Thermodynamic stability of intermetallic compounds in technical aluminum
Автор: Begunov Albert I., Kuzmin Mikhail P.
Журнал: Журнал Сибирского федерального университета. Серия: Техника и технологии @technologies-sfu
Статья в выпуске: 2 т.7, 2014 года.
Бесплатный доступ
For the following systems Al-Ti, Al-Ni, Al-Zr, Al-Cr, Al-Fe, Al-V the wide range of intermetallic compounds based on aluminum was considered. Thermodynamic parameters such as Gibbs energy and formation enthalpy characterizing the properties of resulting intermetallic compounds were calculated. The relations of these two characteristics versus temperature were established in a wide range. On the basis of the obtained relations the comparative assessment of the compound stability for each binary system was carried out. The level of each intermetallic compound stability was revealed on the ground of the affinity with aluminum. The possibilities of practical application of obtained data for actual metallurgical tasks were defi ned.
Formation enthalpy, gibbs energy, intermetallic compound, binary systems, technical aluminum
Короткий адрес: https://sciup.org/146114827
IDR: 146114827 | УДК: 669.713.7,
Текст научной статьи Thermodynamic stability of intermetallic compounds in technical aluminum
It is known that except main impurities of iron and silicon technical aluminum may contain also some impurities of refractory metals especially titanium and vanadium. Behavior of these impurities in crystallization and formation processes is poorly studied and interesting in terms of improving the processes of technical aluminum refining, as present technologies are not sufficiently effective in completeness these metals extraction.
At the iron concentration of 1330 ppm the concentration of vanadium can be 104 ppm, and titanium – 66 ppm [1]. Technical aluminum can also contain impurities of nickel, zirconium and chromium. When the content of impurities is only 50 – 100 ppm or less, the formation of intermetallic compounds (IMC) of these impurities with aluminum can significantly affect the mechanical and strength properties of the metal obtained and products based on it.
Interpretation and discussion of research results
To study the properties of intermetallic compounds formed the main direction of research was studying the characteristics of their thermodynamic stability. Such characteristics as Gibbs energy and
The quantity of intermetallic compounds formed is large and only for precisely identified compounds is: five for Al-Fe system (FeAl, FeAl 2 , FeAl 3 , Fe 2 Al 5 , Fe 3 Al), two for Al-Ti (AlTi, Al 3 Ti), four for Al-Ni (AlNi, AlNi3, Al3Ni, Al3Ni2), one for Al-Zr (AlZr2), three for Al-Cr (CrAl3, CrAl4, CrAl7) and two for Al-V system (AlV 3 , Al 5 V 8 ). A total, there are 17 identified intermetallic compounds of congruent and partially incongruent melting in six considered systems. Even more IMC in these systems cannot be definitively identified [1,2].
For all considered systems the calculations were carried out using classical calculations according to Vant Hoff’s equation and reference data for standard formation enthalpy, entropy, temperature rows of heat capacity and the temperatures and heats of phase change [2-4]. The calculations were carried out in a wide temperature range. The standard temperature of 298K was chosen as initial one and in the capacity of the final temperature, it was chosen the melting temperature of intermetallic compound or metal-impurity.
For some compounds the calculations were made with a help of the following software: “CHS Chemistry 5” and “SELECTOR”. However, these programs does not contain source data for the most part of considered intermetallic compounds. Therefore, for such compounds the special methods of binary systems thermodynamic characteristics calculations were applied [5]. These methods were adapted specifically for intermetallic compounds based on aluminum. Before calculating of studied systems thermodynamic characteristics, the accuracy of every technique was tested on 30 – 60 other intermetallic compounds. The inaccuracy of methods used is negligible – from 1 to 3 % [4].
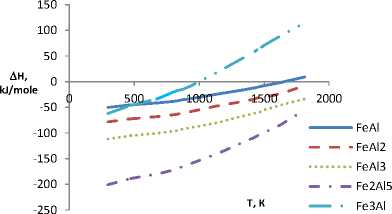
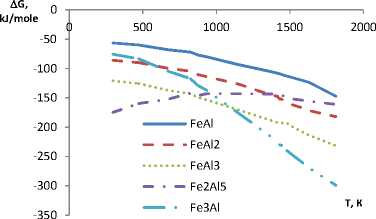
The calculation results indicate that chemical affinity between aluminum and titanium is slightly dependent on temperature and in the aluminum – nickel system this dependence is observed. Formation enthalpy of IMC for Al-Zr, Al-Cr, Al-Fe and Al-V systems in the considered range with increasing temperature shifts in the positive direction. Gibbs energy considerably shifts (3-4 times) in the negative direction, that is the affinity of the elements of these compounds and thermodynamic stability of IMC increases (Fig. 1, 2).
According to the data obtained, all considered compounds are stable in frames of studied temperature range, i.e. in frames of the temperature values aluminum forms IMC of different composition. In system of Al-Ti the most thermodynamically probable is the formation of Al3Ti, in system of Al-Ni – Al 3 Ni 2 . In systems of Al-Zr, Al-Cr, Al-Fe and Al-V are primarily formed compounds AlZr2, CrAl7, Fe3Al and V5Al8 respectively.
The character of enthalpy and Gibbs energy change in four last systems is slightly differs from each other. At the same time for IMC of all six systems Gibbs energy is considerably below zero, i.e. the compounds are quite stable at the temperatures from 298 to 2000 K. This statement refers to the melting temperature of aluminum (933 K) and intermetallic compound (table).
Summary and conclusions
The literature has a few data allowing to track the influence of the temperature on enthalpy. Therefore, the obtained data of the enthalpy versus temperature dependences are interesting due to the opportunity to determine heat capacity of liquid alloys on their basis.
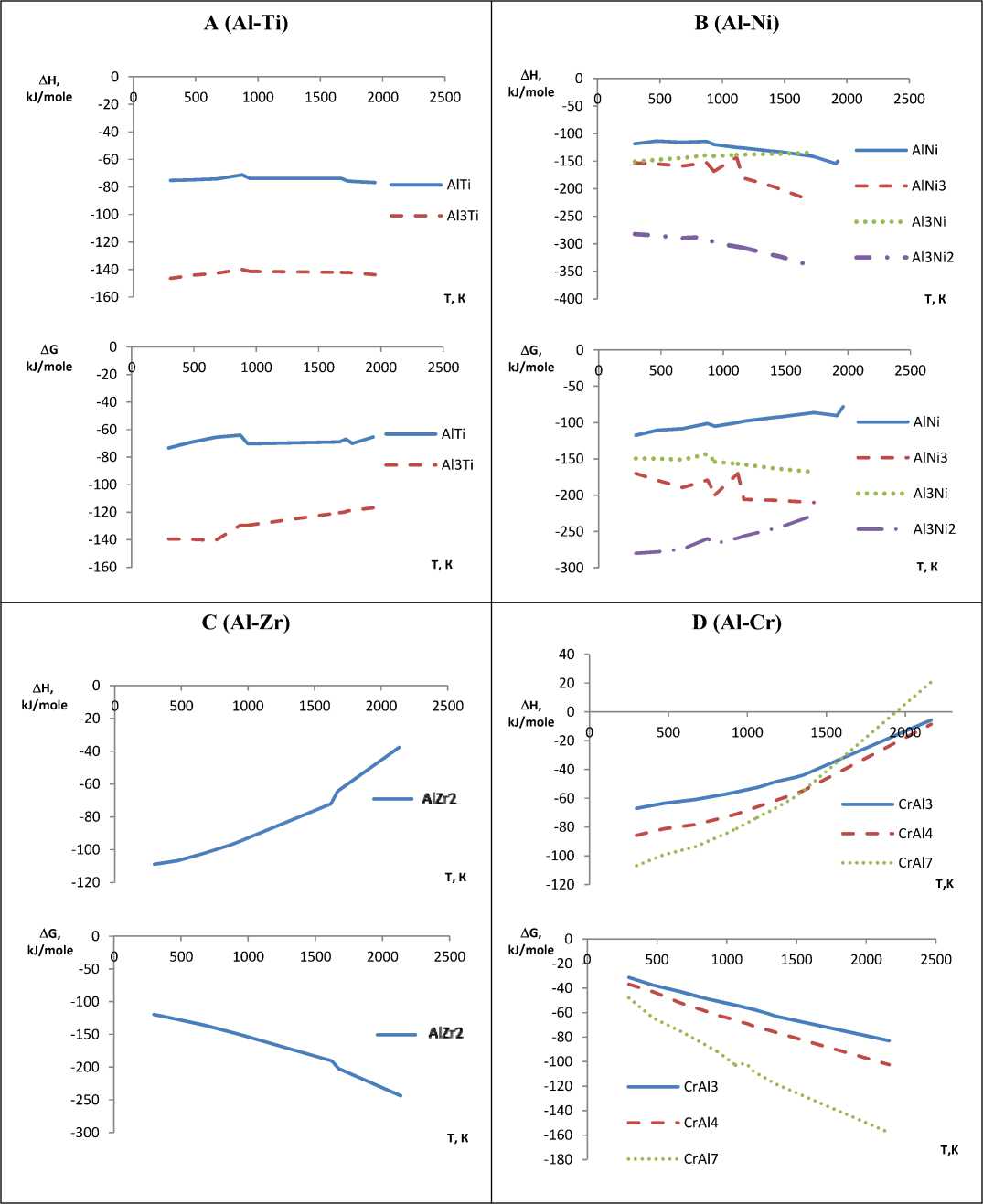
Fig. 1. The change of enthalpy and Gibbs energy of intermetallic compounds in aluminum versus temperature (systems: A – Al-Ti; B – Al-Ni; C – Al-Zr; D – Al-Cr)
F (Al-V)
E (Al-Fe)
H, 0 kJ/mole ‐50
‐100
‐150
‐200
‐250
‐300
‐350
G 0 kJ/mole ‐100
‐200
‐300
‐400
‐500
‐600
‐700
500 1000 1500 2000 2500

500 1000 1500 2000 2500

VAl3
V5Al8
Т, К
VAl3
V5Al8
Т, К
Fig. 2. The change of enthalpy and Gibbs energy of intermetallic compounds in aluminum versus temperature (systems: E – Al-Fe; F – Al-V)
Refractory, heat resisted and mechanically stable extraneous crystals of IMC in aluminum and alloys based on it are undesirable. Therefore, the development of efficient methods of refractory intermetallic compounds refinement is reasonable. It is also advisable to conduct the refinement with a view of further usage of metals-impurities especially such as zirconium, titanium and vanadium. These metals have a high value in the world market so their extraction from the technical aluminum can bring significant economic effect.
The results obtained on the thermodynamic stability and the affinity of the compounds to technical aluminum can be useful for engineering, electroconductivity, mechanical strength problems and also to improve other properties of technical aluminum samples.
Table. ΔH and ΔG values at the melting temperatures of aluminum and intermetallic compounds (IMC)
|
№ |
System |
IMC |
933 K |
При T m. |
т m. IMC,K |
||
|
ΔH, kJ/mole |
ΔG, kJ/mole |
ΔH, kJ/mole |
ΔG, kJ/mole |
||||
|
1 |
Al-Ti |
AlTi |
-73,691 |
-70,369 |
-75,727 |
-67,097 |
1720 |
|
Al 3 Ti |
-141,314 |
-129,539 |
-142,039 |
-120,417 |
1668 |
||
|
2 |
Al-Ni |
AlNi |
-119,804 |
-105,109 |
-154,389 |
-90,406 |
1911 |
|
AlNi 3 |
-168,469 |
-199,435 |
-142,920 |
-170,228 |
1115 |
||
|
Al 3 Ni |
-140,702 |
-154,091 |
-138,747 |
-156,874 |
1115 |
||
|
Al 3 Ni 2 |
-298,049 |
-266,634 |
-320,317 |
-246,106 |
1406 |
||
|
3 |
Al-Zr |
AlZr 2 |
-95,139 |
-150,083 |
-72,086 |
-190,462 |
1623 |
|
4 |
Al-Cr |
CrAl 3 |
-55,558 |
-102,758 |
-49,742 |
-113,875 |
1143 |
|
CrAl 4 |
-70,869 |
-134,819 |
-56,998 |
-154,117 |
1303 |
||
|
CrAl 7 |
-80,927 |
-165,558 |
-73,488 |
-182,786 |
1063 |
||
|
5 |
Al-Fe |
FeAl |
-33,219 |
-80,009 |
-4,04 |
-120,208 |
1583 |
|
FeAl 2 |
-58,385 |
-113,948 |
-37,239 |
-144,555 |
1365 |
||
|
FeAl 3 |
-89,785 |
-153,378 |
-61,239 |
-193,072 |
1430 |
||
|
Fe 2 Al 5 |
-159,908 |
-142,927 |
-106,016 |
-145,076 |
1444 |
||
|
Fe 3 Al |
-8,658 |
-137,018 |
-17,900 |
-117,513 |
825 |
||
|
6 |
Al-V |
VAl 3 |
-100,557 |
-136,710 |
-82,170 |
-167,446 |
1633 |
|
V5Al8 |
-269,018 |
-397,915 |
-179,443 |
-572,148 |
1943 |
||


