Theoretical evaluation of phenyl-substituted aziridines, azirines and epoxides reactivity
Автор: Borodina O.S., Novikov A.S., Zyryanov G.V., Bartashevich E.V.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Физическая химия
Статья в выпуске: 4 т.15, 2023 года.
Бесплатный доступ
The reactivity of three-membered heterocycles such as phenyl-substituted aziridines, azirines, epoxides was studied in comparison with styrenes, azomethines and carbonyl compounds which are most often used in organic synthesis in nucleophilic and electrophilic reactions. In accordance with electronic reactivity indices of nucleophilicity and electrophilicity calculated on the base of DFT approach, the substituted molecules of azirines, aziridines, and epoxides exhibit the similar reactivity to aldehydes and styrenes. In all cases, our predictions were checked against the experimental observations given in literature. All considered compounds mainly characterizes by the properties of medium-strength electrophiles. Aziridines, azirines, epoxides with two aryl groups show the best electrophilic properties. This observation can be used in predictions of reactivity for these classes of compounds. Among three-membered heterocycles, the best nucleophilic properties were observed for substituted epoxy cycles. We have found that the dual descriptor based on Fukui functions cannot be recommended for predictions the reactivity of aziridines and epoxides. The values of the dual descriptor on both carbon atoms in three-membered ring significantly differ, while the experimental data on nucleophilic addition indicate that the reaction proceeds at both carbon atoms. Nevertheless, for phenyl-substituted azirines, the descriptor based on Fukui functions shows its effectiveness quite well.
Aziridines, azirines, epoxides, reactivity indices, fukui functions, dual descriptor, three-membered heterocycles, nucleophilic and electrophilic reactions
Короткий адрес: https://sciup.org/147242676
IDR: 147242676 | УДК: 544.163.2 | DOI: 10.14529/chem230406
Текст научной статьи Theoretical evaluation of phenyl-substituted aziridines, azirines and epoxides reactivity
Aziridines, azirines and epoxides can enter into the same reactions as aldehydes, imines, derivatives of substituted ethylenes, which are the most typical reagents and also products of the transformation of three-membered cycles. At the same time, they can exhibit different reactivity, which has been experimentally shown in reactions of addition, substitution at a double bond under the attack of electrophilic and nucleophilic reagents. Aziridines have been studied in nucleophilic addition reactions with regioselective ring opening by various nucleophiles [1]. Also they have been investigated in nucleophilic addition reactions with allyl zinc halide with comparison with epoxides [2], where allyl zinc halide presumably exhibits ambident nucleophilic properties. Aziridines have been studied in reactions with nitriles too [3]. Azirines have been studied in reactions with electrophiles with regioselective ring opening [4], as well as in the reaction of O -acylation of the H-C(sp3) bond of 3-aryl- 2H -azirines [5], where a hydrogen atom is removed by the acetoxy radical, which leads to the 2H -azirine radical formation.
The comparison of the reactivity of three-membered rings with carbonyl compounds gives that they mainly exhibit electrophilic properties. For example, carbonyl compounds were studied in the reaction of diamine addition and in the synthesis of azomethines, which were involved in the nitroaldol reaction. A rational method for the preparation of imidazo[1,2-a]pyridines can be obtained through the binucleo-philic addition of 2-aminopyridines to nitroalkenes [6]. The addition of various reagents to the double bond of substituted ethylenes has been studied in the following reactions. The reactivity of styrenes upon interaction with the NaIO4/hydroxylamine hydrochloride system was studied. As a result, β-iodohydrins, as well as their esters and acetates were synthesized using this system in the presence of O-nucleophiles (water, alcohol, acetic acid) [7]. The reactivity of other compounds containing C=C fragments activated by the electron accepting substituents was also studied. Thus, a three-component one-pot reaction of thioamination of 1,4-naphthoquinone by C-H functionalization in the presence of (diace-toxy)iodobenzene was developed [8]. The interaction of styrenes with benzoyl substituents with the NaIO4/hydroxylamine hydrochloride reagent system in solvent ethylene glycol leads to the formation of 2,3-disubstituted 1,4-dioxanes. Carrying out the reaction with other activated styrenes in the absence of ethylene glycol led in one step to the formation of diiodo-substituted derivatives. The advantages of these methods of synthesis using three-membered cycles is the availability of reagents and catalysts, as well as a high yield of target products, which allows them to be considered as potential methods of “green chemistry”.
Reactivity indices are used in studies of various compounds based on the theoretical foundations of the concept of electrophilicity and nucleophilicity of organic compounds and to predict their reactivity [9]. List of descriptors contains global reactivity indices such as ionisation potential (I = –E HOMO ), electron affinity (A = –E LUMO ), electronic chemical potential (µ ≈ –(E HOMO + E LUMO )/2), Mulliken electronegativity (χ = –µ). Structures can be characterized by pairs of opposite parameters, such as chemical hardness (η ≈ (E LUMO – E HOMO )/2) and softness (S = 1/η), Gázquez's electroaccepting and electrodonating power, electrophilicity index (ω = µ2/2η) and nucleophilicity indexes (N = E HOMO (Nucleophile) – E HOMO (tetracyanoethylene)), and Roy's nucleophilicity index (N' = 10/ω-). The maximum number of electrons that a compound can acquire (ΔN max = –µ/η) also can be good reactivity descriptor for electrophiles.
Studies of usage in reactions with different compounds classes are necessary for most of reactivity indices. On the example of studies carried out in [10], an application of the method was found to describe the selectivity of substitution at the sp3-hybridized carbon atom in the aromatic and aliphatic series. It was shown that the observed regularities correspond to the concept of Pearson [11] with the possibility of dividing them according to the principle of HSCA (hard and soft acids and bases). In accordance with the concept of reactivity descriptors based on quantum chemical calculations of the electronic structure, local softness and hardness were used to predict the sequences of reaction steps and evaluate the reactivity of carbonyl compounds with respect to nucleophilic attack [12]. The results showed that local hardness can be used as a meaningful parameter. On the other hand, relative electrophilicity and nucleophilicity provide truer trends in intramolecular reactivity describing. In difficult cases, for example, in systems having α,β-unsaturation or a phenyl group, the reaction centers involved in the delocalization of a positive charge on the carbonyl carbon can have comparable values of relative electrophilicity. This suggests that, despite the dominance of one of the electrophilic centers, one should not forget about the influence of the others.
Reactivity indices are also used to predict the behavior of large biomolecular systems [13]. To reliably determine regioselectivity in such systems, the method based on fragmentation was developed [14]. The descriptor used as a key tool in this model is the local hardness, since its dominant component is the electronic contribution to the molecular electrostatic potential, which is effective at relatively large distances from atoms. To solve the problem of calculation performance, the authors of [15] proposed to compare only those fragments for which the number of electrons is the same, for example, in DNA base pairs.
In a number of cases, the applicability of reactivity indices correlated with the orbital occupancy has been shown. For example, the evaluation of molecular descriptors describing the reactivity of 2-substituted nitrobenzenes in hydrogenation showed that the use of such parameters as electron affinity and the volume of substituents in the second position makes it possible to obtain models with good predictive properties [16]. Also, good used reactivity indices are the Fukui functions /(r) =
F dp(r) l
L dN ]„(r) ,
where p(r) is electron density, N is the number of electrons, and v (r) is the external potential (electronnucleus attraction potential plus any other potential applied to the system) [17]. They were used in paper [18] to predict the sites of electrophilic and nucleophilic attack in the series of molecules with a closed shell. In all cases, the atoms with the maximum value of the Fukui functions f- and f+ were defined as the preferred centers of electrophilic and nucleophilic addition respectively. The predictions made in this way agreed with experimental observations and did not depend on calculation methods.
As a key one, the dual descriptor Д/(г) = P^^ ] , which is the response of the electron density to the change in the number of electrons [19], was established in pericyclic reactions, when the reactants simultaneously accept and donate electrons. The dual descriptor proved to be useful for ambiphilic reagents when it was necessary to take into account both the orbital electron transfer effect and the electrostatic potential. The reactivity indices are compared with the rate constants. For example, it was found possible to quantitatively describe the reactions of protonation of radical anions and dianions of aromatic compounds using reactivity indices [20]. The experimental rate constants of the protonation reaction correlated with the descriptors, which were calculated using the values of electron affinity and ionization potential. Thus, prediction of trends in catalytic processes can provide an effective screening of high-performance catalysts using reactivity indices [21].
The aim of our work is to compare the reactivity of aziridines, azirines, epoxides, in comparison with aldehydes, azetidines and substituted styrenes in addition, substitution reactions at double bond under the action of electrophilic and nucleophilic reagents. We have used such reactivity indices as ionization potential, electron affinity, electronic chemical potential, electrophilicity index, nucleophilicity index and Roy's nucleophilicity index, as well as Fukui function and dual descriptor, that take into account the features of the electron density distribution, calculated on the basis of density functional theory methods.
Computational details
We calculated main global reactivity descriptors for optimized equilibrium model structures 1–15 based on conceptual DFT [22], such as the ionisation potential I, electron affinity A, electronic chemical potential µ, electrophilicity index ω, nucleophilicity index N and Roy's nucleophilicity index N'.
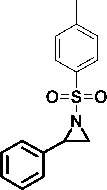
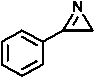
The reactivity indices were calculated for the studied compounds including 1-tosyl-2-phenylaziridine 1 , 2-phenylepoxide 2 and 3-benzoyl-2-phenylepoxide 3 , 2-phenylazirine 4 , 2-methyl-3-phenylazirine 5 and 2.3 -diphenylazirine 6 . Also, for comparison, the same descriptors were calculated for styrene 7 , nitrostyrene 8 , ethoxyacetylstyrene 9 and benzoylstyrene 10 , 2-(benzylideneamino)pyridine 11 and 2-(benzylideneamino)benzene 12 , benzaldehyde 13 , naphthoquinone 14 , 4-hydroxycoumarin 15 , tetracyanoethylene (TCE) as a reference compound in the calculation of nucleophilicity index.

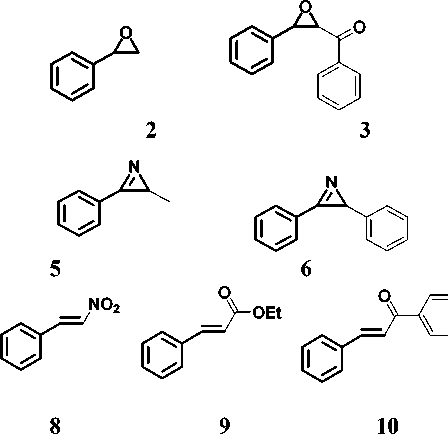
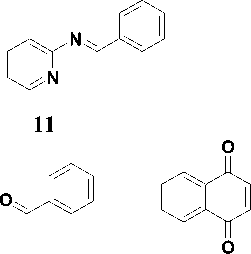
13 14
The electrophilicity ω scale allowed the classification of organic molecules as strong electrophiles with ω > 1.5 eV, moderate electrophiles with 0.8 < ω < 1.5 eV and marginal electrophiles with ω < 0.8 eV [23]. An analysis of a series of common nucleophilic species participating in polar organic reactions allowed a further classification of organic molecules as strong nucleophiles with N > 3.0 eV, moderate nucleophiles with 2.0 < N < 3.0 eV and marginal nucleophiles with N < 2.0 eV [24]. The quantum chemical calculations were carried out at the B3LYP/6-31G(d) level of theory in Gaussian-09 [25]. No symmetry restrictions were applied during the geometry optimization procedure. The Hessian matrices were calculated for all optimized model structures to prove the location of correct minima on the potential energy surface (no imaginary frequencies were found in all cases).
To identify the nucleophilic and electrophilic regions of the molecules that will be the first to be attacked by the reagents, the Fukui functions f – and the dual descriptor Δ f were calculated using the Mul-tiwfn software [26].
Results and Discussion
Azirines, aziridines, epoxides, and imines have similar reactivity to keto-compounds, which were included in the comparison sample. Let's analyze the calculated reactivity descriptors given in Table 1. According to the values of the electronic chemical potential µ among three-membered cycles, one can notice, that 2-benzoyl-3-phenylepoxide 3 is more reactive than 2-phenylepoxide 2 , and among azirines, on the contrary, 2-phenylazirine 4 is the most reactive. Keto-compounds of our sample naphthoquinone 14 and benzaldehyde 13 have the highest values of µ (Fig. 1a), but the electronic chemical potential of 4-hydroxycoumarin 15 is much lower. Ethylenes also show rather high values. Especially, nitrostyrene 8 is the most reactive in this indicator. Then there is a tendency to decrease when replacing a substituent by benzoyl and ester group. Styrene 7 is the least reactive.
In order to compare the reactivity of compounds 1–15 in reactions with electrophilic reagents, such as in reactions from [4], let's compare their nucleophilicity indices N [12] and Roy's nucleophilicity N' index [28] (Fig. 1b). According to the nucleophilicity scale [24], styrene 7, 2,3-diphenylazirine 6 and azomethines 11, 12 are strong nucleophiles. Naphthoquinone 14 is a weak nucleophile. A ziridines, azirines, epoxides are medium strength nucleophiles.
Table 1
Global reactivity descriptors for optimized equilibrium model structures 1–15, all values are given in eV
|
Structure |
E HOMO |
E LUMO |
I |
A |
µ |
ω |
N |
N' |
|
1 |
–6.554 |
–1.026 |
6.55 |
1.03 |
–3.79 |
2.60 |
2.57 |
2.57 |
|
2 |
–6.500 |
–0.202 |
6.50 |
0.20 |
–3.35 |
1.78 |
2.62 |
2.98 |
|
3 |
–6.498 |
–1.729 |
6.50 |
1.73 |
–4.11 |
3.55 |
2.62 |
2.26 |
|
4 |
–6.788 |
–1.449 |
6.79 |
1.45 |
–4.12 |
3.18 |
2.33 |
2.32 |
|
5 |
–6.716 |
–1.404 |
6.72 |
1.40 |
–4.06 |
3.10 |
2.40 |
2.36 |
|
6 |
–6.038 |
–1.537 |
6.04 |
1.54 |
–3.79 |
3.19 |
3.08 |
2.47 |
|
7 |
–6.031 |
–0.832 |
6.03 |
0.83 |
–3.43 |
2.26 |
3.09 |
2.86 |
|
8 |
–6.946 |
–2.632 |
6.95 |
2.63 |
–4.79 |
5.32 |
2.18 |
1.79 |
|
9 |
–6.339 |
–1.695 |
6.34 |
1.70 |
–4.02 |
3.48 |
2.78 |
2.31 |
|
10 |
–6.312 |
–2.094 |
6.31 |
2.09 |
–4.20 |
4.19 |
2.81 |
2.12 |
Table 1 (end)
|
Structure |
E HOMO |
E LUMO |
I |
A |
µ |
ω |
N |
N' |
|
11 |
–6.094 |
–1.775 |
6.09 |
1.77 |
–3.93 |
3.58 |
3.03 |
2.33 |
|
12 |
–5.903 |
–1.553 |
5.90 |
1.55 |
–3.73 |
3.20 |
3.22 |
2.50 |
|
13 |
–6.943 |
–1.712 |
6.94 |
1.71 |
–4.33 |
3.58 |
2.18 |
2.17 |
|
14 |
–7.174 |
–3.171 |
7.17 |
3.17 |
–5.17 |
6.68 |
1.95 |
1.56 |
|
15 |
–6.397 |
–1.590 |
6.40 |
1.59 |
–3.99 |
3.32 |
2.72 |
2.35 |
|
TCE |
–9.121 |
–4.959 |
9.12 |
4.96 |
–7.04 |
11.91 |
0.00 |
1.00 |
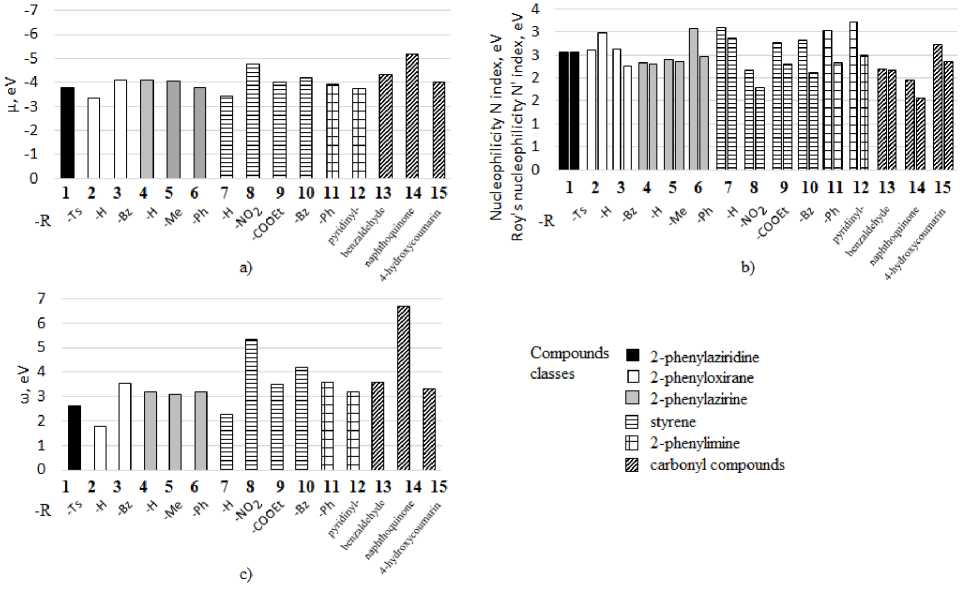
Fig. 1. Values of a) electronic chemical potential µ; b) nucleophilicity indexes; c) electrophilicity index in the series of studied structures
According to traditional estimates, for a high probability of electrophilic attack, the reaction centers must have high value of the Fukui function f –- . The most positive part of the function f – shown in Fig. 2 in compounds with three-membered cycles 1 – 6 is localized on heteroatoms, but in 2,3-diphenylazirine 6 it also is localized on the carbons of the azirine ring. At the same time, all heterocycles, except aziridine 1 , are characterized by different f – value on two carbon atoms of the cycles. It shows their inequivalence in reactions with electrophilic reagents. In compounds of other classes increased values of f – are observed on the atoms at double bond C=C, C=N and C=O because of these sites are favorable for electrophilic attack.
The dual descriptor shows a favorable center of electrophilic attack if Δf < 0. These sites (Fig. 3), in three-membered cycles are also located on heteroatoms and, in addition, on carbons in epoxedes add azirines. At the same time, in epoxide 2 unsaturated carbon is more nucleophilic, but in epoxide 3 carbon with more electron acceptor benzoyl group is more nucleophilic. The same is observed for azirines. Decreased values of the function is characterized only for carbon saturated by electron acceptor group in 2,3-diphenylazirine 6 . In structures with double bonds decreased values of Δf are localized at the C=C site, on the nitrogen atoms of C=N bond and carbonyl oxygen.
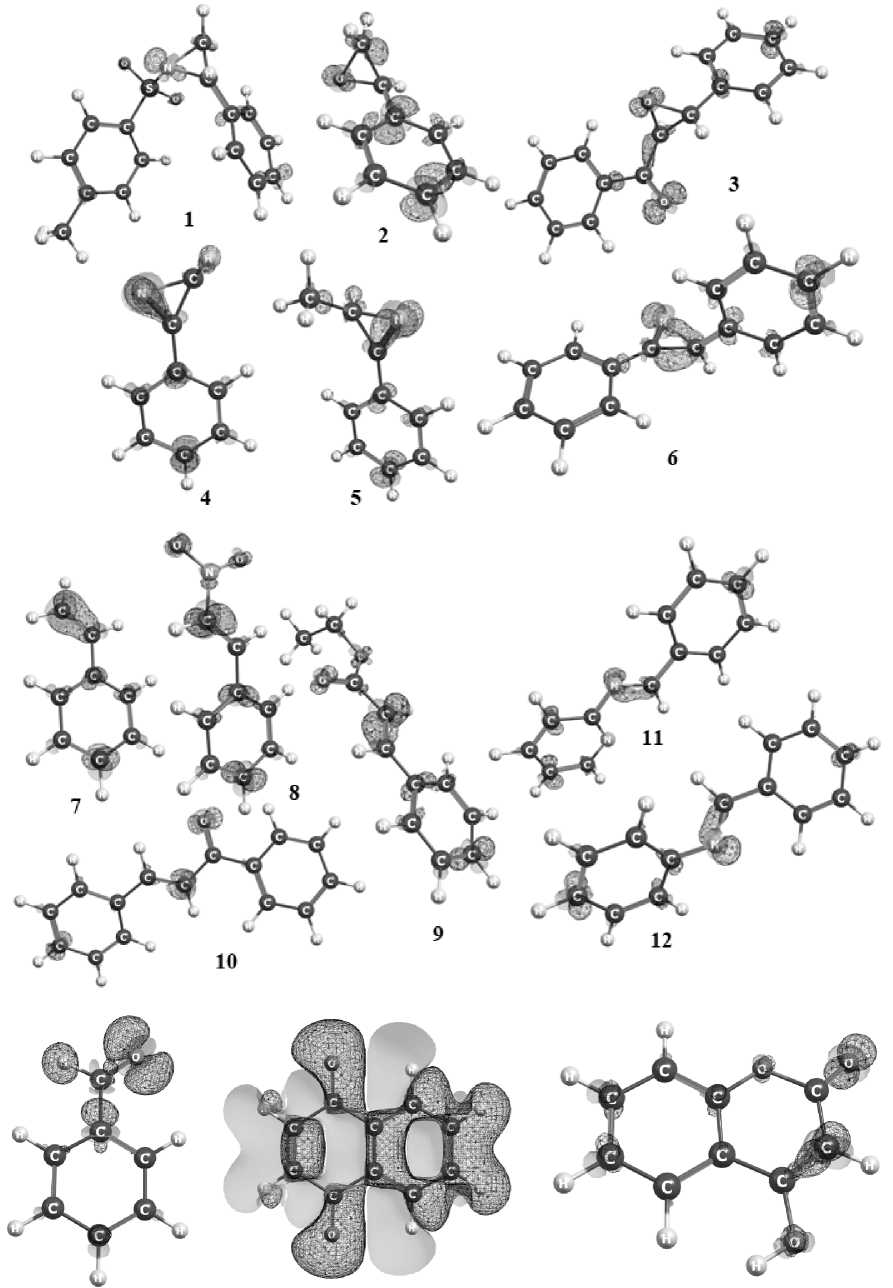
Fig. 2. Fukui function f – with a contour of 0.007 at.u. On the maps, dark lines and a light surface correspond to the positive and negative areas of the f – function, respectively
To compare the electrophilic reactivity of compounds 1–15 in addition reactions with nucleophilic reagents, as well as in aza-Henry and Michael reactions, it is necessary to pay attention to the electrophilicity index ω (Fig. 1c). For all studied compounds, this parameter is greater than 1.5. This means that, according to the electrophilicity scale [23], all compounds are enough strong electrophiles. At the same time, the values of the electrophilicity index for different classes of substances (azirines, epoxides, imines, alkenes, carbonyl compounds) lie approximately in the same range. However, within each class studied, the values vary greatly depending on the substituents. Thus, among carbonyl compounds, naphthoquinone 14 is the best electrophile; among ethylenes, nitrostyrene 8 and benzoylstyrene 10 are the best, among three-membered cycles, 3-benzoyl-2-phenylepoxide 3 is the best. The tendency of this descriptor change is mostly like evaluations of global reactivity calculated by electron chemical potential µ (Fig. 1a). This suggests that all the studied compounds mainly exhibit the properties of electrophiles.
The dual descriptor Δ f > 0 usually indicates a favorable site for nucleophilic attack. These sites shown in Fig. 3, among three-membered cycles inside ring are typical only for azirines and located on carbon atom at С=N bond. Also, they are bright in naphthoquinone 14 on the carbonyl carbon atoms of the benzaquinone ring, in structures 8–10 they are located on carbon with the phenyl substituent at C=C bond.
In reactions where hydrogen atom abstraction occurs [5], compounds with low bond polarity and low electron affinity will have the best reactivity. According to the relevant descriptor A (Table 1), such among those studied are epoxides, aziridine and azirines.
In reactions of addition, the nucleophile or electrophile attacks one atom at the double bond. Comparison of the experimental data for each compound with the values of Δ f (Table 2) shows that in most cases Δ f reproduces attack centers with nucleophilic and electrophilic reagents, except for 2-phenyl-N-tosylazirine 1 and 2-phenylepoxide 2 . Also, for compound 10 , the reaction with formation first of a three-membered ring, and then with a nucleophilic attack [7] occurs on each carbon atom. However, the calculated Δ f data show that these carbon atoms differ significantly in their electro- and nucleophilicity.
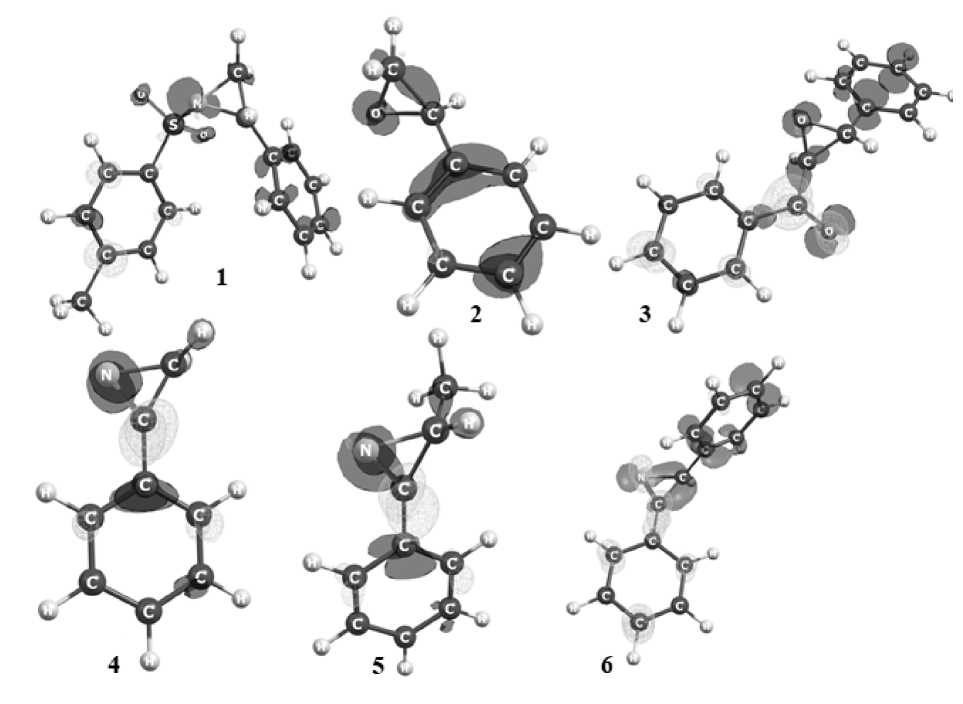
Fig. 3. Dual descriptor Δf with contour 0.007 a.u. On the maps, light and dark surfaces correspond to the positive and negative regions of the Δf function, respectively (see also p. 156)
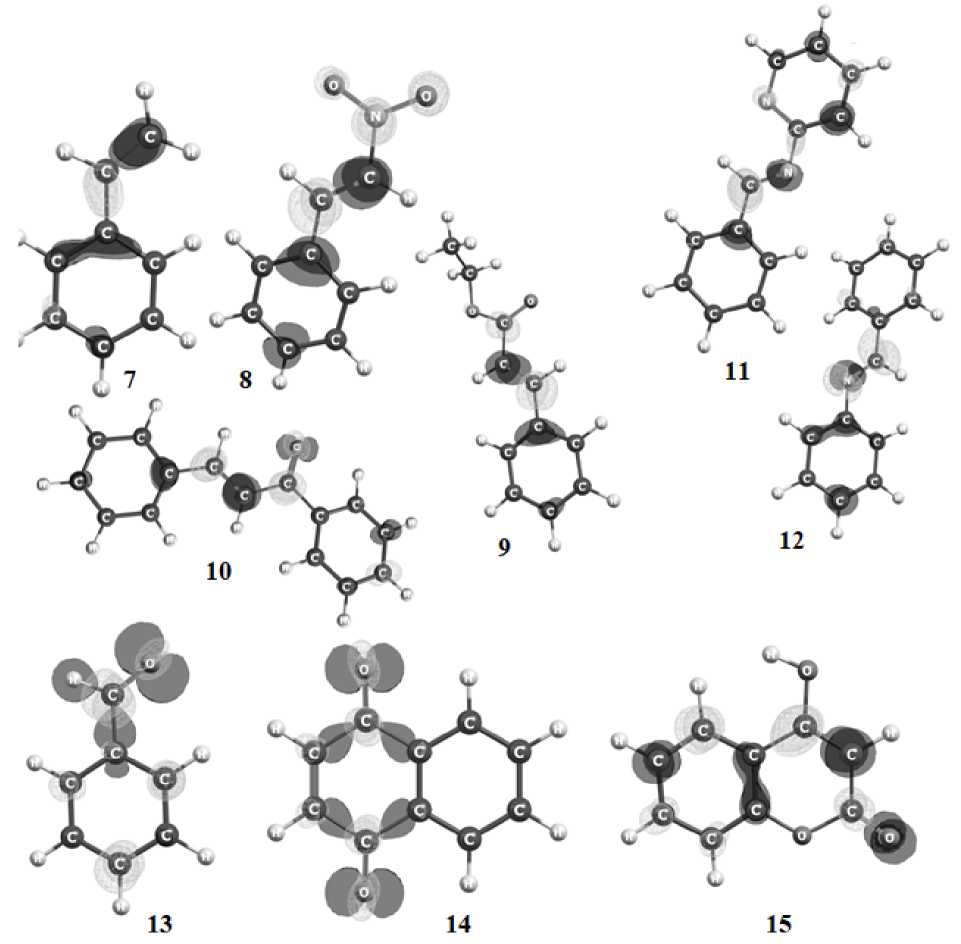
Fig. 3. End
Table 2 Comparison of calculated and experimental data. Gray indicates where the double descriptor does not reproduce or does not fully reproduce
|
Structure |
AN reaction |
Reaction electrophilic center on attack by Nu– (exp.) |
Center with more positive value of Δ f |
|
1 |
[1], [2], [3] |
N(Ts)- C (Ar) |
N (Ts)-C(Ar); both atoms are prone to nucleophilic attack |
|
2 |
[2] |
O- C (R) |
O - C (R); both atoms are prone to nucleophilic attack |
|
7 |
[7] |
C (Ar)=C |
C (Ar)=C |
|
8 |
[7] |
С (Ar)-С(=O) |
С (Ph)-С(=O) |
|
[7] |
C =C(NO 2 ) |
C =C(NO 2 ) |
|
|
9 |
[7] |
С (Ar)=С-С(=O) |
С (Ph)=С-С(=O) |
|
10 |
[7] |
C (Ar)=C-C(=O) |
C (Ph)=C-C(=O) |
|
[7] |
C (Ar)- C -C(=O) of intermediate |
C (Ar)=C-C(=O) |
|
|
[7] |
С (Ar)-С(=O) |
Table 2 (end)
|
Structure |
A N reaction |
Reaction electrophilic center on attack by Nu– (exp.) |
Center with more positive value of Δ f |
|
|
11 |
[6] |
N= C |
N= C |
|
|
13 |
[6] |
C =O |
C =O |
|
|
14 |
[8] |
C = C |
C = C |
|
|
Structure |
A E reaction |
Reaction nucleophilic center on attack by E (exp.) |
Center with more positive value of f - |
Center with more negative value of Δ f |
|
4 |
[4] |
N =C(Ar) |
N =C(Ar) |
N =C(Ar) |
|
5 |
[4] |
N =C(Ar) |
N =C(Ar) |
N =C(Ar) |
|
6 |
[4] |
N =C(Ar) |
N =C(Ar) |
N =C(Ar) |
|
10 |
[7] |
C (Ar)= C -C(=O) with triatomic cycle formation |
C(Ar)= C- C(=O) |
C(Ar)= C- C(=O) |
Conclusion
The reactivity of the studied molecules of azirines, along with substituted styrenes, azomethines and carbonyl compounds, can be described using such descriptors as the nucleophilicity index, electrophilicity index, electronic chemical potential, and the Fukui functions.
Azirines, aziridines, and epoxides show similar reactivity to aldehydes and styrenes in reactions with electrophiles and nucleophiles according nucleophilicity index and electrophilicity index. This observation can be used in reactivity predictions for these classes of compounds. Epoxides have the best nucleophilic properties in list of three-membered heterocycles according nucleophilicity index and Fukui functions. The considered compounds mainly exhibit the properties of electrophiles. Structures with aryl substituents have the best electrophilic properties in all classes of the considered compounds.
The dual descriptor of the Fukui function cannot be used in describing the reactivity of aziridines and epoxides, since the difference in the value of the function on both atoms at the considered C–C bond in the triatomic cycle gives the second kind error: carbon atoms carry a significantly different value of the descriptor, while the experimental data on nucleophilic addition indicate that the reaction proceeds at both atoms.
Список литературы Theoretical evaluation of phenyl-substituted aziridines, azirines and epoxides reactivity
- Chatterjee R., Santra S., Chakraborty Ghosal N. et al. // ChemistrySelect. 2020. V. 5, no. 15. P. 4525–4529. DOI: 10.1002/slct.202000853.
- Chatterjee R., Samanta S., Mukherjee A. et al. // Tetrahedron Letters. 2019. V. 60, no. 3. P. 276–283. DOI: 10.1016/j.tetlet.2018.12.027.
- De A., Santra S., Kovalev I.S. et al. // Mendeleev Commun. 2020. V. 30, no. 2. P. 188–189. DOI: 10.1016/j.mencom.2020.03.019.
- De A., Santra S., Zyryanov G.V. et al. // Org. Lett. 2020. V. 22, no. 10. P. 3926–3930. DOI: 10.1021/acs.orglett.0c01206.
- De A., Santra S., Hajra A. et al. // J. Org. Chem. 2019. V. 84, no. 18. P. 11735–11740. DOI: 10.1021/acs.joc.9b01625.
- Zyryanov G.V., Kopchuk D.S., Kovalev I.S. et al. // Mendeleev Commun. 2020. V. 30, No. 5.
- P. 537–554. DOI: 10.1016/j.mencom.2020.09.001.
- Chakraborty N., Santra S., Kundu S.K. et al. // RSC advances. 2015. V. 5, no. 70. P. 56780–56788. DOI: 10.1039/C5RA11092K.
- Pal S., Chatterjee R., Santra S. et al. // Adv. Synth. Catal. 2021. V. 363, no. 23. P. 5300–5309. DOI: 10.1002/adsc.202100796.
- Zevatskiy Y. New calculation method physical and chemical properties of organic compounds. Saint-Petersburg: Saint-Petersburg State Institute of Technology (Technical University), 2020. 59 p. DOI: 10.2139/ssrn.4453611.
- Krylov E.N., Zubanova E.A., Ivanova Yu.M. // Ivanovo State University bulletin. Series Natural, social sciences. 2012. No. 2. P. 58–67. (In Russ.). EDN: PBJGAN.
- Chattaraj P.K. (Ed.) Chemical reactivity theory: A Density Functional View (1st ed.). Boka Raton: CRC Press., 2009. 576 p. DOI: 10.1201/9781420065442.
- Roy R.K., Krishnamurti S., Geerlings P. et al. // J. Phys. Chem. A. 1998. V. 102, No. 21. P. 3746–3755. DOI: 10.1021/jp973450v.
- Roy R.K., Saha S. // Annu. Rep. Prog. Chem., Sect. C: Phys. Chem. 2010. V. 106, No. 118. DOI:10.1039/b811052m.
- Saha S., Roy R. // Phys. Chem. Chem. Phys. 2008. V. 10. P. 5591. DOI: 10.1039/B802966K.
- Volkova T.G., Sterlikova I.O., Klyuev M.V. // Russ. J. Gen. Chem. 2011. V. 81, No. 10. P. 2121–2124. DOI: 10.1134/S1070363211100148.
- Parr R.G., Yang W. // J. Am. Chem. Soc. 1984. V. 106. P. 4049–4050. DOI: 10.1021/ja00326a036.
- Oláh J., Van Alsenoy C., Sannigrahi A.B. // J. Phys. Chem. A. 2002. V. 106, No. 15. P. 3885–3890. DOI: 10.1021/jp014039h.
- Cárdenas C., Rabi N., Ayers P.W. et al. // J. Phys. Chem. A. 2009. V. 113, No. 30. P. 8660–8667. DOI: 10.1021/jp902792n.
- Mendkovich A.S., Syroeshkin M.A., Mikhailov M.N. et al. // Russ. Chem. Bull. 2010. V. 59, No. 11. P. 2068–2071. DOI: 10.1007/s11172-010-0356-0.
- Zhao Z.J., Liu S., Zha S. et al. // Nat. Rev. Mater. 2019. V. 4, No. 12. P. 792–804. DOI: 10.1038/s41578-019-0152-x.
- Domingo L.R., Ríos-Gutiérrez M., Pérez P. // Molecules. 2016. V. 21, No. 6. P. 748. DOI: 10.3390/molecules21060748.
- Domingo L.R., Aurell M.J., Pérez P. et al. // Tetrahedron. 2002. V. 58, No. 22. P. 4417–4423. DOI: 10.1016/S0040-4020(02)00410-6.
- Jaramillo P., Domingo L.R., Chamorro E. et al. // J. Mol. Struct.: THEOCHEM. 2008. V. 865, No. 1–3. P. 68–72. DOI: 10.1016/j.theochem.2008.06.022.
- Frisch M.J., Trucks G.W., Schlegel H.B. et al. // Gaussian 09, Revision D. 01, Gaussian, Inc., Wallingford CT. 2009. URL.: http://www. gaussian. com (date of access: 01.09.2023).
- Lu T., Chen F. // J. Comput. Chem. 2012. V. 33, No. 5. P. 580–592. DOI: 10.1002/jcc.22885.
- Contreras R., Andrés J., Safont V.S. et al. // J. Phys. Chem. A. 2003. V. 107, No. 29. P. 5588–5593. DOI: 10.1021/jp0302865.


