis (dialysis), osmotic equilibrium and reverse osmosis
The hydrostatic pressure required to stop the water flow is called the osmotic pressure (я). If pressures greater than the osmotic pressure are applied to the salt side of the membrane, then the flow of water is reversed, and water begins to flow from the salt solution to the pure water side of the membrane. This process is called reverse osmosis, which is an important method of producing pure water from salt solutions [1].
Compared to other methods reverse osmosis has the following advantages:
-
1) there are no phase transitions in the separation of impurities, which allows to minimize the energy consumption for conducting the processes;
-
2) separation can be carried out at low water temperatures, which are determined by the properties of the membrane;
-
3) if to avoid clogging of the membrane processes are continuous in nature;
-
4) can be carried out without additives of chemical reagents (if additives are introduced in very small amounts);
-
5) apparatus for carrying out these processes are relatively simple and have no moving parts. The amount of energy needed for membrane processes do not usually exceed 2-2,5 kW·h/m3 of filtrate [2].
In connection with increasing importance of protecting waterbodies from the discharges of various contaminants, the problem of reducing runoff is one of the urgent. This indicates the need for reconstruction of the chemical plant and the transition to chemical free methods for desalination of water.
Список литературы The use of low-waste technologies of desalination of water in the reconstruction of the chemical plant
- Baker R.W. Membrane technology and application. Second edition.
- Стерман Л. С, Покровский В. Н. Физические и химические методы обработки воды на ТЭС: Учебник для вузов.- М.: Энергоатомиздат, 1991.- 328 с.


