Thermal properties of dipicolinic acid and dipicolinic acid sodium salt NaH(COO)2C5H3N·H2(COO)2C5H3N·3H2O
Автор: Rajakumar K., Sharutin V.V., Zherebtsov D.A., Osipov A.A., Lutsenko A.I.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Физическая химия
Статья в выпуске: 2 т.15, 2023 года.
Бесплатный доступ
Pyridine-2,6-dicarboxylic acid (dipicolinic acid) are actively studied by scientists. Dicarboxylic acid can be used as a special biomarker of anthrax. The crystal structure of NaH(COO)2C5H3N·H2(COO)2C5H3N·3H2O (1) was redetermined from single crystal. In the structure of the sodium salt of dipicolinic acid, sodium is the central atom having two molecules of dipicolinic acid and three molecules of water as ligands. The dipicolinic anion coordinate sodium cation by both oxygens and one nitrogen atom. A network of hydrogen bonds connects water and dipicolinic acid molecules. Hydrogen bonds take part in the formation of building blocks and arranging them into chains which, in their turn, make up layers. The sodium salt of dipicolinic acid as well as its precursor - dipicolinic acid were studied by IR spectroscopy and simultaneous thermal analysis. According to thermal analysis dipicolinic acid is anhydrous substance, that decompose on melting at 252 °C forming only gaseous products, presumably CO2 and pyridine. Opposite to that, thermolysis of its sodium salt follow much more complicated way. According to thermogram the 1 has first stage of three water molecules removal at 105 °C. The measured mass loss is very close to expected mass loss. Heat of dehydratation reaction is 151 kJ/mole. On a further heating the sodium salt of dipicolinic acid was melted at 256 °C with large mass loss. The nature of products at 268 and 315 °C is hard to state. EDX elemental analysis of black residue after 700 °C state brutto-formula of “NaNC5O2” - Na cyanate or carbonate in glassy carbon matrix
Dipicolinic acid, carboxylates, thermolysis, edx
Короткий адрес: https://sciup.org/147240922
IDR: 147240922 | УДК: 547-32 | DOI: 10.14529/chem230212
Текст научной статьи Thermal properties of dipicolinic acid and dipicolinic acid sodium salt NaH(COO)2C5H3N·H2(COO)2C5H3N·3H2O
Thermal decomposition of organic substances in inert atmosphere may open a way to new forms of carbon or carbon composites [1–4]. Key method for that is simultaneous thermal analysis, suitable to detect both mass change and thermal effects on heating.
Dipicolinic acid (pyridine-2,6-dicarboxylic acid or PDC and DPA) and its salts are actively studied by scientists in various fields of chemistry [5–7]. This acid constitutes approximately 10 % of Bacillus subtilis spore dry weight and has been shown to play a significant role in the survival of B. subtilis spores exposed to wet heat and to 254 nm UV radiation in the laboratory [8–9]. 2,6-dipicolinic acid is the unique component of anthrax spores and it can be used as a special biomarker of anthrax [10].
It have been reported thermal analysis results of cobalt (II), zirconium (IV), calcium (II) complexes with dipicolinic acid and imidazole derivatives [11]. Beyond those thermal analyses of metal-organic frameworks of Mn (II) and Zn (II) with dipicolinic acid [12], as well as polymers based on dipicolinic acid and 3,4-diaminopyridine [13] were investigated.
Aqueous solutions of the calcium and sodium salts of dipicolinic acid (DPA) were shown to have weak fluorescence when excited at wavelengths near 300 nm [14].
Experimental sectionSynthesis of the dipicolinic acid sodium salt NaH(COO)2C5H3N·H2(COO)2C5H3N·3H2O
The dipicolinic acid sodium salt NaH(COO)2C5H3N · H2(COO)2C5H3N · 3H2O 1 was prepared by neutralisation of hot concentrated dipicolinic acid water solution with 15 mass % NaOH and natural cooling. Product was collected as large clear crystals examined by single crystal X-Ray diffractometer.
Single crystal X-ray diffraction studies were carried out with BRUKER D8 Quest diffractometer (Mo-Kα fine-focus sealed tube). Refinement of unit cell parameters, and absorption correction were performed using SMART and SAINT-Plus programs [15]. All structure calculations and refinement were carried out using the SHELXL/PC [16] and Olex2 [17] programs.
Crystal data and measurement parameters are presented in Table 1. Full tables of atomic coordinates, bond lengths and bond angles have been deposited with the Cambridge Crystallography Data Center (No. 2061501; ; . Structure could be described as complexes of Na as central atom with two dipicolinic acid ligands and three water ligands building up a capped octahedron around sodium cation (Fig. 1).
Table 1
Crystal data and structure refinement for 1
|
Empirical formula |
C 14 H 15 N 2 O 11 Na |
|
Formula weight |
410.27 |
|
Temperature / K |
293.15 |
|
Crystal system |
triclinic |
|
Space group |
P– 1 |
|
a / Å |
6.937(6) |
|
b / Å |
11.154(7) |
|
c / Å |
11.228(8) |
|
α / ° |
85.48(3) |
|
β / ° |
82.01(4) |
|
γ / ° |
87.06(3) |
|
Volume / Å3 |
856.9(11) |
|
Z |
2 |
|
ρ calc / g/cm3 |
1.590 |
|
μ / mm–1 |
0.159 |
|
F (000) |
424.0 |
|
Crystal size / mm3 |
0.38 × 0.21 × 0.14 |
|
Radiation |
MoKα ( λ = 0.71073) |
|
2Θ range for data collection / ° |
5.94 to 71.52 |
|
Index ranges |
–11 ≤ h ≤ 11, –18 ≤ k ≤ 18, –18 ≤ l ≤ 18 |
|
Reflections collected |
45446 |
|
Independent reflections |
7893 [ R int = 0.0487, R sigma = 0.0447] |
|
Data/restraints/parameters |
7893/0/262 |
|
Goodness-of-fit on F 2 |
1.025 |
|
Final R indexes [ I >2 σ ( I )] |
R 1 = 0.0605, wR 2 = 0.1400 |
|
Final R indexes [all data] |
R 1 = 0.1136, wR 2 = 0.1627 |
|
Largest diff. peak / hole / e Å–3 |
0.46/–0.49 |
The IR spectra of compound 1 and 2 (dipicolinic acid) were recorded on a Shimadzu IRAffinity-1S IR spectrometer in KBr tablets in the region of 4000–400 cm–1 (Table 2).
Table 2
IR absorption bands of 1 and 2
|
Vibration, cm–1 |
1 |
2 |
|
O–H (ν) carboxylic |
3545, 3482 |
3549, 3482, 3414 |
|
H–O–H (ν) |
3364 |
– |
|
C–H (ν) |
3094 |
3105, 3069 |
|
C=O (ν) |
1728, 1703 |
1700 |
The thermal analysis was performed using Netzsch STA 449F1 Jupiter .
The powder X-ray diffraction (PXRD) were performed on a diffractometer Rigaku Ultima IV in the angular range from 10 to 80° 2 θ using filtered CuKα radiation.
The analysis of the elemental composition and morphology was investigated using the Jeol JSM-7001F, EDS Oxford INCA X-max 80.
Results and discussion
The structure of 1 was reported several times earlier [18–20], still it should be briefly described here. One molecule of dipicolinic acid in a structure is single time substituted, forming anion, while second molecule of dipicolinic acid presented as nondissociated molecule (Fig. 1). The dipicolinic anion coordinate sodium cation by both oxygens and one nitrogen atom. Nondissociated dipicolinic acid molecule coordinate cation with single carbonile oxygen. The carboxylic groups and water molecules are involved into hydrogen bond network. First, hydrogen bonds arranges building blocks into chains, next, these chains form layers (Fig. 1a). Additional hydrogen bonds connect layers in bulk (Fig. 1b).
b)
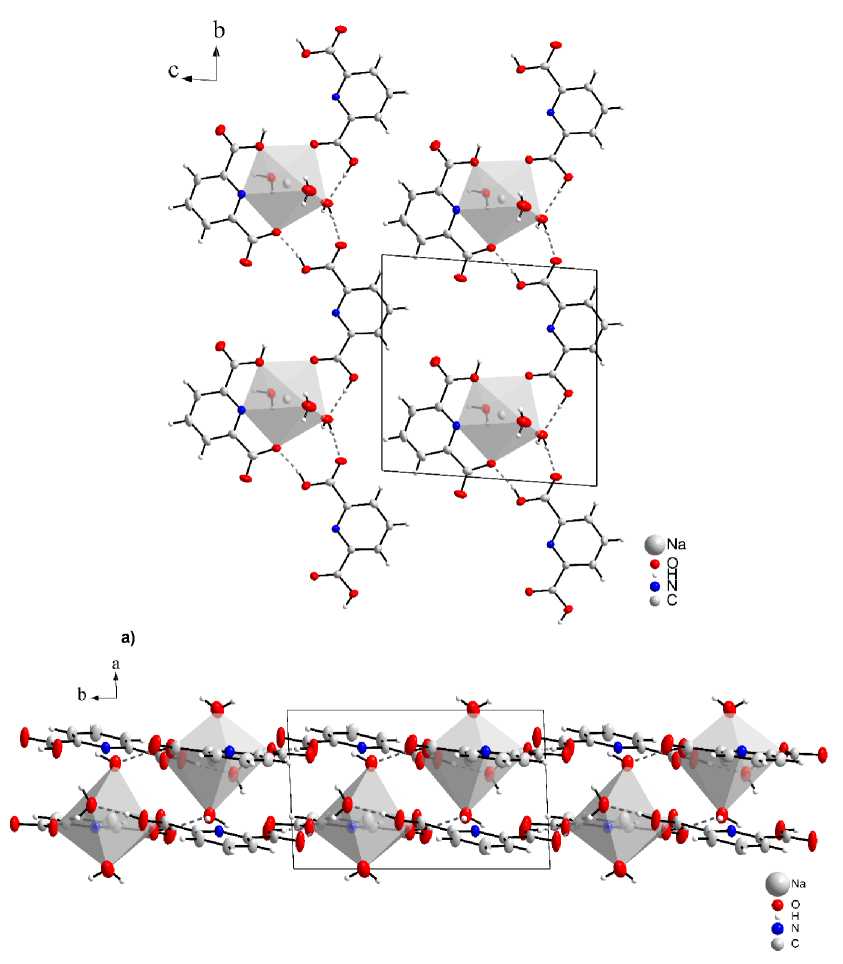
Fig. 1. Structure of NaH(COO) 2 C 5 H 3 N · H 2 (COO) 2 C 5 H 3 N · 3H 2 O. Dash lines depict hydrogen bonds:
a – the influence of hydrogen bonds on the structure of the compound (1); b – layered arrangement of molecules
The compound 1 and the dipicolinic acid 2 were examined by IR spectroscopy (Fig. 2). Due to same main molecule in both crystals, the absorption spectra of 1 and 2 have many similar bands (Fig. 2, Table 2).
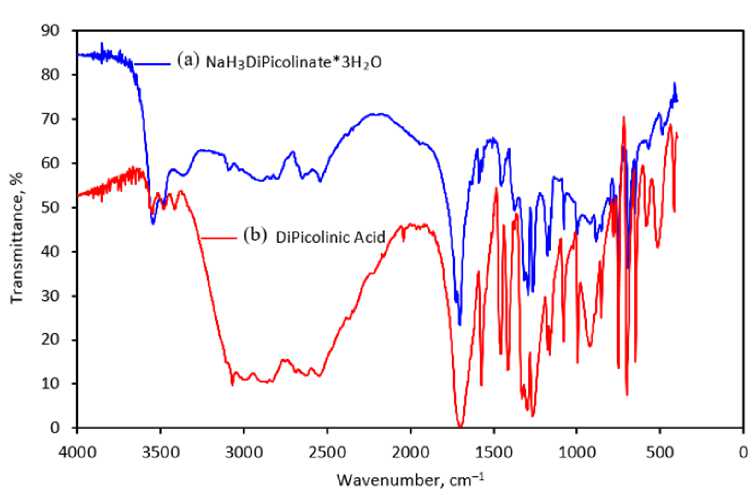
Fig. 2. IR spectra of 1 (a) and 2 (b) compounds
The 1 and a parent compound 2 were subjected to thermal analysis using Netzsch STA 449F1 Jupiter to discover further similarity. To avoid oxidation reactions, the thermal analysis was done in argon atmosphere. The thermogram of 2 is much simpler than that of 1 (Fig. 3, 4).
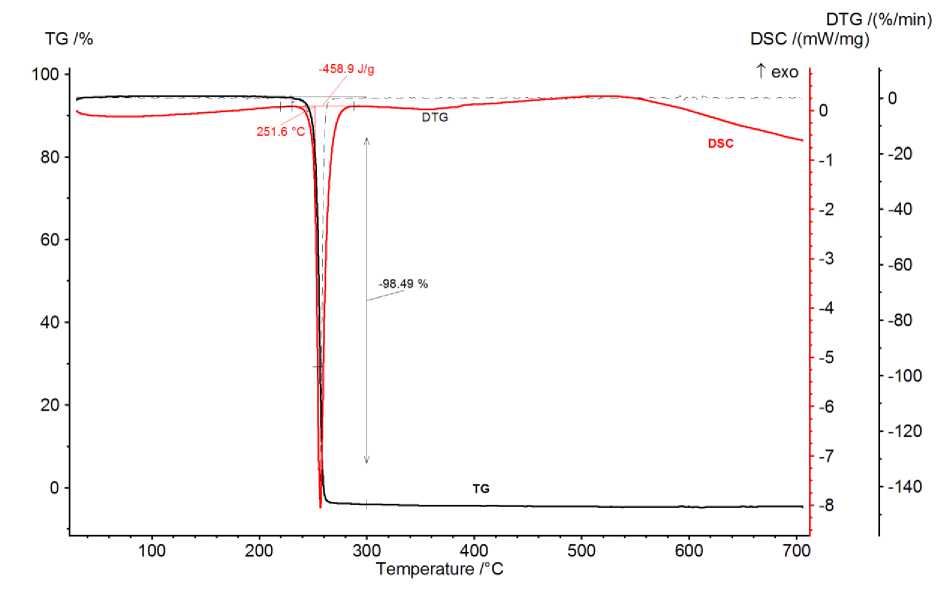
Fig. 3. Thermogram of dipicolinic acid (2) in Ar
On melting of dipicolinic acid at 252 °C it is immediately occurring its complete decomposition by reaction:
H 2 (COO) 2 C 5 H 3 N = C 5 H 5 N + 2CO 2
Decarboxylation of carboxylic acids on heating is well known for many of them like benzoic [21], anthranilic [22], pyridinecarboxylic [23] or maleic acid [24], thus forming benzene, aniline, pyridine and acetylene correspondingly. Overall heat measured from the DSC peak area of dipicolinic acid including heat of melting, enthalpy of decomposition and heat of pyridine vaporisation ΔH(DSC) = ΔH(melting) + ΔH(decomposition) + ΔH(vaporisation) is 459 J/g or 76.7 kJ/mole. Note that pyridine vaporisation takes 35.1 kJ/mole [25], so the enthalpy of decomposition reaction ΔH(decomposition) (forming liquid pyridine) is less than 41.6 kJ/mole. The uncertainties are ±1 °C on temperature and ±5 % on enthalpy.
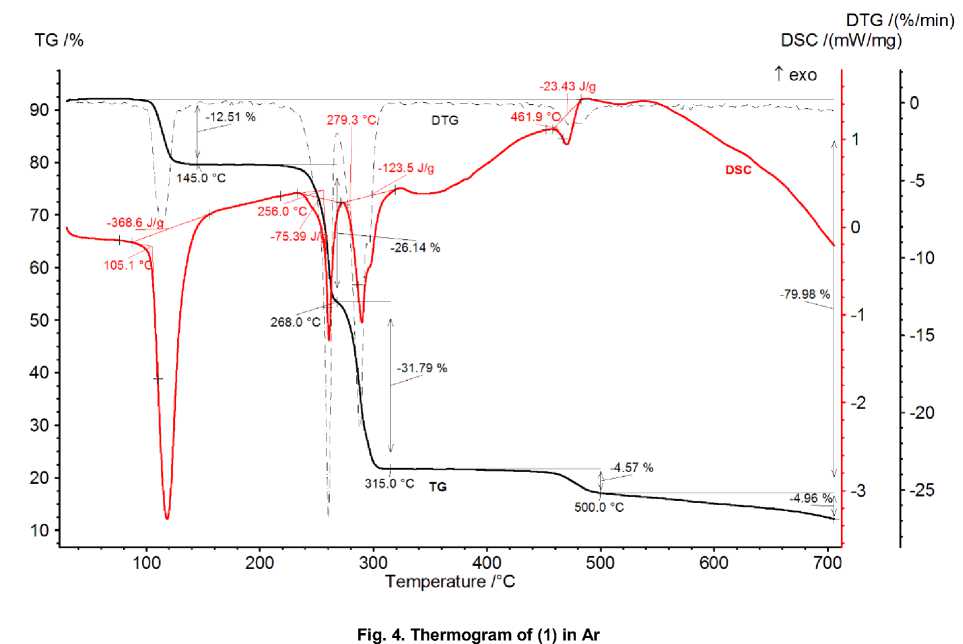
According to thermogram (Fig. 4) the 1 has first stage of three water molecules removal at 105 °C. The measured mass loss of 12.51 mass % is very close to expected 11.70 mass % by equation:
NaH(COO) 2 C 5 H 3 N · H 2 (COO) 2 C 5 H 3 N · 3H 2 O = NaH(COO) 2 C 5 H 3 N · H 2 (COO) 2 C 5 H 3 N + 3H 2 O
Heat of dehydratation reaction is 369 J/g or 151 kJ/mole. That is close to 122 kJ/mole needed for evaporation of three moles of water [25].
On a further heating the Na salt was melted at 256 °C with large mass loss, similar to decomposition at 252 °C of pure dipicolinic acid. From the first glance that stage could be attributed to removal from crystal and decomposition of nondissociated dipicolinic acid. But the measured mass loss of 26.14 mass % is much smaller than expected 40.73 mass % according to reaction
NaH(COO) 2 C 5 H 3 N · H 2 (COO) 2 C 5 H 3 N = NaH(COO) 2 C 5 H 3 N + C 5 H 5 N + 2CO 2
Hence, the nature of products at 268 and 315 °C is hard to state. Moreover, next stage at 462 °C with mass loss of 4.57 mass % could be attributed to decomposition of certain organic salt of sodium, like oxalate or cyanamide. The nitrogen containing salt is more preferable since EDX elemental analysis of black residue after 700 °C state brutto-formula of “NaNC5O2”, where carbon present both in Na-containing particles (probably, carbonate or cyanate NaCNO) in glassy carbon matrix. Powder X-Ray diffraction of product does not fit to any known Na salt. Total loss of 79.98 mass % can be attributed to expected loss of 78.31 mass % for scheme:
NaH(COO) 2 C 5 H 3 N*H 2 (COO) 2 C 5 H 3 N*3H 2 O = NaCNO + 2C + volatiles.
Conclutions
In this paper, the thermal properties of dipicolinic acid 1 and dipicolinic acid sodium salt NaH(COO) 2 C 5 H 3 N · H 2 (COO) 2 C 5 H 3 N · 3H 2 O 2 were investigated. It was shown that the thermogram of 2 is much simpler than that of 1 . The melting points of compounds 1 and 2 are equal to 256 °C and 252 °C. A scheme describing the decomposition of compounds 1 and 2 when heated to 700 °C in an argon atmosphere was shown.
Acknowledgments
Elemental, IR and thermal analyzes and diffraction studies were carried out at the scientific and educational center “Nanotechnology” SUSU.
Список литературы Thermal properties of dipicolinic acid and dipicolinic acid sodium salt NaH(COO)2C5H3N·H2(COO)2C5H3N·3H2O
- Jordan R.S., Li Y.L., Lin C.-W., McCurdy R.D. Synthesis of N = 8 Armchair Graphene Nanorib-bons from Four Distinct Polydiacetylenes. J. Am. Chem. Soc. 2017;139(44):15878-15890. DOI: 10.1021/jacs.7b08800.
- Hou Y.-L. Li M.-Q, Cheng S., Diao Y. Dramatic Improvement of Stability by in situ Linker Cyc-lization of a Metal-Organic Framework. Chem. Commun. 2018;54(68):9470-9473. DOI: 10.1021/jacs.7b08800.
- Zherebtsov D.A., Nayfert S.A., Polozov M.A., Morozov R.S. Features of Thermolysis of Aromatic Compounds. Russ. J. Phys. Chem. 2021;95(12):2460-2470. DOI: 10.1134/S0036024421120232.
- Tolstoguzov D.S., Zherebtsov D.A., Gruba O.N., Avdin V.V. Synthesis of Co, Ni, Cu Composites in a Glassy Carbon Matrix. Russ. J. Phys. Chem. A. 2022;96(1):139-244. DOI: 10.1134/S0036024422010241.
- Garin A.B., Rakaric D., Andric E.K., Kosanovic M.M. Synthesis of monosubstituted dipicolinic acid hydrazide derivative and structural characterization of novel Co (III) and Cr (III) complexes. Polyhedron. 2019;166:226-232. DOI: 10.1016/j.poly.2019.03.059.
- Hiendrawan S., Veriansyah B., Widjojokusumo E., Soewandhi S. Simultaneous cocrystallization and micronization of paracetamol-dipicolinic acid cocrystal by su-percritical antisolvent (SAS). Int. J. Pharm. Sci. 2016; 8(2): 89-98.
- Agarwal S.K., Pant T.K. Spectral and thermal characterization of mixed-ligand coordination complexes of Fe (II) and Fe (III) with 2-furan thiocarboxyhydrazide as primary and picolinic/dipicolinic acid as co-ligand. Thermochim. Acta. 1991; 185(1): 177-182. DOI: 10.1016/0040-6031(91)80128-6.
- Slieman T.A., Nicholson W.L. Role of Dipicolinic Acid in Survival of Bacillus subtilis Spores Exposed to Artificial and Solar UV Radiation. Appl. Environ. Microbiol. 2001; 67(3): 1274-1279. DOI: 10.1128/AEM.67.3.1274-1279.2001.
- Dikec J., Pacheco M., Lavaud M., Winckler P. Uptake of UVc induced photoproducts of dipico-linic acid by Bacillus subtilis spores-Effects on the germination and UVc resistance of the spores. J. Photochem. Photobiol., B. 2022;236:112569. DOI: 10.1016/j.jphotobiol.2022.112569.
- X. Chen,. Xu J, Li Y., Zhaob T., Zhang L. Two birds with one stone: Visual colorful assessment of dipicolinic acid and Cu2+ by Ln-MOF hybrid attapulgite nano-probe. Appl. Surf. Sci. 2022; 605: 154665. DOI: 10.1016/j.apsusc.2022.154665.
- Abdolmaleki S., Ghadermazi M., Ashengroph M., Saffari A. Cobalt (II), zirconium (IV), calcium (II) complexes with dipicolinic acid and imidazole deriva-tives: X-ray studies, thermal analyses, evaluation as in vitro antibacterial and cytotoxic agents. Inorg. Chim. Acta. 2018;480:70-82. DOI: 10.1016/j.ica.2018.04.047.
- Çolak A.T., Çolak F., Yeçilel O.Z., Büyükgüngör O. Synthesis, characterization, crystal structure and biological activities of supramolecular com-pounds of Mn (II) and Zn (II) with dipicolinic acid and 8-hydroxyquinoline. J. Coord. Chem.2009;62(10): 1650-1660. DOI: 10.1080/00958970802676656.
- Derikvand Z., Bruno G., Rudbari H.A., Shokrollahi A. Synthesis, crystal structures, spectros-copic, thermal analysis, electrochemical and solution studies of two new mixed metal coordination polymers based on dipicolinic acid and 3,4-diaminopyridine. Inorg. Chim. Acta. 2014;410: 221-229. DOI: 10.1016/j .ica.2013.11.004.
- Nudelman R., Bronk B.V., Efrima S. Fluorescence emission derived from dipicolinic acid, its sodium, and its calcium salts. Applied Spectroscopy. 2000;54(3): 445-449. DOI: 10.1366/0003702001949564.
- Bruker. SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. Bruker AXS Inc., Madison, Wisconsin, USA, 1998.
- Bruker. SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data. Bruker AXS Inc., Madison, Wisconsin, USA, 1998.
- Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A.K., Puschmann H. OLEX2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009;42:339-341. DOI: 10.1107/S0021889808042726.
- Browning K., Abboud K.A., Palenik G.J. Sodium Hydrogen Dipicolinate Dipicolinic acid Tri-hydrate: Synthesis, Structure, and Valence Bond Sums. J. Chem. Crystallogr. 1995;25(12):851-855. DOI: 10.1007/BF01671082.
- Laid P., Gourdon A., Launay J.P. Chemistry of Iron with Dipicolinic Acid. 1. Mononuclear Complexes of Iron (II) or Iron (III). Inorg. Chem. 1995;34(21):5129-5137. DOI: 10.1021/ic00125a009.
- Santra S., Das B., Baruah J.B. Assembly of Dipicolinic Acid by Alkali-Metal Cations. J. Chem. Crystallogr. 2011 ;41(12): 1981-1987. DOI: 10.1007/s10870-011-0187-3.
- Winter K., Barton D. The Thermal Decomposition of Benzoic Acid. Can. J. Chem. 1979;48(24): 3797-3801. DOI: 10.1139/v70-641.
- Stevens W.H., Pepper J.M., Lounsbury M. The Decarboxylation of Anthranilic Acid. Can. J. Chem. 1952;30(7):529-540. DOI: 10.1139/v52-065.
- Dunn G.E., Thimm H.F. Kinetics and Mechanism of Decarboxylation of Some Pyridinecarbox-ylic Acids in Aqueous Solution. II. Can. J. Chem. 1977;55(8):1342-1347. DOI: 10.1139/v77-185.
- Sakthi Dharan C.P., Polozov M.A., Polozova V.V., Nayfert S.A. Features of the Thermolysis of Li, Na, and Cd Maleates. Russ. J. Phys. Chem. A. 2020;94(7): 1311-1318. DOI: 10.1134/S0036024420070250.
- Majer V., Svoboda V. Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation. Oxford: Blackwell Scientific Publications. 1985:172.


